Abstract
In the field of depression, inflammation-associated depression stands up as an exception since its causal factors are obvious and it is easy to mimic in an animal model. In addition, quasi-experimental studies can be carried out in patients who are treated chronically with recombinant cytokines for a medical condition since these patients can be studied longitudinally before, during and after stimulation of the immune system. These clinical studies have revealed that depression is a late phenomenon that develops over a background of early appearing sickness. Incorporation of this feature in animal models of inflammation-associated depression has allowed the demonstration that alterations of brain serotoninergic neurotransmission do not play a major role in the pathogenesis. This is in contrast to the activation of the tryptotphan degrading enzyme indoleamine 2,3 dioxygenase that generates potentially neurotoxic kynurenine metabolites such as 3-hydroxy kynurenine and quinolinic acid. Although the relative importance of peripherally versus centrally produced kynurenine and the cellular source of production of this compound remain to be determined, these findings provide new targets for the treatment of inflammation-associated depression that could be extended to other psychiatric conditions mediated by activation of neuroimmune mechanisms.
Keywords: Depression; inflammation; interferon-alpha; indoleamine 2,3 dioxygenase; tryptophane; kynurenine; quinolinic acid; kynurenic acid
From depression to animal models of depression
Venturing into the field of depression is akin to an adventure into the unknown. Depression by itself is not a disease sensu stricto since its etiology and mechanisms have not yet been elucidated. In addition, depression is not associated with any characteristic structural alteration (despite the emphasis on possible hippocampal atrophy), and there is no biomarker of depression (in particular escape from the dexamethasone suppression test is not a consistent feature). Depression is therefore defined by a list of symptoms that differ according to the classification. Many symptoms of depression are unfortunately ambiguous in nature since the disorder can manifest by an excess or an insufficiency (e.g., alterations in appetite or in sleep). Even when one adheres to a category of symptom clusters such as the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV TR for “Text Revision”), it is obvious that what is usually called depression actually encompasses a number of disorders, the so-called major depressive disorders. This disparity attests of the diversity of forms of depression. In addition, several symptoms of depression, including depressed mood, can also be found in other disorders such as dysthymia (a minor form of chronic depression), adjustment disorders with depressed mood and bipolar disorders. There is even a temporary category in which patients whose condition has not yet been fully characterized fit, the so-called “depressive disorder not otherwise specified”.
Our understanding is equally unclear when it comes to pathophysiology. The monoamine theory of depression has dominated the neuropharmacology of depression for several decades. According to this theory, major depressive disorders are caused by a deficit in monoaminergic neurotransmission that can be treated by administering drugs that correct this deficit. Tricyclic antidepressants such as imipramine and desipramine alleviate the symptoms of depression by enhancing levels of norepinephrine and serotonin in the synaptic cleft. Selective serotonin reuptake inhibitors such as fluoxetine and paroxetine are claimed to achieve a similar action by inhibiting the presynaptic serotonin transporter. This theory has been useful to the drug industry for developing antidepressant drugs. As a consequence, psychiatrists now have a long list of treatment options that can be used in order to adjust treatment to the particular symptoms presented by each patient. Indeed, psychiatric disorders were the first to utilize what is now called, “personalized medicine.” However, the hypothesis that depression is due to a deficiency in monoaminergic neurotransmission and furthermore that antidepressants alleviate symptoms of depression by normalizing monoaminergic neurotransmission has never been demonstrated to be true and is probably wrong.
Many review papers have already been published on animal models of depression. Most of the tests that have been developed to assess depressive-like behavior in rodents are actually nothing other than pharmacological bioassays in the sense that they are able to screen potential antidepressant drugs with relatively good sensitivity and specificity. This applies in particular to the forced swim and tail suspension tests. In the forced swim test, laboratory rodents are immersed into a tank filled with water try to climb on the walls, swim around and ultimately remain immobile as assessed by floating. This is anthropomorphically interpreted as resignation in the absence of any possibility to escape. Antidepressant drugs administered acutely or chronically typically decrease the duration of immobility whereas anxiolytic and anti-stress drugs have no effect. The same principle applies to the tail suspension test. In all these tests, pretreatment with a potential antidepressant drug decreases the duration of immobility. By extension, it is tempting to claim that any condition, whether it be pharmacological, environmental or genetic, that results in the same effect reveals an antidepressant phenotype. Conversely, any condition resulting in the opposite effect reveals a depression-like phenotype. An apparent exception to this tautology is the test of sucrose preference. Exposure to a context that is supposed to mimic predisposing factors for development of depression (e.g., chronic inescapable stress) leads to a decrease in the spontaneous preference for the rewarding effects of a sucrose solution. The sucrose reward can be replaced by any other rewarding stimulus, ranging from electrical stimulation of the so-called brain reward centers (e.g., the lateral hypothalamus) to presentation of a sexually attractive female to a male. The symptom of depression that is modeled in this type of test is the loss of capacity to feel pleasure, also known as anhedonia.
All of this would be expected to discourage any reasonable thinking scientist to invest in research on depression outside the context of psychopharmacology of antidepressant drugs. However, this is not the case. The number of original research articles describing antidepressant or depressant behavior phenotypes induced by specific gene or environmental alterations is still increasing even in high impact factor journals. This trend is evident in the absence of any obvious link between the condition under study and depression. The situation is fortunately different in the case of inflammation. Clinical studies have revealed that activation of the immune system in physically ill patients is associated with an increased prevalence of clinical depression. This is particularly the case in patients infected with the hepatitis C virus that are chronically administered interferon-alpha (IFNα) along with the anti-viral agent ribavarin. The same side effects occur in patients receiving immunotherapy for kidney cancer or malignant melanoma. These patients respond to the massive doses of IFNα and/or interleukin-2 by exhibiting symptoms of depression. Based on these clinical findings, it has been possible to go back to the bench and carry out preclinical studies on development of depressive-like behavior in laboratory animals in which the immune system has been activated. Of course, depressive-like behavior has been assessed in these studies by the same types of behavioral assays as those described earlier, and with the same limitations. However, face validity is no longer the critical issue.
The purpose of the present review is to discuss the main results that have emerged from the study of inflammation-associated depression and their clinical relevance for the understanding of depression.
Inflammation-associated depression – Human studies
The possibility that depression is not just a disorder of the mind but rather a consequence of physical illness has haunted medicine for a long time. Melancholia, an extreme form of depression, was originally supposed to be caused by an imbalance in the four humors that control emotions, in this case an excess of black bile as its etymology refers to. In modern literature, black bile has been replaced by cortisol of which the brain effects are studied by a myriad of researchers throughout the world. However, the therapeutic efficacy of inhibitors of cortisol synthesis or antagonists of glucocorticoid receptor activity has not yet fulfilled its premises in psychiatry (Blasey et al 2009; Kling et al 2009) and the pathogenesis of depression still remains elusive.
