Abstract
MBL structurally contains a type II-like collagenous domain and a carbohydrate recognition domain (CRD). We have recently generated three novel recombinant chimeric lectins (RCL), in which varying length of collagenous domain of mannose-binding lectin (MBL) is replaced with that of L-ficolin (L-FCN). CRD of MBL is used for target recognition because it has a broad spectrum in pathogen recognition compared with L-FCN. Results of our study demonstrate that these RCLs are potent inhibitors of influenza A virus (IAV). RCLs, against IAV, show dose-dependent activation of the lectin complement pathway, which is significantly higher than that of recombinant human MBL (rMBL). This activity is observed even without MBL-associated serine proteases (MASPs, provided by MBL deficient mouse sera), which have been thought to mediate complement activation. These observations suggest that RCLs are more efficient in associating with MASP-2, which predominantly mediates the activity. Yet, additional serum further increases the activity while RCL-mediated coagulation-like enzyme activities are diminished compared with rMBL, suggesting reduced association with MASP-1, which has been shown to mediate coagulation-like activity. These data suggest that RCLs may interfere less with host coagulation, which is advantageous to be a therapeutic drug. Importantly, these RCLs have surpassed rMBL for anti-viral activities, such as viral aggregation, reduction of viral hemagglutination (HA) and inhibition of virus-mediated HA and neuraminidase (NA) activities. These results are encouraging that novel RCLs could be used as anti-IAV agents with less side effect and that RCLs would be suitable candidates in developing a new anti-IAV therapy.
Keywords: innate immunity, mannose-binding lectin, ficolin, influenza A virus, complement, coagulation
1. Introduction
IAV is an RNA virus whose surface is enveloped with glycoproteins containing neuraminidase (NA) and hemagglutinin (HA), which have glycosylation sites [1]. IAV infection is a common infection that could result in fatal complications, even in individuals who are appeared to be healthy [2, 3]. Mortality and hospitalization are estimated to exceed annually more than 30,000 and 200, 000, respectively in the United States alone [2]. Prevention is currently relied upon immunization, however vaccines are less effective in elderly and are not approved by the FDA for infants younger than 6 months of age [3, 4]. Resistance to antiviral agents has developed in seasonal and pandemic IAV strains [2, 5]. Thus, there is a need for new effective anti-IAV therapeutics.
The first line of host defense is the innate immunity, which recognizes pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors and soluble molecules that include lectins [6]. One of such lectins is MBL, which is primarily synthesized in the liver and circulates in the blood [7–9]. MBL belongs to the collectin family that is structurally characterized by consisting of a type II-like collagenous domain at N-terminus, followed by a neck region and a carbohydrate recognition domain (CRD) at C-terminus [10]. The collectin family includes lung surfactant protein (SP)-A and SP-D [11]. These surfactant proteins have anti-viral functions [12–17] and mice lacking SP-A or SP-D have increased susceptibility to AIV infection [14, 18].
In early 1990, MBL was identified as a β-inhibitor, which had been discovered as an IAV inactivating serum factor in the 1940’s [19]. Since then, many studies have described MBL’s anti-IAV functions, including inhibition of viral hemagglutination, inhibition of HA and NA and viral neutralization [20–23]. MBL activates complement via the lectin complement pathway, which is a key biologic function along with other complement pathways, such as the classical and the alternative complement pathway. The classical complement pathway is mediated by C1rs proteases, which are replaced by MASP-1, MASP-2 and/or MASP-3, in the lectin complement pathway [24]. Both pathways cleave C4 and C2 to generate the C3 convertase, C4bC2a [25, 26].
MBL-MASP complex also initiates coagulation via thrombin-like activity [27, 28]. Coagulation is a primitive yet effective host defense mechanism. For example, tachylectins in horseshoe crab hemolymph provide immune protection by clotting lipopolysaccharide and β-glucan, pattern recognition molecules of pathogens (PAMPs of Gram negative bacteria and fungus, respectively) [29].
The collectin family also includes L-FCN and H-FCN, which are also circulating serum pattern recognition molecules of innate immune system [30]. Like MBL, both FCNs contain the collagenous domain while CRD is replaced with fibrinogen-like domain, which preferentially recognizes acetylated molecules and sialic acid [31, 32]. In contrast, MBL’s target recognition is broad, including mannose, which is widely expressed on many pathogens [33]. It has been shown that other chimeric lectins consisting of MBL-CRD and the collagenous domain of SP-D gain anti-IAV activities, such as viral aggregation, inhibition of HA, NA and viral infectivity [22, 34].
We have previously generated three RCLs consisting of L-FCN and MBL, in which various length of the collagenous domain was replaced with that of L-FCN [23]. Previous characterization study has demonstrated that these RCLs are either comparable to or surpassed rMBL for several biologic activities, including their binding to Nipah, Hendra and Ebola viruses [23]. Here, we further characterized biologic activities of these lectins against IAV using in vitro system and will discuss our findings.
2. Materials and methods
2.1. Recombinant chimeric lectins
Chimeric lectins were produced as previously described [23]. In this study, these lectins are named RCL1, RCL2 and RCL3, corresponding to L-FCN/MBL126, L-FCN/MBL76 and L-FCN/MBL64, respectively in the previous publication. All RCLs have MBL-CRD while MBL-collagenous domain was replaced with 126, 76 or 64 amino acids of L-FCN’s collagenous domain, resulting in total amino acid length of 251, 255 or 254 for RCL1, RCL2 or RCL3, respectively. Thus, overall amino acid length is similar while RCL1 has the longest L-FCN collagenous domain followed by RCL2 and then RCL3. The junction of two proteins in RCL2 is located at the middle of a putative MASP-binding domain.