Based on the observation that cytokines produced by macrophages induce symptoms of depression when given to volunteers and have brain effects including activation of the hypothalamic pituitary-adrenal axis, Smith first proposed the macrophage theory of depression (Smith 1991). According to this theory, excessive production of inflammatory mediators by macrophages plays a causal role in depression. In accordance with the theory, Maes (Maes 1999; Maes et al 1995) was able to collect biochemical evidence for activation of the innate immune system in depressed patients. This was in contrast to the previously emphasized immunodepression that had been reported in these patients, based on natural killer cell activity and lymphocyte proliferation assays (Irwin 1999).
The association between depression and inflammation has been confirmed in various clinical populations. However, it accounts for only a small amount of the variance and there is still no consensus as to the best biomarkers to reveal systemic inflammation (Dantzer et al 2008a; Irwin & Miller 2007). The association between depression and inflammation has actually turned out to be easier to observe in physically ill patients than in patients with psychiatric disorders who are otherwise considered medically healthy. This is the case in patients with coronary heart disease (Frasure-Smith et al 2007; Joynt et al 2003; Kiecolt-Glaser & Glaser 2002; Lesperance & Frasure-Smith 2007). A similar association between biomarkers of inflammation and depression, especially when measured with psychological instruments (e.g., the Beck Depression Inventory or the Montgomery-Asberg Depression Rating Scale), is commonly found during aging and is exacerbated by obesity and type II diabetes (Bremmer et al 2008; Milaneschi et al 2009; Penninx et al 2003).
The first recombinant human cytokines became available in the 1980s, and they were first tested as therapy in a variety of cancer patients. However, the side effects of systemic cytokine therapy revealed numerous CNS-associated symptoms, such a disorentiation, mood changes, sleep, fever and fatigue (reviewed in (Dantzer & Kelley 1989)). We proposed that endogenous cytokines could cause similar symptoms, but the prevailing view at that time was that these CNS-side effects were caused only by pharmacological, not physiological, doses of recombinant cytokines. Besides these early correlative findings, the basic assumption that immune activation can cause depression stemmed from studies of cognitive and mood disorders in cancer patients receiving cytokine therapy (Meyers 1999). Major progress in the field took place when categorical evaluation of mood disorders was replaced by dimensional analysis aiming at assessing the degree of intensity of various dimensions of symptoms (Capuron et al 2000; Capuron et al 2004). With this approach, cytokine immunotherapy was found to rapidly induce a core of neurovegetative or sickness-like symptoms that included fatigue, pain, decreased appetite, and sleep disorders in most of the cancer or hepatitis C patients treated with IFNα and/or IL-2. After a few days or weeks, depending on the cytokine and dose administered, 30 to 50 percent of the patients developed psychological symptoms including depressed mood, feelings of worthlessness, guilt and even suicidal ideation that culminated in a true major depressive episode. Psychological symptoms of depression, in contrast to neurovegetative symptoms, were found to be sensitive to preventive antidepressant treatment with paroxetine (Capuron et al 2002a).
Since not all patients receiving immunotherapy ultimately develop clinical depression, it is important to determine the risk factors for these side effects. In patients with cancer, individuals at risk for developing cytokine-induced depression had a greater depressed mood at baseline, as expressed by feelings of sadness and pessimism, together with sleep disorders and low social support (Capuron & Ravaud 1999; Capuron et al 2004). In an independent study, poor sleep together with high IL-6 was confirmed as a risk factor for developing IFNα-induced depression (Prather et al 2009). Patients at risk of developing depression also displayed an exaggerated reactivity of the pituitary-adrenal axis in response to the very first injection of IFNα (Capuron et al 2003b). Genetic vulnerability factors in the form of functional polymorphisms in the genes coding for the serotonin transporter and for IL-6 have also been found to be associated with the risk of developing depression in response to IFNα (Bull et al 2009; Lotrich et al 2009).
The relevance of cytokine immunotherapy-induced clinical depression as a model to understand the relationship between inflammation and depression can be questioned based on the drastic nature of the immune changes induced by the massive doses of cytokines that are administered to patients. However, more recent studies on volunteers administered low doses of endotoxin acutely (Reichenberg et al 2001) or vaccinated against typhoid (Strike et al 2004; Wright et al 2005) confirmed the basic premises of the hypothesis, i.e., that inflammation can cause depressed mood in medically healthy subjects. In both cases, the occurrence of depressed mood was associated with increased production of proinflammatory cytokines.
A few intervention studies have confirmed a role for inflammation in depression associated with medical illness. Administration of the TNFα antagonist etanercept to patients with moderate to severe psoriasis resulted in an improvement of symptoms of depression measured by the Beck Depression Inventory (Tyring et al 2006). This improvement was independent of the effect of etanercept on joint pain. Blockade of the inducible cyclooxygenase-2 enzyme that mediates the synthesis of prostaglandins of the E2 series in response to cytokines enhanced the antidepressant effect of the specific norepinephrine re-uptake inhibitor reboxetine, although the dropout rate was particularly elevated in this study (Muller et al 2006).
Several mechanisms have been proposed to account for the depressogenic action of cytokines. They include induction of extrahypothalamic CRF and vasopressin, development of glucocorticoid resistance, activation of the tryptophan degrading enzyme indoleamine 2,3 dioxygenase (IDO), and increased expression of the serotonin transporter (Miller 2008; Miller 2009). Activation of IDO was initially proposed to account for the drastic fall in plasma tryptophan levels that occurs in patients with inflammatory disorders as well as in patients who receive cytokine immunotherapy (Bonaccorso et al 2002; Capuron et al 2003a; Capuron et al 2002b; Widner et al 2002). Capuron et al. observed a linear relationship between decreased plasma tryptophan levels and scores of depression after 26 days of immunotherapy in cancer patients (Capuron et al 2002b). Because this relationship was very suggestive of a causal relationship between these two events, it prompted a series of experimental studies on the possible role of IDO activation in the transition from cytokine-induced sickness to depression (Capuron & Dantzer 2003; Dantzer et al 2008b; Godbout et al 2008)
The brain areas that are the targets of cytokines have been identified by functional brain imaging techniques. They are remarkably similar to those whose activity is altered in depressed patients. Patients treated with IFNα for chronic hepatitis C displayed hypometabolism in the prefrontal cortex, as measured by [18F] deoxygluocose positron emission tomography (Juengling et al 2000). This hypometabolism was correlated with increases in depression scores. Hypermetabolism was observed in the putamen. Patients with malignant melanoma treated with IFNα displayed decreased glucose metabolism in dorsal prefrontal cortex and increased glucose metabolism in the basal ganglia and cerebellum (Capuron et al 2007). In these patients, the increased glucose metabolism in the left nucleus accumbens and putamen was correlated to self-reported fatigue. Patients treated with IFNα for chronic hepatitis C exhibited a significantly higher activation in the dorsal part of the anterior cingulate cortex, as measured by functional magnetic resonance imaging. The degree of activation of this structure highly correlated with the number of errors they made in a task of visuo-spatial attention (Capuron et al 2005).
Once more, massive levels of immune activation do not need to be achieved to detect correlations between cytokine levels and neural responses. Volunteers were injected intravenously with a small dose of endotoxin sufficient to increase proinflammatory cytokines and depress mood. During fMRI, they were asked to participate in a ball-tossing video game during which they experienced social exclusion. In females, but not males, increases in IL-6 were associated with increases in activity of the dorsal anterior cingulate cortex and the anterior insula (Eisenberger et al 2009). Healthy male volunteers who received typhoid vaccination were asked to perform an implicit emotional face perception task. Functional magnetic resonance imaging revealed enhanced activity in the subgenual anterior cingulate cortex during emotional face processing. These changes were correlated with circulating IL-6 (Harrison et al 2009).
Correlations between inflammation and depression-like neural activity can be even seen in the absence of immune stimulation by antigens. In line with the observation that proinflammatory cytokines can be activated by psychosocial stressors (Bierhaus et al 2003; Wolf et al 2009), women undergoing the emotional stress of bereavement were exposed to a grief elicitation task during a session a functional magnetic resonance imaging. As expected, they displayed activation of brain areas involved in emotional processing including the subgenual anterior cingulate cortex, and this activation was positively correlated to salivary concentrations of IL-1β and the soluble tumor necrosis factor receptor II (O'Connor et al 2009d).
Cytokines and depression - Animal studies
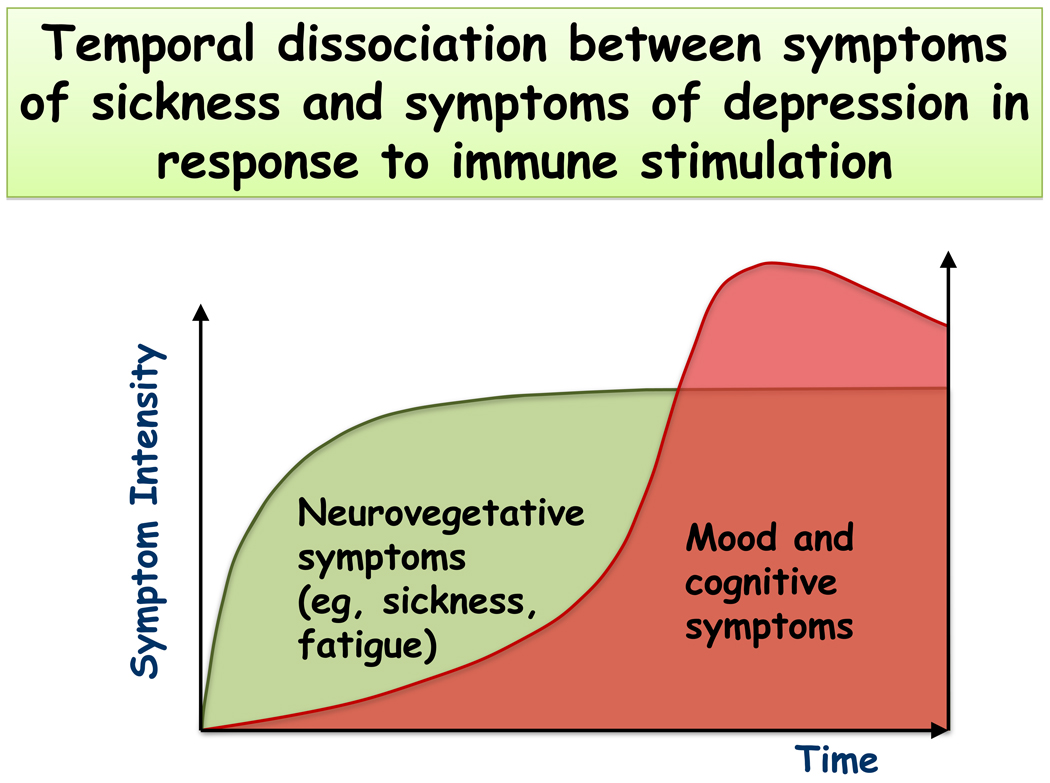
Because of the overwhelming evidence that treatment of patients with IFNα can cause depression in humans, the effects of this cytokine have also been studied in mice and rats. The problem is that recombinant mouse or rat IFNα has not been readily available so that most researchers have used recombinant human IFNα. However, it is well known that human IFNα is not biologically active in the mouse or rat system because it lacks a consensus sequence. As could have been expected, pegylated human IFNα-2a or 2b that is used for the treatment of chronic hepatitis C did not alter rat behavior and weight gain (De La Garza et al 2005; Loftis et al 2006) although positive results have been obtained by other authors using chronic administration of human IFNα-2a 3 times a week over 4–5 weeks (Fahey et al 2007; Myint et al 2007c). A single injection of rat recombinant IFNα was found to induce sickness behavior up to 4 days following treatment (Kentner et al 2006). Using a hybrid recombinant IFNα A/D that is active in the mouse system, Dunn and Crnic observed a highly reliable depression of motor activity (Dunn & Crnic 1993). The group of Dunn also confirmed that recombinant human IFNα had no effect on behavior in mice. In contrast, a single injection of homologous IFNα decreased the time spent immobile in the forced swim test and tended to decrease time spent in open arms in the elevated plus-maze test without altering general activity. There was no change in the tail suspension test nor in the light/dark exploration test. The same treatment increased concentrations of tryptophan and 5-hydoxyindoleacetic acid/serotonin ratios in selected brain regions. These data indicate that homologous IFNα is behaviorally active in rodent models although its activity over time and its mechanisms of action remain to be elucidated. However, there has been no systematic attempt to model what has been observed in human malignant melanoma or hepatitis C (plus ribivarin) patients treated with IFNα using longitudinal studies. This aspect is important since a bedside-to-bench strategy should allow building an animal model of cytokine-induced depression that is not biased by the occurrence of sickness behavior. This can be done by capitalizing on the temporal dissociation that is observed between sickness behavior and depression in patients who undergo cytokine immunotherapy (Fig. 1). One possibility is to administer an acute injection of LPS and test for depressive-like behavior only when sickness behavior has dissipated. Another possibility is to inoculate a microbial pathogen that induces chronic infection and assess depressive-like behavior after sickness has disappeared.