2.2. Virus preparations
IAV (A/Phillipines/82(H3N2)) was prepared as previously described [35]. Briefly, IAV was grown in the chorioallantoic fluid of chicken eggs and purified on a discontinuous sucrose gradient (Sigma-Aldrich, St. Louis, MO). Virus stocks were dialyzed against PBS (Sigma-Aldrich, St. Louis, MO) and aliquots were stored at −80°C. HA titers were determined by titration with human type O, Rh− red blood cells (RBCs) in PBS.
2.3. MBL binding assay
This assay was performed using previously described methods with a minor modification [36]. IAV concentration was arbitrary defined as 1,000 units/ml, whish was determined to be optimal for many in vitro studies base on dose response experiments. Briefly, 96 well plates were coated with mannan (Sigma-Aldrich, St. Louis, MO) or IAV and then blocked. Following wash, the wells were incubated with indicated concentrations of recombinant lectins. After wash, bound MBL was detected by mouse anti-hMBL monoclonal Ab (2A9, a gift from Dr. Gregory Stahl)[37], followed by alkaline-phosphatase conjugated anti-mouse Ab (Promega, Madison, WI) and pNTP substrate (Sigma-Aldrich, St Louis, MO). Reaction was read at 415 nm using SpectraMax M5 (Molecular Devices, Sunnyvale, CA) and expressed as OD415 nm reading. Assays were performed in triplicates and were repeated at least twice.
2.4. Mouse sera
MBL null mice were previously generated and fully backcrossed onto C57Black/6J [36, 38]. Sera were collected and stored at −80°C prior to the study. All animal experiments were performed under a protocol approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital, Boston, MA.
2.5. Assays of the lectin complement activity
The lectin pathway assay was performed with a minor modification of previously described method [36]. Briefly, 96 well plates were coated with mannan or IAV as above. After wash and block, the wells were incubated with various concentrations of lectins with or without 1% MBL null sera (MASP source) diluted in a binding buffer, 10 mM Tris, pH 7.8, 10 mM CaCl2, 1M NaCl (all chemicals were purchased from Sigma-Aldrich, St. Louis, MO). After wash, the wells were incubated with human C4 and incubated at 37° C. After wash, the wells were incubated with rabbit anti-hC4c Ab (Dako, Carpinteria, CA) followed by biotin-conjugated anti-rabbit Ab, alkaline phosphatase-conjugated biotin-avidin (ABC-AP system, Vector Labs, Burlingame, CA) and then with pNTP (Sigma-Aldrich, St. Louis, MO). The plates were read at 415 nm. Binding activity was expressed as OD 415 nm reading. Pooled human serum with known MBL concentration and C4 activity, which was arbitrarily defined as 1,000 U/ml (State Serum Institute, Denmark), was used to generate a standard curve on mannan-coated wells. Assays were performed in triplicates and were repeated twice.
2.6. Assay of thrombin-like and factor Xa-like activities
These activities were assayed using previously described methods [39]. Briefly, 384 well plates were coated with mannan or IAV as above. After wash, the wells were incubated with various concentrations of lectins with or without 1% MBL null mouse serum or 1% MASP-1/3 null mouse serum (MASP source) [40] diluted in the binding buffer. After wash, wells were incubated with rhodamine 110-thrombin substrate (R22124, Invitrogen, Carlsbad, CA) or amino-4-methylcoumarin acetate (AMC)-factor Xa substrate (222F, American Diagnostica Inc., Stamford, CT) and read at 500 nm excitation/520 nm emission or 360 nm excitation/440 nm emission, respectively, using SpectraMax M5. The results were expressed as arbitrary units (AUs). Assays were performed in triplicates and were repeated twice.
2.7. Viral neutralizing assay
The assay was performed as previously described [41]. Briefly, viruses were pre-incubated with lectins and washed and then incubated with Madin-Darby Canine Kidney (MDCK) cells. Infection was assayed by FITC-conjugated anti-IAV antibody (Ab)(Millipore, Billerica, MA). Virus neutralizing activity as %inhibition was calculated by the formula: FFC in test samples/FFC in saline control × 100. Data from 5 experiments were combined.
2.8. HA and HA inhibition assay
The assay was previously described [42]. Briefly, virus was incubated with lectins at various concentrations and assayed for HA titer using human type O, Rh− RBCs. HA inhibition of IAV by recombinant lectins at 2 μg/ml was measured in round-bottom 96-well plates (Serocluster U-Vinyl plates; Costar, Cambridge, MA). HA inhibition was detected as the formation of a RBC pellet. To enable graphical comparisons of HA inhibition, data were mathematically converted and expressed as the number of HA units inhibited by the lectins. Four experiments were combined.
2.9. NA inhibition assay
The assay was performed using Amplex Red Neuraminidase Assay kit (A22178, Molecular Probes, CA), according to the manufacture’s instructions. IAV (500 units/ml) was mixed with recombinant lectins at at 2 μg/ml and Amplex Red reagents in 40 μl reaction volume. Assay was performed in triplicate. Reaction was read at 530 nm excitation/590 nm emission using SpectraMax M5. The results were expressed as %inhibition calculated by the formula: [(IAV−(IAV + recombinant lectins)) × 100 ]/IAV.
3.9. Viral aggregation assay
The assay was previously described [43]. Briefly, at time 0, viral suspensions were mixed with 800 ng/ml of lectins in PBS++ in a final volume of 1.0 ml. Under continuous stirring, the light transmission was monitored at excitation/emission 350 nm during 12 min using an SLM/Aminco 8000 C (SLM Instrument, Urbana, IL) spectrofluorometer. A decline in light transmission correlates with viral aggregation. Results are expressed as percent of control light transmission (virus without lectins). Six experiments were combined.
2.10 Statistical analysis
All data was analyzed by ANOVA or Wilcoxon/Kruskal-Wallis tests depending on data distribution using JMP software (SAS institute Inc., Cary, NC). P values less than 0.05 was considered to be significant.