Fig. 1.
Temporal dissociation between sickness and depression in response to peripheral immune stimulation. Note that sickness develops earlier whereas depressive symptoms occur later, with a time interval varying from hours to weeks depending on the eliciting condition.
This strategy has been employed in situations of both acute and chronic immune activation. To induce acute immune stimulation, mice were administered LPS which, as expected, induced sickness as measured by reduced locomotor activity, decreased food intake and loss of body weight during the first hours after treatment. Twenty-four hours later, when sickness had apparently disappeared, mice were submitted to tests of depressive-like behavior. They displayed increased duration of immobility in the forced swim test and the tail suspension test (Frenois et al 2007; O'Connor et al 2009c). Furthermore, depressive-like behavior was increased not in intensity but in duration by the chronic inflammation associated with aging (Godbout et al 2008).
To induce chronic immune stimulation, mice were inoculated with Bacillus Calmette-Guerin (BCG), an attenuated form of Mycobacterium bovis. BCG-treated mice showed an acute episode of sickness that lasted until 3–4 days post BCG. Their duration of immobility in the forced swim and tail suspension tests was increased for up to 21 days post-inoculation of BCG, and their preference for sucrose was decreased at the same time (Moreau et al 2008).
In terms of mechanisms, LPS as well as BCG are able to activate IDO in both the periphery and brain (Lestage et al 2002; Moreau et al 2005) with a time course that is compatible with the development of depressive-like behavior. The hypothesis that IDO mediates cytokine-induced depressive-like behavior was tested by blocking either the inducible expression of cytokines (presumed responsible for upregulating IDO) in response to LPS or by bypassing cytokines and directly blocking IDO activation. In the first case, the expected result was that the treatment should block both sickness and depressive-like behavior while, in the second case, it should block only depressive-like behavior and leave sickness behavior intact. This is exactly what was observed when mice were pretreated with minocycline, a tetracycline derivative that has anti-inflammatory properties. Minocycline pretreatment abrogated LPS-induced expression of cytokines and IDO and blocked development of both sickness behavior and depressive-like behavior as measured by increased duration of immobility in the forced swim and tail suspension test (O'Connor et al 2009c). Conversely, administration of the IDO competitive antagonist 1-methyl-D,L- tryptophan (1MT) had no effect on LPS-induced proinflammatory cytokines and sickness behavior but abrogated LPS-induced depressive-like behavior (O'Connor et al 2009c). Administration of 1MT also blocked the development of BCG-induced depressive-like behavior without altering the proinflammatory cytokine response and sickness (O'Connor et al 2009b).
IDO is activated robustly by IFNγ and TNFα. Mice deficient in the IFNγ receptor responded less to BCG inoculation in terms of IDO expression and depressive-like behavior whereas their acute sickness response was unchanged (O'Connor et al 2009a). Antagonism of TNFα with etanercept attenuated BCG-induced IDO activation and depressive-like behavior but to a lesser extent (O'Connor et al 2009a).
These experimental results are important because they confirm that inflammation-associated depression develops on a background of sickness behavior but does not totally confound it. Furthermore, they indicate that inflammation-associated depression is dependent on activation of the tryptophan degrading enzyme IDO.
Our initial hypothesis was that the enhanced degradation of tryptophan would result in a relative decrease in tryptophan bioavailabity, thereby setting the stage for a decrease in serotoninergic neurotransmission. Tryptophan is the limiting factor for the synthesis of serotonin. In order to enter the brain, tryptophan needs to be imported via a transporter that is relatively non-specific since it also transports other large neutral amino acids such as phenylalanine, leucine, isoleucine, valine and tyrosine. Tryptophan is hydroxylated by tryptohan hydroxylase into the intermediate product 5-hydroxy-tryptophan. This enzyme is only 50% saturated with its substrate, so increasing or decreasing the level of bioavailable tryptophan should have an immediate impact on brain serotonin levels. Support for this hypothesis is provided by experiments using acute tryptophan depletion. Acute tryptophan depletion can be achieved by consumption of a tryptophan-free amino acid drinking solution. This results in a lowering of plasma tryptophan to 10–50% of original levels, which is accompanied by a decline in central serotonin, as shown by experiments in animals and healthy volunteers (Moore et al 2000). In response to rapid tryptophan depletion, full clinical relapse occurred in 47% of depressed patients who were recently medicated and partially remitted, and 9% of the patients who were either non-medicated or fully remitted. The placebo procedure had no effect (Van der Does 2001). When mood alterations were taken into consideration rather than the incidence of clinical depression, acute tryptophan depletion was found to cause significantly greater depressed mood in healthy subjects with a family history of depression than in healthy subjects without such a family history, and this effect was in general more pronounced in women than in men (Benkelfat 1994; Benkelfat et al 1994). Similar results have been reported in subjects with a genetic or a personal history of depression by several other groups (e.g., (Riedel 2002; Riedel et al 2002)). The assessment of the negative effects of tryptophan depletion on mood has been proposed as a means to predict relapse after treatment discontinuation in recovered patients and treatment refractoriness in symptomatic patients (Van der Does 2001).
As expected, activation of IDO by administration of LPS or BCG to mice is accompanied by decreased circulating levels of tryptophan and increased levels of kynurenine, resulting in an increase in the kynurenine to tryptophan ratio (O'Connor et al 2009b; O'Connor et al 2009c). The same effect was observed in the brain but it was due to an increase in kynurenine without any concomitant decrease in tryptophan. Paradoxically, brain tryptophan did not decrease in these conditions, rather, LPS actually increased brain tryptophan and the turnover of brain serotonin, as measured by the ratio of 5-hydroxy-indoleacetic acid to serotonin (O'Connor et al 2009c). This increase was even more pronounced in aged than in young adult mice and this was an associated with a longer duration of LPS-induced depressive-like behavior, as measured by increased duration of immobility in the forced swim test (Godbout et al 2008). Furthermore, pretreatment with the anti-inflammatory agent minocycline or with the IDO competitive antagonist 1MT attenuated LPS-induced increases in the ratio of kynurenine to tryptophan in the plasma and brain and abrogated LPS-induced depressive-like behavior but had no effect on LPS-induced increases in brain tryptophan and serotonin turnover (O'Connor et al 2009c). The observation that inflammatory stimuli increase brain tryptophan and stimulate the metabolism of brain serotonin in a manner similar to stress had already been made by Dunn and his group several years before (Dunn et al 1999), although most of these studies were carried out only a few hours following administration of LPS or cytokines. Obviously, the brain can compensate for the decrease in circulating tryptophan induced by acute or chronic inflammation, although the mechanism of this effect is still unknown. Whatever the case, this made the hypothesis of a decreased serotoninergic neurotransmission being responsible for inflammation-induced depression no longer tenable.
An alternative hypothesis would be that cytokines decrease the amount of serotonin available for neurotransmission by increasing activity of the serotonin transporter (SERT). Neuronal SERT activity was found to be up regulated by IL-1β and TNFα in a p38-dependent manner (Zhu et al 2006). Administration of the synthetic double-stranded RNA Poly I:C to rats increased expression of IFNα and SERT in the brain at the same time as it induced fatigue, as measured by decreased voluntary running wheel activity (Katafuchi et al 2006). Increased expression of SERT in response to IFNα was also observed in immune cells and in a T cell line (Tsao et al 2006). In the Jurkat T cell line, this effect was mediated by activation of mitogen-activated protein kinases (p38, ERK and JNK) (Tsao et al 2008). An effect of cytokines on serotonin receptors is also possible. As an example, chronic administration of IFNα A/D to rats increased binding capacity of the low affinity 5-HT1A receptor sites (Abe et al 1999).
Independently of the respective role of serotonin and kynurenine metabolites in inflammation-induced depression, investigations on the involvement of IDO itself have become more complicated since the discovery of another gene coding for indoleamine 2,3 dioxygenase-like protein (Ball et al 2009). The product of this gene is called IDO2 to distinguish it from the first IDO that was discovered and which is now called IDO1. The genes coding for IDO1 and IDO2 are adjacent to each other on chromosome 8 in both humans and mice, with IDO2 being probably more ancient in terms of phylogeny than IDO1. IDO2 shares 43% homology at the amino acid level with IDO1. IDO2 is expressed mainly in the kidney and reproductive system although transcripts for IDO2 are also found in the brain. IDO2 appears to be less efficient to metabolize tryptophan than IDO1 but the exact cofactors required for its activity still remain to be elucidated. In terms of inhibition, levo-1-methyltryptophan appears to be a more effective inhibitor of IDO2 than dextro-1-methyltryptophan (Yuasa et al). However, opposite results have been reported (Lob et al 2008).
Another complicating factor is the recent discovery that tryptophan 2,3 dioxygenase (TDO) previously known as tryptophan pyrrolase and of which the expression was claimed to be restricted to the liver has now been found to be present in other organs including the brain. Cortisol and corticosterone are potent activators of TDO and it is therefore possible that they also regulate the activity of TDO in the brain, although this has not yet been investigated. There are at least two variants of the tdo gene and its transcripts in the mouse brain, with differential expression according to the stage of development (Kanai et al 2009). Although much still remains to be done at the bench level to understand the respective role of TDO, IDO1 and IDO2 in the regulation of inflammation in the brain, it should already be obvious that at the practical level the simple measurement of kynurenine metabolites is no longer sufficient to have a full picture of the origin and modalities of activation of the kynurenine pathway. Future studies will need to determine which exact enzyme(s) are responsible for the observed changes in kynurenine metabolites. Examples of this are provided in the next section.
Depression and activation of the kynurenine pathway – Back to the clinics
As already mentioned, the first clinical studies on the relationship between immune-mediated activation of the tryptophan degradation pathway and depression focused on the possible negative consequences of lowered tryptophan levels on brain serotoninergic neurotransmission. This hypothesis was formalized in a review paper published by the Fuch’s group in 2002 (Widner et al 2002). However, the prediction that decreased circulating tryptophan negatively impacts on brain tryptophan and serotonin was not tested. Actually, a recent study in IFNα-treated patients showed that CSF tryptophan levels remained stable despite decreased blood levels of tryptophan (Raison et al).
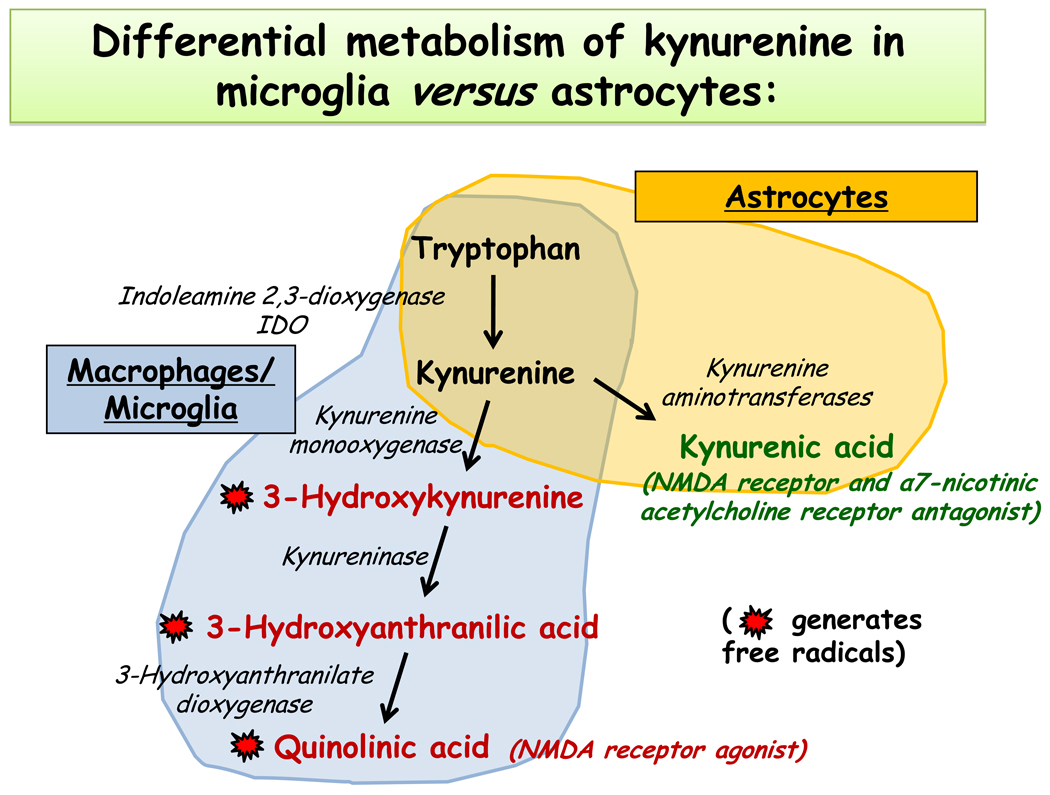
The possibility that tryptophan metabolites can play a role independently of serotonin came later in the equation mainly because of the evidence that was already available on the neurotropic action of some of these metabolites. The degradation of tryptophan by IDO induces the synthesis of the intermediate metabolite known as kynurenine. Kynurenine is inactive in itself. It is further degraded into different metabolites, the nature of which depends on the cell type in which kynurenine is produced or transported (Guillemin et al 2007; Schwarcz 2004; Ting et al 2007) (Fig. 2). Microglia degrade kynurenine into 3-hydroxykynurenine and quinolinic acid. These two last compounds are neurotoxic because of their ability to generate oxidative radicals and to act as agonists of the NMDA receptor. Astrocytes degrade kynurenine into kynurenic acid that is neuroprotective by acting as an antagonist of the NMDA and alpha-7 nicotinic acetylcholine receptor. Intact neurons can degrade kynurenine into picolinic acid that is neuroprotective. The production of kynurenine metabolites can be modulated by endogenous factors, the levels of which could vary according to inflammation. A so-called “glia depressing factor” that exerts an inhibitory activity on the production of kynurenic acid by depressing activity of kynurenine aminotransferase I (the main enzyme that degrades kynurenine into kynurenic acid) has for instance been identified in the human CSF and brain (Baran et al 2010). The levels of this factor appear to increase during inflammation since CSF from patients with multiple sclerosis has a lower ability to inhibit kynurenine aminotransferase I than CSF from control subjects.