3. Results
3.1. RCLs bind IAV as efficient as rMBL
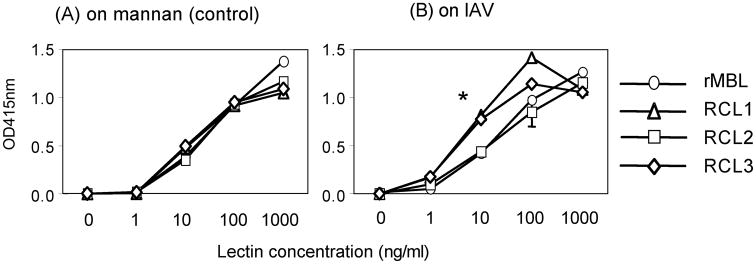
We first confirmed RCLs’ binding ability to IAV by comparing to mannan because all RCLs have MBL-CRD, which preferentially recognizes mannan [23]. Therefore, mannan was used as a positive control throughout the investigation. All RCLs bound to mannan at comparable efficiency as rMBL in a dose dependent manner (Fig. 1A). Similarly, all RCLs bound to IAV in a dose dependent manner although RCL1 and RCL3 demonstrated significantly more efficient binding at 10 ng/ml compared with RCL2 and rMBL (Fig. 1B). Thus, all RCLs bound to mannan and IAV in a dose response manner, demonstrating that all RCLs were biologically functional for the target binding activity.
Fig. 1.
Lectin binding assay to mannan (A) or IAV (B). Bound lectins on mannan (control) or influenza A virus (IAV) were detected by monoclonal anti-MBL antibody, which detect all RCLs. Open circles, rMBL; open triangles, RCL1; open squares, RCL2; and open diamonds, RCL3. Data were expressed as mean ± SD, all of which are smaller than sizes of symbols. *, p<0.05.
3.2. RCLs activate the lectin complement pathway more efficiently than rMBL
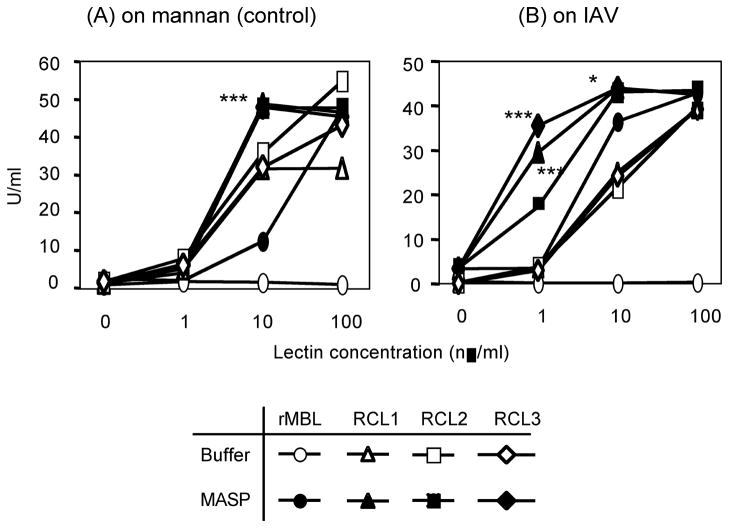
On mannan, rMBL showed C4 deposition in a dose dependent manner only when MASPs (MBL null serum) was supplied as expected (open circles vs. closed circles in Fig. 2A). In contrast, all RCLs even without MASPs showed significant C4 deposition activity. C4 deposition activity by RCLs alone was significantly (p<0.0001) higher than that of rMBL even at 10 ng/ml (p < 0.05, 0.005 and 0.0001 for RCL2, RCL1 and RCL3, respectively). At 100 ng/ml, C4 deposition activity of RCL2 was highest followed by RCL3 and then RCL1 (P<0.005 for both RCL4 vs. RCL3 and RCL3 vs. RCL1)(Fig. 2A). Addition of MASPs significantly (p<0.0001) increased C4 deposition activity reaching to plateau at 10 ng/ml. All RCLs demonstrated comparable activities and were significantly stronger than rMBL at 10 ng/ml (Fig. 2A).
Fig. 2.
The lectin complement pathway activation activity on mannan (A) or IVA (B). The lectin pathway activity was assayed as C4 deposition (U/ml) as described in the materials and method. Open circles, rMBL; open triangles, RCL1; open squares, RCL2; and open diamonds, RCL3. Closed symbols are with MBL null sera to supply MASP. C4 deposition activities (U/ml) were expressed as mean ± SE, most of which are smaller than sizes of symbols. *, p < 0.05; ***, p < 0.0001.
On IAV, C4 deposition activity of RCLs alone was also observed while it was not detectable by rMBL alone (Fig. 2B). Similar to their activity on mannan, all RCLs alone activated C4 even at 1 ng/ml (p < 0.005 and 0.05 for RCL2 and RCL 3, respectively and no significance for RCL 3)(Fig. 2B). Once again, addition of MASP significantly (p<0.0001) enhanced the C4 deposition activity even at 1 ng/ml on IAV, unlike on mannan (Fig. 2A vs. 2B) and reached to plateau at 10 ng/ml. At 1 ng/ml, C4 deposition activity by RCLs with MASPs was significantly (p<0.005) stronger than rMBL/MASP complex (Fig. 2B).
Taken together, these results demonstrated that RCLs were more efficient in activating the lectin pathway mediated C4 deposition on both mannan and IAV.