Fig. 2.
Degradation of kynurenine varies according to the enzymatic equipment of the cells. Note that astrocytes produce mainly kynurenic acid whereas microglia transform kynurenine into 3-hydroxy kynurenine and quinolinic acid. Kynurenic acid is considered to be neuroprotective whereas 3-hydroxy kynurenine and quinolinic acid are neurotoxic.
Myint and Kim proposed that activation of IDO during inflammation switches the metabolism of kynurenine toward the production of neurotoxic metabolites to the detriment of the neuroprotective kynurenic acid (Myint & Kim 2003). The same hypothesis was reformulated by Müller and Schwartz (Muller & Schwarz 2008). They proposed that depression is associated with a predominance of the microglial kynurenine degradation pathway over the astrocytic pathway whereas schizophrenia would be associated with the opposite pattern (Muller & Schwarz 2008).
In patients with chronic hepatitis C, up to 24 weeks of treatment with IFNα increased as expected the kynurenine over tryptophan ratio at the same time as it increased symptoms of depression (Wichers et al 2005). The ratio of tryptophan to competing amino acids did not change at the periphery despite decreased levels of tryptophan, indicating that the availability of tryptophan to the brain was not modified. The kynurenine over kynurenic acid ratio increased mainly because of the increased kynurenine levels and the lack of any concomitant increase in kynurenic acid, which was interpreted as an index of decreased neuroprotection. These changes in kynurenine and its metabolites do not remain restricted to the periphery since another study in patients with hepatitis C treated for 12 weeks with IFNα showed evidence of enhanced levels of kynurenine, quinolinic acid and kynurenic acid in the cerebro-spinal fluid (CSF) of these patients in addition to increased signs of IDO activation at the periphery (Raison et al). Increases in CSF kynurenine and quinolinic acid were correlated with increased CSF IFN-alpha, soluble tumor necrosis factor-alpha receptor 2 and monocyte chemoattractant protein-1 as well as increased depressive symptoms (Raison et al).
Independently of the research carried out on inflammation-associated depression, the search for the possible occurrence of biomarkers of activation of the kynurenine pathway in depressed patients had already been in the past a very active field of research in biological psychiatry. One of the first reports in this area was actually from the group of Curzon and colleagues. This study was carried out at the time at which the only known tryptophan degrading enzyme was TDO. Curzon and Bridges gave an oral load of tryptophan to endogenously depressed patients and control subjects. They observed that female depressed patients excreted significantly more kynurenine and 3-hydroxykynurenine than did female control subjects but this did not extend to 3-hydroxyanthralinic acid (Curzon & Bridges 1970). These results were not always reproducible in other laboratories, e.g., (Moller et al 1982), and the issue remained dormant in the biological psychiatry literature until the discovery of IDO and its association with inflammation.
Fuchs and colleagues reported an association between neuropsychiatric symptoms and activation of IDO in macrophages (as measured by increased circulating kynurenine over tryptophan ratio and neopterin levels) in HIV seropositive patients (Fuchs et al 1990). Heyes and colleagues proposed in 1992 that quinolinic acid and kynurenic acid that are elevated in the central nervous system of HIV-1-infected patients as a consequence of IDO activation act as mediators of the neuronal dysfunction and nerve cell death that occur in these patients (Heyes et al 1992). In depressed patients without any comorbid somatic disease, Myint et al. reported a decreased tryptophan to competing amino acids ratio, an increased kynurenine to tryptophan ratio and a decreased kynurenic acid over kynurenine ratio, the so-called neuroprotection index (Myint et al 2007b). This last decrease was mainly due to a decrease in kynurenic acid levels. There was no major change during the course of the treatment, as attested by the lack of differences in these indexes between admission and discharge besides an increase in the ratio of tryptophan to competing amino acids (Myint et al 2007b). However, the results are far from being always as clear in other studies on patients with major depressive disorders. Mackay et al. reported for instance that the relationship between kynurenine metabolites and depression emerged only after fluoxetine treatment while it was absent in drug-naïve patients (Mackay et al 2009). The clinical form of depression itself could play a role since decreased tryptophan and elevated kynurenine over tryptophan ratios were found in adolescent depression with melancholic but not non-melancholic features independently of treatment (Gabbay et al).
The situation in bipolar disorders is not easier to grasp. In the study published by the group of Myint et al., drug free bipolar manic patients displayed a lower tryptophan index (tryptophan over competing amino acids) compared to controls and after 6 weeks of treatment they had lower tryptophan and tryptophan index but higher kynurenine over tryptophan ratios (Myint et al 2007a). The neuroprotective ratio was not different between groups. However, in another study by a different group the levels of kynurenic acid in the cerebrospinal fluid of bipolar patients were found to be higher when sampled during the euthymic phase (Olsson et al).