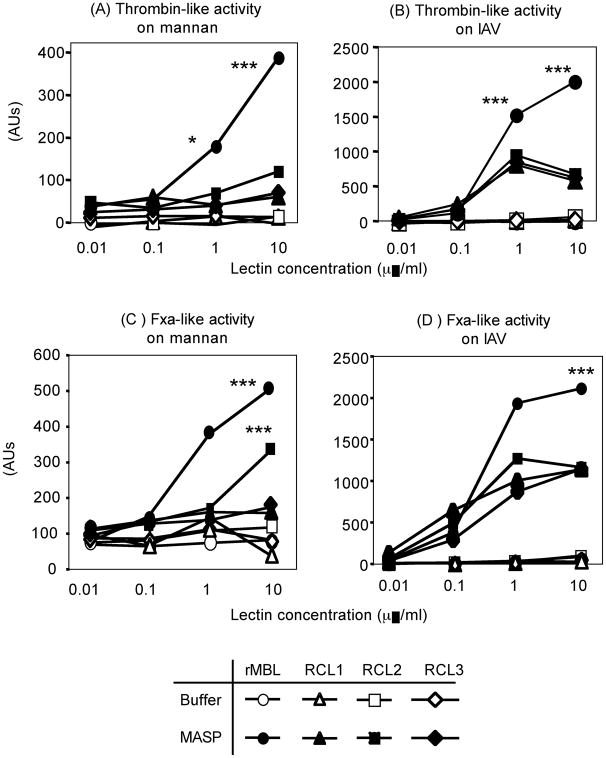
3.3. rMBL strongly activates coagulation enzyme-like activities compared with all RCLs
On mannan, thrombin-like activity was observed in a dose dependent manner by rMBL when MASP was supplemented as expected (Fig. 3A). Unlike C4 deposition activity, no thrombin-like activity was detected without MASP by any recombinant lectin on both mannan and IAV (Fig 3A and 3B). Even when MASP was supplied, only MBL mediated significant thrombin-like activity on mannan (Fig. 3A). In contrast, all recombinant lectins activated thrombin-like activity on IAV at 1 and 10 μg/ml. rMBL-mediated activity was significantly stronger than that of RCLs (Fig. 3B).
Fig. 3.
Thrombin-like activities on mannan (A) or IAV (B) and FXa-like activities on mannan (C) or IAV (D). These activities were assayed using enzyme-specific peptide substrates, which become fluorescent upon enzyme digestion. Open circles, rMBL; open triangles, RCL1; open squares, RCL2; and open diamonds, RCL3. Closed symbols are with MBL null sera to supply MASP. Activities (arbitrary units, AUs) were expressed as mean ± SE, most of which are smaller than sizes of symbols. *, p < 0.05; ***, p < 0.0001.
Similar to thrombin-like activity, when MASP was supplied rMBL and RCL2 significantly activated FXa-like activity on mannan compared with R1 and R3. However, RCL2 showed the activity only at 10 μg/ml while rMBL was significantly active even at 1 μg/ml (Fig. 3C). Once again, all recombinant lectins activated FXa-like activity on IAV at as low as 0.1 μg/ml when MASP was supplied. MBL-mediated activity was significantly stronger than that of RCLs (Fig. 3D).
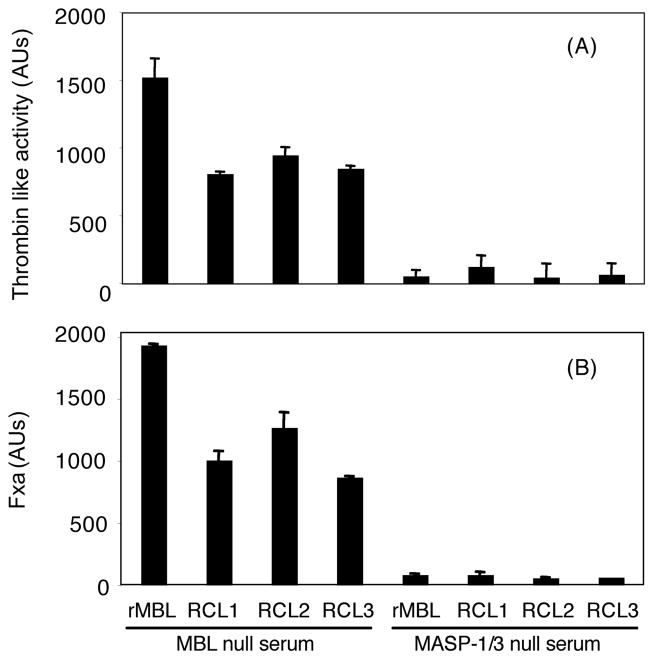
For both thrombin-like and FXa-like activities, when MASP-1/3 null mouse serum was used as MASP source these activities were abrogated to nearly undetectable levels as this was expected from our previous study (Fig. 4)[39]
Fig. 4.
MASP-1/3 dependent thrombin-like (A) and FXa-like (B) activities. Assays were performed as in Fig. 3. Lectins were used at 1 μg/ml and mixed with 1% of MBL null or MASP- 1/3 null mouse sera. Data were expressed as mean ± SE, most of which are smaller than sizes of symbols. The data shown is the representative result of three experiments.
3.4. RCLs demonstrate efficient anti-IAV biologic functions
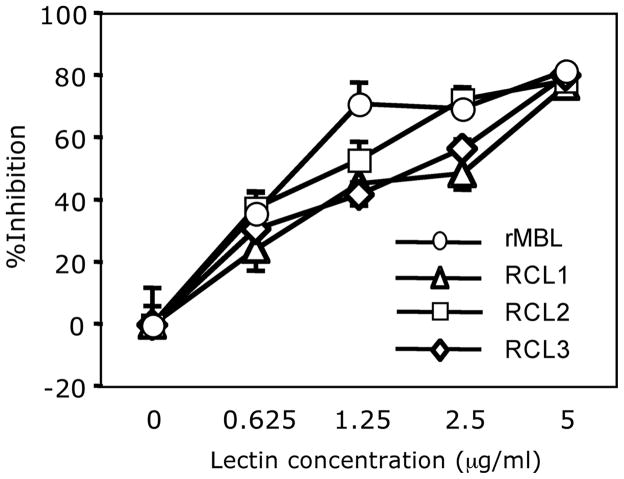
All RCLs inhibited MDCK cell infection in a dose dependent manner. The activity was comparable to rMBL (Fig. 5). IC50s (μg/ml ± SE) for RCL1, RCL2, RCL3, and rhMBL were 1.12 ± 0.15, 0.72 ± 0.15, 1.09 ± 0.14, and 0.81 ± 0.16, respectively. Thus, all lectins were active in inhibiting IAV infection to MDCK epithelial cells.
Fig. 5.
IAV neutralizing assay. Neutralizing activity was assayed as %inhibition of viral infection to MDCK cells by comparing lectin-pretreatment to no lectin-pretreatment. Open circles, rMBL; open triangles, RCL1; open squares, RCL2; and open diamonds, RCL3. Results were expressed as mean ± SE of %inhibition. *, p<0.05.
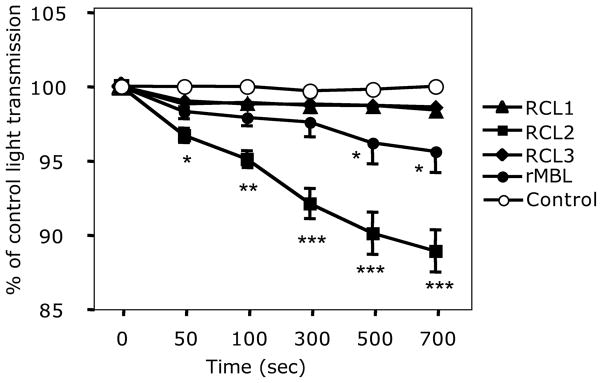
In IAV aggregation activity, RCL2 exceeded all other RCLs and rMBL (Fig. 6). IAV aggregation by RCL2 was detected as early as 50 seconds into incubation (Fig. 6). rMBL aggregated IAV better than both RCL1 and RCL3 after incubation time at 500 and 700 seconds (p < 0.05 for both time points, Fig. 6).
Fig. 6.
Viral aggregation assay by lectins. Viral aggregation by lectins was assayed by the reduction of light transmittance and expressed as % of control (saline) light transmittance. Data were expressed as mean ± SE. *, p < 0.05; **, p < 0.005; ***, p < 0.0001.
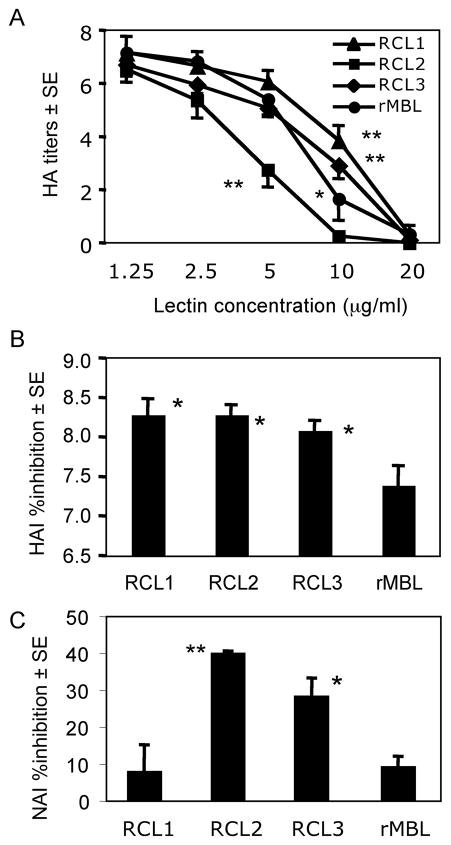
RCLs inhibited HA titers as potent as rMBL. Similar to viral aggregation results, RCL2 significantly (p < 0.001) reduced HA titer at 5 μg/ml compared with other RCLs and rMBL (Fig. 7A). RCL2 abolished HA titer at 10 μg/ml while RCL1, RCL3 and rMBL required 20 μg/ml to achieve the same effect (Fig. 7A).
Fig. 7.
HA titers and HA inhibition (HAI) and NA inhibition (NAI) by lectins. (A) HA titers are inhibited by lectins. Closed circles, rMBL; closed triangles, RCL1, closed squares, RCL2; and closed diamonds, RCL3. The results were expressed as mean ± SE. (B) HAI assay complements lectin’s effects on HA titers in Fig. 7A. Bars indicated mean ± SE. *, p<0.05 against rMBL. (C) NAI assay examines lectin’s effect on viral NA activity. Bars indicated mean ± SE. **, p<0.005; *, p<0.05. Both statistics are against RCL1 and rMBL.
As complementing lectins ability to reduce HA titer, HA inhibition was assayed at 2 μg/ml for all chimeric lectins. The results showed that all RCLs significantly inhibited HA compared with rMBL (Fig. 7B), demonstrating that all RCLs were significantly more efficient in inhibiting IAV-mediated HA.
One of other anti-viral activity is inhibition of NA. RCL2 and RCL3 significantly inhibited NA compared with RCL1 and rMBL, the latter two of which showed similar NA inhibitory activity (Fig 7C). These results suggested that RCL2 and RCL3 were significantly effective in inactivating NA. Taken together, these results further suggest that RCLs, in particular RCL2 was effective in preventing IAV infection into host cells.
4. Discussion
In this study, we have demonstrated that novel RCLs, in particular, RCL2, is efficient anti-IAV agent as we summarize our findings in Table 1. RCLs have been generated by replacing various portion of the collagenous domain of MBL with that of L-FCN, thus utilizing MBL-CRD for target recognition. MBL-CRD is chosen based on our understanding that MBL-CRD has broader target recognition than L-FCN, which preferentially recognizes acetylated compounds [44]. These results support our recent findings that these RCLs have a better binding activity against Ebola, Nipah and Hendra viruses [23]. Taken together, these results demonstrate that novel RCLs have broad and potent anti-viral activities compared with its parent rMBL.
Table 1.