There is clearly a need for a more systematic approach of biomarkers of inflammation and kynurenine pathways in psychiatric patients. Ideally, measurements should be carried out both at the periphery and in the cerebrospinal fluid. These measurements should take into consideration classical markers of inflammation (C reactive protein and probably IL-6 although there is no standardization of IL-6 assay). In addition it would be useful to determine which enzyme is actually involved in the observed changes. In the case of post-partum depression for instance, the already reported association between increased blood kynurenine over tryptophan ratios and symptoms of depression and anxiety (Maes et al 2002) is more likely to be related to TDO than to IDO since it occurs in the absence of any sign of activation of macrophages as measured by plasma neopterin (Kohl et al 2005; Schrocksnadel et al 2003). Extrahepatic TDO could also be involved in some instances since a higher density of TDO-positive glial cells associated with higher kynurenine levels was found in the gray and white matter of the anterior cingulate cortex of patients with bipolar disorders (Miller et al 2006). Note that this does not apply to pre-partum depression since there are both high CRP levels and IDO activity at this stage of pregancy (Schrocksnadel et al 2003; Scrandis et al 2008).
Conclusions
The use of animal models for studying inflammation-associated symptoms of depression is plagued with many difficulties, including the lack of a real animal model of depression and the huge confounding intervention of performance factors that are sensitive to sickness behavior. In addition, it should not be forgotten that depression remains a condition that is exclusively defined by a list of obligatory and facultative symptoms, and for which the causal factors remain unknown.
It might appear somewhat incongruous if not inappropriate to relate a relatively nonspecific response, inflammation, to symptoms of depression that still tend to be considered by many psychiatrists as the expression of a specific psychiatric entity. However, this view is obsolete since it does not take into account that symptoms of depression are transnosographic, in the sense that they are present in many clinical psychiatric and somatic conditions (Baruch et al 1992).
Inflammation-associated depression is a clinical entity that has taken long to be recognized but has received much attention during the recent years. Based mainly on the quasi-experimental model of cytokine immunotherapy, its nosology is now well established. Furthermore, it provides an invaluable source of insight on the dynamics of interaction between peripheral and brain mechanisms since the same subjects can be studied at different stages of development of depression, and those subjects who become depressed can be compared to those who remain depression-free. Based on what has been learned at the clinical level, reverse translational studies can now be carried out in animal models. This makes possible the study of cellular and molecular mechanisms of inflammation-associated depression and opens the perspective of developing new antidepressant drugs that target the brain cytokine system or its downstream processes.
Acknowledgement
Supported by grants from the National Institutes of Health to RD (MH 079829 and MH 71349) and KWK (MH 51569 and AG 029573)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Robert Dantzer has received honorarium from Astra-Zeneca, Bristol-Myers-Squibb and Lundbeck and is currently working as a consultant for Lundbeck laboratories.
Keith W. Kelley has received honorarium from Astra-Zeneca.
References
- Abe S, Hori T, Suzuki T, Baba A, Shiraishi H, Yamamoto T. Effects of chronic administration of interferon alpha A/D on serotonergic receptors in rat brain. Neurochem Res. 1999;24:359–363. doi: 10.1023/a:1020929415443. [DOI] [PubMed] [Google Scholar]
- Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467–471. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Baran H, Kepplinger B, Draxler M. Endogenous kynurenine aminotransferases inhibitor is proposed to act as "glia depressing factor" (GDF) Int J Tryptophan Res. 2010;3:13–22. doi: 10.4137/ijtr.s3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch P, Filteau MJ, Bouchard RH, Pourcher E, Vincent P, Jouvent R. The dimensional approach to clinical psychopharmacology: a polysemous concept. J Psychiatry Neurosci. 1992;17:55–60. [PMC free article] [PubMed] [Google Scholar]
- Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51:687–697. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51:687–697. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasey CM, Debattista C, Roe R, Block T, Belanoff JK. A multisite trial of mifepristone for the treatment of psychotic depression: a site-by-treatment interaction. Contemp Clin Trials. 2009;30:284–288. doi: 10.1016/j.cct.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2009;14:1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17 Suppl 1:S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002a;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003a;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003b;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient's initial affective state. N Engl J Med. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002b;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Curzon G, Bridges PK. Tryptophan metabolism in depression. J Neurol Neurosurg Psychiatry. 1970;33:698–704. doi: 10.1136/jnnp.33.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, et al. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008a;33:18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci. 1989;44:1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008b;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Asnis GM, Pedrosa E, Stearns C, Migdal AL, et al. Recombinant human interferon-alpha does not alter reward behavior, or neuroimmune and neuroendocrine activation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:781–792. doi: 10.1016/j.pnpbp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. 1999;461:117–127. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Crnic LS. Repeated injections of interferon-alpha A/D in Balb/c mice: behavioral effects. Brain Behav Immun. 1993;7:104–111. doi: 10.1006/brbi.1993.1011. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey B, Hickey B, Kelleher D, O'Dwyer AM, O'Mara SM. The widely-used anti-viral drug interferon-alpha induces depressive- and anxiogenic-like effects in healthy rats. Behav Brain Res. 2007;182:80–87. doi: 10.1016/j.bbr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Irwin MR, Sauve C, Lesperance J, Theroux P. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302–308. doi: 10.1016/j.biopsych.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D, Moller AA, Reibnegger G, Stockle E, Werner ER, Wachter H. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J Acquir Immune Defic Syndr. 1990;3:873–876. [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, et al. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry. 51:935–943. doi: 10.1111/j.1469-7610.2010.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115(Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- Irwin M. Immune correlates of depression. Adv Exp Med Biol. 1999;461:1–24. doi: 10.1007/978-0-585-37970-8_1. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Joynt KE, Whellan DJ, O'Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54:248–261. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, et al. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 2000;152:383–389. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- Kanai M, Nakamura T, Funakoshi H. Identification and characterization of novel variants of the tryptophan 2,3-dioxygenase gene: differential regulation in the mouse nervous system during development. Neurosci Res. 2009;64:111–117. doi: 10.1016/j.neures.