Summary of RCL activities
| Activities | Recombinant lectins |
|||
|---|---|---|---|---|
| RCL1 | RCL2 | RCL3 | rMBL | |
| Binding | + | + | + | + |
| LCP activation activity | ++ | +++ | ++ | + |
| Thrombin-like activity | + | + | + | +++ |
| FXa-like activity | + | + | + | +++ |
| Viral aggregation | + | +++ | + | + |
| Reduction of HA titer | + | ++ | + | + |
| HA inhibition | ++ | ++ | ++ | + |
| NA inhibition | + | +++ | ++ | + |
| Viral infectivity (in vitro) | + | + | + | + |
Note: LCP, lectin complement pathway; FXa, factor X-activated; HA, hemaggulutination; NA, neuraminidase. Activities are scored as follows:
positive;
strong;
very strong.
Binding ability of all RCLs on mannan (a positive control) was in a dose dependent manner and was comparable to that of rMBL. Thus, RCLs maintain MBL-CRD binding activity and introduction of the collagenous domain of L-FCN does not alter the MBL-CRD function. Similar to on mannan, RCLs bind IAV in a dose dependent manner at comparable levels to rMBL, confirming that MBL-CRD is functional in binding to IAV.
MBL in a complex with MASPs initiates the lectin complement pathway, one of the key functions of MBL. By replacing various portion of collagen domain with that of L-ficolin, all RCLs without MASP activate the lectin complement pathway, which is also observed by rMBL but requires 10 μg/ml, 1,000-fold more protein (unpublished observation). Importantly, RCL-mediated lectin complement pathway activation activity is augmented by MASP supplementation, suggesting that the lectin complement pathway activation activity would be efficiently augmented in vivo. We have previously shown that the lectin complement pathway activation activity correlates with host protection from bacterial infection, including S. aureus infection in vivo [36, 45]. Taken together, these observations suggest that RCLs are efficient activators of the lectin complement pathway and could be administered with smaller dose than rMBL. Further investigation is required to determine in vivo efficiency.
Although SP-A and SP-D belong to the collectin family and share structural and functional similarity with MBL and FCNs these surfactant proteins do not activate the lectin complement pathway [46]. SP-A rather inhibits complement activation [47]. The chimeric protein of SP-D collagenous domain and MBL-CRD has increased anti-viral characteristics compared with its parents [34], however complement activation activity has not been examined. Interestingly, when Wallis and his colleagues introduced MASP binding sequence of MBL/FCN into SP-A the chimeric SP-A became constitutively active in the lectin complement pathway. This is not the case for MBL/FCN chimera because their activities are dose dependent and correlate with ligand binding. Nevertheless, superb lectin complement pathway activities of RCLs may be explained by the idea that altering MASP-binding region might have allowed to increase MASP-2 binding to RCLs and also MASP-2 activity itself because the lectin complement pathway is predominantly mediated by MASP-2 [24].
Conversely, compared with rMBL, all RCLs now have reduced thrombin-like and FXa-like coagulation enzyme activities, which are mediated by MASP-1/3. MASP-1 has been linked to coagulation enzyme-like activities as we also have demonstrated in this study, confirming previous findings of our own and others [28, 39]. One can speculate that while all RCLs efficiently bind to MASP-2 their MASP-1 binding is diminished either by competition or structural conformational change. Another possibility is due to triggering different amplification loop. It has been proposed that the alternative pathway amplifies the classical pathway and the lectin pathway [48–50]. There could be other such amplification loop as it has become clear that interaction of complement pathway and coagulation pathway is more complex. Further detailed investigations are required to dissect out such pathways and cascades.
The reduced coagulation enzyme-like activities of RCLs is advantageous over rMBL for the following reasons: Blood fluidity is maintained on a fine balance of clotting (coagulation/thrombosis) and bleeding, thus, tip over to one side would trigger coagulation disorders, including disseminated intravascular coagulation [51]. So far, a phase 1 clinical study of rMBL did not result in adverse effect in healthy volunteers [52]. Taken together, our results suggest that RCLs have low coagulation enzyme-like activities, such as thrombin-like and FXa-like activities. This should be advantageous in clinical use, as it would have less risk to trigger coagulation disorder and related side effects.
MBL has been known to neutralize virus as this protein was initially discovered as a serum factor, the β-inhibitor of IAV in 1940s and re-identified to be MBL in 1990s [19]. Viral neutralization is assayed by infectivity of lectin-treated virus to MDCK cells, thus the activity indicates integrity of virus [41]. All RCLs neutralize IAV at similar level to rMBL, suggesting that all RCLs are at least functionally as efficient as rMBL in vitro. Further investigations would require assessing these activities in more physiological way, in which primary cells would be used in the presence of serum as well as in vivo infection studies.
Further characterization has revealed that these RCLs, in particular RCL2 surpasses rMBL for important aspects of anti-IAV functions. For example, aggregation of virus is an efficient anti-viral mechanism as aggregated virus loses infectivity [20]. In regard to this activity, RCL2 surpassed all other lectins, including other two RCLs. RCL2’s superb anti-viral activity is also evident in reduction of HA titer. RCL2’s activity is further confirmed by HA inhibition, which is an important anti-viral activity as it suggests that RCL2 would inhibit viral attachment to the host cells [20], thereby preventing infection. Similarly, RCL2 inhibits NA, suggesting that RCL inactivate NA that digests sialic acid, which is expressed on intact host cells, and promotes infection [22]. The finding confirms and extends the previous report that MBL not only binds to NA but also inactivates its activity [22]. The RCL2’s superior activities may be partly attributed to significantly higher binding affinity to mannan and GlcNAc, another favored MBL-ligand, compared with other lectins [23].
In conclusion, our results demonstrate that RCLs, in particular RCL2, are more potent anti-IAV reagents than MBL as summarized in the Table 1. We also speculate that these RCLs would also recognize infected host cells that may be expressing HA and NA. Our studies also provide new insights into understanding how collagenous domains would contribute to biologic functions, such as complement activation and coagulation, which are mediated by MASPs. Further in vivo investigation is encouraged to examine their efficiency to use as a new innate immune therapeutics against IAV infections.
Acknowledgments
We would like to thank Enzon pharmaceuticals for providing rMBL. We also thank Dr. Gregory Stahl, Center for Experimental Therapeutics and Reperfusion Injury, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA02115, for providing mouse anti-human MBL monoclonal antibody (2F9). This work was supported by NIH grants UO1 AI074503-01 and R21 AI077081-01A1 (KT) and UO1 AI070330-01 (EVS).
Footnotes
All authors have no financial conflict.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nayak DP, Hui EK, Barman S. Assembly and budding of influenza virus. Virus Res. 2004;106:147–65. doi: 10.1016/j.virusres.2004.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch JP, 3rd, Walsh EE. Influenza: evolving strategies in treatment and prevention. Sem Respir Crit Care Med. 2007;28:144–58. doi: 10.1055/s-2007-976487. [DOI] [PubMed] [Google Scholar]
- 3.Munoz FM. Influenza virus infection in infancy and early childhood. Paed Respir Rev. 2003;4:99–104. [PubMed] [Google Scholar]
- 4.Bouree P. Immunity and immunization in elderly. Pathol Biol (Paris) 2003;51:581–5. doi: 10.1016/j.patbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Saito R, Sato I, Suzuki Y, Baranovich T, Matsuda R, Ishitani N, et al. Reduced Effectiveness of Oseltamivir in Children Infected With Oseltamivir-Resistant Influenza A(H1N1) Viruses With His275Tyr Mutation. Pediatr Infect Dis J. 2010 doi: 10.1097/INF.0b013e3181de9d24. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 7.Uemura K, Saka M, Nakagawa T, Kawasaki N, Thiel S, Jensenius JC, et al. L-MBP is expressed in epithelial cells of mouse small intestine. J Immunol. 2002;169:6945–50. doi: 10.4049/jimmunol.169.12.6945. [DOI] [PubMed] [Google Scholar]
- 8.Hansen S, Thiel S, Willis A, Holmskov U, Jensenius JC. Purification and characterization of two mannan-binding lectins from mouse serum. J Immunol. 2000;164:2610–8. doi: 10.4049/jimmunol.164.5.2610. [DOI] [PubMed] [Google Scholar]
- 9.Oka S, Ikeda K, Kawasaki T, Yamashina I. Isolation and characterization of two distinct mannan-binding proteins from rat serum. Arch Biochem Biophys. 1988;260:257–66. doi: 10.1016/0003-9861(88)90448-1. [DOI] [PubMed] [Google Scholar]
- 10.Weis WI, Drickamer K. Trimeric structure of a C-type mannose-binding protein. Structure. 1994;2:1227–40. doi: 10.1016/S0969-2126(94)00124-3. [DOI] [PubMed] [Google Scholar]
- 11.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 12.Crouch E, Hartshorn K, Ofek I. Collectins and pulmonary innate immunity. Immunol Rev. 2000;173:52–65. doi: 10.1034/j.1600-065x.2000.917311.x. [DOI] [PubMed] [Google Scholar]
- 13.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Ame Thorac Soc. 2007;4:252–7. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol. 2001;167:5868–73. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- 15.Crouch E, Hartshorn K, Horlacher T, McDonald B, Smith K, Cafarella T, et al. Recognition of mannosylated ligands and influenza A virus by human surfactant protein D: contributions of an extended site and residue 343. Biochem. 2009;48:3335–45. doi: 10.1021/bi8022703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartshorn KL, Webby R, White MR, Tecle T, Pan C, Boucher S, et al. Role of viral hemagglutinin glycosylation in anti-influenza activities of recombinant surfactant protein D. Respir Res. 2008;9:65. doi: 10.1186/1465-9921-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tecle T, White MR, Sorensen G, Gantz D, Kacak N, Holmskov U, et al. Critical role for cross-linking of trimeric lectin domains of surfactant protein D in antiviral activity against influenza A virus. Biochem J. 2008;412:323–9. doi: 10.1042/BJ20071663. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Siddiqui J, Hendry M, Akiyama J, Edmondson J, Brown C, et al. Surfactant protein-A--deficient mice display an exaggerated early inflammatory response to a beta-resistant strain of influenza A virus. Am J Respir Cell Mol Biol. 2002;26:277–82. doi: 10.1165/ajrcmb.26.3.4584. [DOI] [PubMed] [Google Scholar]
- 19.Anders EM, Hartley CA, Jackson DC. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci U S A. 1990;87:4485–9. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartshorn KL, Sastry K, Brown D, White MR, Okarma TB, Lee YM, et al. Conglutinin acts as an opsonin for influenza A viruses. J Immunol. 1993;151:6265–73. [PubMed] [Google Scholar]
- 21.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204–12. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kase T, Suzuki Y, Kawai T, Sakamoto T, Ohtani K, Eda S, et al. Human mannan-binding lectin inhibits the infection of influenza A virus without complement. Immunol. 1999;97:385–92. doi: 10.1046/j.1365-2567.1999.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michelow IC, Dong M, Mungall BA, Yantosca LM, Lear C, Ji X, et al. A Novel L-ficolin/Mannose-binding Lectin Chimeric Molecule with Enhanced Activity against Ebola Virus. J Biol Chem. 2010;285:24729–39. doi: 10.1074/jbc.M110.106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi M, Mori S, Shigeta S, Fujita T. Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway. Adv Exp Med Biol. 2007;598:93–104. doi: 10.1007/978-0-387-71767-8_8. [DOI] [PubMed] [Google Scholar]
- 25.Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, Poulsen K, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krarup A, Wallis R, Presanis JS, Gal P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS ONE. 2007;2:e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Presanis JS, Hajela K, Ambrus G, Gal P, Sim RB. Differential substrate and inhibitor profiles for human MASP-1 and MASP-2. Mol Immunol. 2004;40:921–9. doi: 10.1016/j.molimm.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Muta T, Iwanaga S. Clotting and immune defense in Limulidae. Prog Mol Subcell Biol. 1996;15:154–89. doi: 10.1007/978-3-642-79735-4_8. [DOI] [PubMed] [Google Scholar]