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Kondo T, Take S, Yoshimura M. Brain cytokines and the 5-HT system during poly I:C-induced fatigue. Ann N Y Acad Sci. 2006;1088:230–237. doi: 10.1196/annals.1366.020. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Miguelez M, James JS, Bielajew C. Behavioral and physiological effects of a single injection of rat interferon-alpha on male Sprague-Dawley rats: a long-term evaluation. Brain Res. 2006;1095:96–106. doi: 10.1016/j.brainres.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Kling MA, Coleman VH, Schulkin J. Glucocorticoid inhibition in the treatment of depression: can we think outside the endocrine hypothalamus? Depress Anxiety. 2009;26:641–649. doi: 10.1002/da.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl C, Walch T, Huber R, Kemmler G, Neurauter G, et al. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J Affect Disord. 2005;86:135–142. doi: 10.1016/j.jad.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N. Depression and heart disease. Cleve Clin J Med. 2007;74 Suppl 1:S63–S66. doi: 10.3949/ccjm.74.suppl_1.s63. [DOI] [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152–2154. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Wall JM, Pagel RL, Hauser P. Administration of pegylated interferon-alpha-2a or -2b does not induce sickness behavior in Lewis rats. Psychoneuroendocrinology. 2006;31:1289–1294. doi: 10.1016/j.psyneuen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol Psychiatry. 2009;65:344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay GM, Forrest CM, Christofides J, Bridel MA, Mitchell S, et al. Kynurenine metabolites and inflammation markers in depressed patients treated with fluoxetine or counselling. Clin Exp Pharmacol Physiol. 2009;36:425–435. doi: 10.1111/j.1440-1681.2008.05077.x. [DOI] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–116. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpe S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71:1837–1848. doi: 10.1016/s0024-3205(02)01853-2. [DOI] [PubMed] [Google Scholar]
- Meyers CA. Mood and cognitive disorders in cancer patients receiving cytokine therapy. Adv Exp Med Biol. 1999;461:75–81. doi: 10.1007/978-0-585-37970-8_5. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Corsi AM, Penninx BW, Bandinelli S, Guralnik JM, Ferrucci L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biol Psychiatry. 2009;65:973–978. doi: 10.1016/j.biopsych.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain, Behavior Immunity. 2008;23:149–1458. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23:149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Moller SE, Kirk L, Honore P. Tryptophan tolerance and metabolism in endogenous depression. Psychopharmacology (Berl) 1982;76:79–83. doi: 10.1007/BF00430761. [DOI] [PubMed] [Google Scholar]
- Moore P, Landolt HP, Seifritz E, Clark C, Bhatti T, et al. Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology. 2000;23:601–622. doi: 10.1016/S0893-133X(00)00161-5. [DOI] [PubMed] [Google Scholar]
- Moreau M, Andre C, O'Connor JC, Dumich SA, Woods JA, et al. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun. 2008;22:1087–1095. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, et al. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis. 2005;192:537–544. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: schizophrenia and major depression as inflammatory CNS disorders. Eur Arch Psychiatry Clin Neurosci. 2008;258 Suppl 2:97–106. doi: 10.1007/s00406-008-2012-3. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK. Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med Hypotheses. 2003;61:519–525. doi: 10.1016/s0306-9877(03)00207-x. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Park SH, Scharpe S, et al. Tryptophan breakdown pathway in bipolar mania. J Affect Disord. 2007a;102:65–72. doi: 10.1016/j.jad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007b;98:143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Myint AM, O'Mahony S, Kubera M, Kim YK, Kenny C, et al. Role of paroxetine in interferon-alpha-induced immune and behavioural changes in male Wistar rats. J Psychopharmacol. 2007c;21:843–850. doi: 10.1177/0269881107077165. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009a;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009b;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009c;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Irwin MR, Wellisch DK. When grief heats up: pro-inflammatory cytokines predict regional brain activation. Neuroimage. 2009d;47:891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson SK, Samuelsson M, Saetre P, Lindstrom L, Jonsson EG, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J Psychiatry Neurosci. 35:195–199. doi: 10.1503/jpn.090180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav Immun. 2009;23:1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Riedel WJ, Klaassen T, Schmitt JA. Tryptophan, mood, and cognitive function. Brain Behav Immun. 2002;16:581–589. doi: 10.1016/s0889-1591(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Riedel WJ, Klaassen T, Schmitt JA. Tryptophan, mood, and cognitive function. Brain Behav Immun. 2002;16:581–589. doi: 10.1016/s0889-1591(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Schrocksnadel K, Widner B, Bergant A, Neurauter G, Schennach H, et al. Longitudinal study of tryptophan degradation during and after pregnancy. Life Sci. 2003;72:785–793. doi: 10.1016/s0024-3205(02)02304-4. [DOI] [PubMed] [Google Scholar]
- Schwarcz R. The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol. 2004;4:12–17. doi: 10.1016/j.coph.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Scrandis DA, Langenberg P, Tonelli LH, Sheikh TM, Manogura AC, et al. Prepartum Depressive Symptoms Correlate Positively with C-Reactive Protein Levels and Negatively with Tryptophan Levels: A Preliminary Report. Int J Child Health Hum Dev. 2008;1:167–174. [PMC free article] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Strike PC, Wardle J, Steptoe A. Mild acute inflammatory stimulation induces transient negative mood. J Psychosom Res. 2004;57:189–194. doi: 10.1016/S0022-3999(03)00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting KK, Brew B, Guillemin G. The involvement of astrocytes and kynurenine pathway in Alzheimer's disease. Neurotox Res. 2007;12:247–262. doi: 10.1007/BF03033908. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Cheng JT, Chang WW, Chen CL, et al. Serotonin transporter mRNA expression is decreased by lamivudine and ribavirin and increased by interferon in immune cells. Scand J Immunol. 2006;63:106–115. doi: 10.1111/j.1365-3083.2005.01715.x. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Cheng JT, Lin CF, Wu HT, et al. Interferon-alpha-induced serotonin uptake in Jurkat T cells via mitogen-activated protein kinase and transcriptional regulation of the serotonin transporter. J Psychopharmacol. 2008;22:753–760. doi: 10.1177/0269881107082951. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Van der Does AJ. The effects of tryptophan depletion on mood and psychiatric symptoms. J Affect Disord. 2001;64:107–119. doi: 10.1016/s0165-0327(00)00209-3. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav Immun. 2002;16:590–595. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Rohleder N, Bierhaus A, Nawroth PP, Kirschbaum C. Determinants of the NF-kappaB response to acute psychosocial stress in humans. Brain Behav Immun. 2009;23:742–749. doi: 10.1016/j.bbi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yuasa HJ, Ball HJ, Austin CJ, Hunt NH. 1-L-methyltryptophan is a more effective inhibitor of vertebrate IDO2 enzymes than 1-D-methyltryptophan. Comp Biochem Physiol B Biochem Mol Biol. 157:10–15. doi: 10.1016/j.cbpb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]