- 30.Endo Y, Takahashi M, Fujita T. Lectin complement system and pattern recognition. Immunobiol. 2006;211:283–93. doi: 10.1016/j.imbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Honore C, Rorvig S, Hummelshoj T, Skjoedt MO, Borregaard N, Garred P. Tethering of Ficolin-1 to cell surfaces through recognition of sialic acid by the fibrinogen-like domain. J Leukoc Biol. 2010 doi: 10.1189/jlb.1209802. [DOI] [PubMed] [Google Scholar]
- 32.Endo Y, Nakazawa N, Liu Y, Iwaki D, Takahashi M, Fujita T, et al. Carbohydrate-binding specificities of mouse ficolin A, a splicing variant of ficolin A and ficolin B and their complex formation with MASP-2 and sMAP. Immunogenetics. 2005;57:837–44. doi: 10.1007/s00251-005-0058-1. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Ezekowitz RA. The role of the mannose-binding lectin in innate immunity. Clin Infect Dis. 2005;41 (Suppl 7):S440–4. doi: 10.1086/431987. [DOI] [PubMed] [Google Scholar]
- 34.White MR, Crouch E, Chang D, Sastry K, Guo N, Engelich G, et al. Enhanced antiviral and opsonic activity of a human mannose-binding lectin and surfactant protein D chimera. J Immunol. 2000;165:2108–15. doi: 10.4049/jimmunol.165.4.2108. [DOI] [PubMed] [Google Scholar]
- 35.Hartshorn KL, Collamer M, Auerbach M, Myers JB, Pavlotsky N, Tauber AI. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988;141:1295–301. [PubMed] [Google Scholar]
- 36.Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, et al. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–90. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collard CD, Montalto MC, Reenstra WR, Buras JA, Stahl GL. Endothelial oxidative stress activates the lectin complement pathway: role of cytokeratin 1. Am J Pathol. 2001;159:1045–54. doi: 10.1016/S0002-9440(10)61779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller-Kristensen M, Hamblin MR, Thiel S, Jensenius JC, Takahashi K. Burn injury reveals altered phenotype in mannan-binding lectin-deficient mice. J Invest Dermatol. 2007;127:1524–31. doi: 10.1038/sj.jid.5700748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi K, Chang WC, Takahashi M, Pavlov V, Ishida Y, La Bonte L, et al. Mannose-binding lectin and its associated proteases (MASPs) mediate coagulation and its deficiency is a risk factor in developing complications from infection, including disseminated intravascular coagulation. Immunobiol. 2010 doi: 10.1016/j.imbio.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi M, Iwaki D, Kanno K, Ishida Y, Xiong J, Matsushita M, et al. Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway. J Immunol. 2008;180:6132–8. doi: 10.4049/jimmunol.180.9.6132. [DOI] [PubMed] [Google Scholar]
- 41.Hartshorn KL, Sastry KN, Chang D, White MR, Crouch EC. Enhanced anti-influenza activity of a surfactant protein D and serum conglutinin fusion protein. Am J Physiol Lung Cell Mol Physiol. 2000;278:L90–8. doi: 10.1152/ajplung.2000.278.1.L90. [DOI] [PubMed] [Google Scholar]
- 42.van Eijk M, White MR, Crouch EC, Batenburg JJ, Vaandrager AB, Van Golde LM, et al. Porcine pulmonary collectins show distinct interactions with influenza A viruses: role of the N-linked oligosaccharides in the carbohydrate recognition domain. J Immunol. 2003;171:1431–40. doi: 10.4049/jimmunol.171.3.1431. [DOI] [PubMed] [Google Scholar]
- 43.Hartshorn KL, Sastry K, White MR, Anders EM, Super M, Ezekowitz RA, et al. Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest. 1993;91:1414–20. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi K. Encyclopedia of Life Sciences. Chichester: John Wiley & Sons, Ltd; 2008. Ficolins. [Google Scholar]
- 45.Moller-Kristensen M, Ip WK, Shi L, Gowda LD, Hamblin MR, Thiel S, et al. Deficiency of Mannose-Binding Lectin Greatly Increases Susceptibility to Postburn Infection with Pseudomonas aeruginosa. J Immunol. 2006;176:1769–75. doi: 10.4049/jimmunol.176.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 47.Watford WT, Wright JR, Hester CG, Jiang H, Frank MM. Surfactant protein A regulates complement activation. J Immunol. 2001;167:6593–600. doi: 10.4049/jimmunol.167.11.6593. [DOI] [PubMed] [Google Scholar]
- 48.Banda NK, Takahashi K, Wood AK, Holers VM, Arend WP. Pathogenic complement activation in collagen antibody- induced arthritis in mice requires amplification by the alternative pathway. J Immunol. 2007;179:4101–9. doi: 10.4049/jimmunol.179.6.4101. [DOI] [PubMed] [Google Scholar]
- 49.Ganter MT, Brohi K, Cohen MJ, Shaffer LA, Walsh MC, Stahl GL, et al. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28:29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 50.Brouwer N, Dolman KM, van Zwieten R, Nieuwenhuys E, Hart M, Aarden LA, et al. Mannan-binding lectin (MBL)-mediated opsonization is enhanced by the alternative pathway amplification loop. Mol Immunol. 2006;43:2051–60. doi: 10.1016/j.molimm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma-Injury Infect Crit Care. 2008;65:748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 52.Petersen KA, Matthiesen F, Agger T, Kongerslev L, Thiel S, Cornelissen K, et al. Phase I safety, tolerability, and pharmacokinetic study of recombinant human mannan-binding lectin. J Clin Immunol. 2006;26:465–75. doi: 10.1007/s10875-006-9037-z. [DOI] [PubMed] [Google Scholar]