Studies using models of total ischemia have corroborated the importance of oxygen as the limiting factor during occlusion. In the present study, the authors present a novel system for vitreal oxygenation using electrolysis of the water content of the vitreous gel. Specifically, the aim was to evaluate the potential role of anterior vitreal oxygenation, through oxygen-generating electrodes, for reversing ischemic insult in a rabbit model with ischemia reperfusion.
Abstract
Purpose.
To study the feasibility of anterior vitreal oxygenation for the treatment of acute retinal ischemia.
Methods.
Twenty rabbits were randomized into an oxygenation group, a sham treatment group, and a no treatment group. Baseline electroretinography (ERG) and preretinal oxygen (Po2) measurements were obtained 3 to 5 days before surgery. Intraocular pressure was raised to 100 mm Hg for 90 minutes and then normalized. The oxygenation group underwent vitreal oxygenation for 30 minutes using intravitreal electrodes. The sham treatment group received inactive electrodes for 30 minutes while there was no intervention for the no treatment group. Preretinal Po2 in the posterior vitreous was measured 30 minutes after intervention or 30 minutes after reperfusion (no treatment group) and on postoperative days (d) 3, 6, 9, and 12. On d14, rabbits underwent ERG and were euthanatized.
Results.
Mean final (d12) Po2 was 10.64 ± 0.77 mm Hg for the oxygenation group, 2.14 ± 0.61 mm Hg for the sham group, and 1.98 ± 0.63 mm Hg for the no treatment group. On ERG, scotopic b-wave amplitude was significantly preserved in the oxygenation group compared with the other two groups. Superoxide dismutase assay showed higher activity in the operated eyes than in the nonoperated control eyes in the sham treatment group and no treatment group only. Histopathology showed preservation of retinal architecture and choroidal vasculature in the oxygenation group, whereas the sham-treated and nontreated groups showed retinal thinning and choroidal atrophy.
Conclusions.
In severe total ocular ischemia, anterior vitreal oxygenation supplies enough oxygen to penetrate the retinal thickness, resulting in rescue of the RPE/choriocapillaris that continues to perfuse, hence sparing the retinal tissue from damage.
Several ocular and systemic diseases (e.g., central retinal artery occlusion, carotid artery disease, diabetic retinopathy, and possibly glaucoma) are accompanied by retinal ischemia, which is a common cause of visual impairment and blindness.1 Retinal ischemia results in neuronal cell degeneration and activation of glial cells. Neuronal cell degeneration is caused by oxygen and substrate deprivation during ischemia and by reperfusion injury.1,2
Apoptosis (programmed cell death without necrosis) has been cited as a potential pathway of retinal ganglion cell death in both human glaucoma and experimental primate glaucoma associated with elevated IOP.2–4 In the eye, growth factor deprivation of retinal ganglion cells results from the blockade of retrograde transport at the lamina cribrosa, preventing growth factors from reaching their site of action in the cell body.5,6
When the IOP is elevated, the choroidal blood flow decreases, leading to a reduction in approximately 0.5 mm Hg choroidal partial oxygen pressure/1 mm Hg perfusion pressure.7–10 This reduction leads to a reduction in photoreceptor oxygen availability and consumption.10 Similarly, anything that decreases choroidal blood flow would be expected to have a negative impact on retinal oxygenation and on photoreceptor function. Experimental glaucoma has been shown to affect photoreceptors,11 possibly because of a reduction in the photoreceptor oxygen supply. There is also direct evidence of retinal damage by reduced choroidal blood flow in birds.12
Several studies have shown that retinal oxygenation can be partially or completely restored during arterial occlusion by making the animal hyperoxic.13–16 Studies using models of total ischemia have corroborated the importance of oxygen as the limiting factor during occlusion. Anderson and Saltzman17 have shown that if human subjects breathed oxygen before IOP elevation, their vision was sustained longer than if they breathed room air. Blair et al.18 have shown that perfusing the vitreous with an oxygenated solution after total occlusion can maintain the structural and electrophysiological integrity of the retina. Despite these positive experimental data, the clinical experience with hyperoxia has been mixed, with only few studies recommending hyperoxia as a treatment for vascular occlusion.19
In the present study, we present a novel system for vitreal oxygenation using electrolysis of the water content of the vitreous gel. Specifically, our aim was to evaluate the potential role of anterior vitreal oxygenation, by oxygen-generating electrodes, for reversing ischemic insult in a rabbit model with ischemia reperfusion.
Methods
Twenty Dutch pigmented rabbits, each weighing 2 to 3 kg, were used in this study. All animal experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Southern California.
For all animal procedures, the rabbits were anesthetized with subcutaneous injection of a mixture of ketamine hydrochloride (25 mg/kg) and xylazine hydrochloride (6 mg/kg) that was repeated as necessary. The pupils were dilated with a topical application of phenylephrine hydrochloride 2.5% and tropicamide 0.5% eyedrops.
Animal Groups
Twenty rabbits were divided randomly into three groups: an oxygenation group (n = 10) that underwent oxygenation after ischemia, a sham treatment group (n = 5) that received inactive oxygen-generating electrode implantation after ischemia, and a no treatment group (n = 5) that did not receive any intervention after ischemia. Both the sham treatment and the no treatment groups acted as controls for the oxygenation group. In all 20 rabbits, baseline electroretinograms and preretinal Po2 (measured 4 disc diameters below the optic disc) was evaluated 3 to 5 days before surgery. Five of the 10 animals in the oxygenation group were evaluated by ERG 2 weeks after surgery, whereas all five animals in the sham treatment group and all five animals in the no treatment group were evaluated by ERG 2 weeks after surgery.
Electroretinography
Full-field ERG was performed 3 to 5 days before surgery and 2 weeks after surgery. Rabbits were dark adapted for 20 minutes before recordings. Stimuli were delivered by a handheld mini-Ganzfeld stimulator (ColorBurst; Diagnosys LLC, Lowell, MA).
The rabbits were positioned on the operating table, contact lens electrodes (ERGjet; Universo Plastique SA, Le Crêt-du-Locle, Switzerland) were placed on the anesthetized cornea, and reference electrodes were inserted into the anterior scalp just above the upper eyelid, with sublingual grounding electrodes in place. Dark-adapted ERG was performed with a white stimulus; a total of eight responses were averaged. The electroretinogram was analyzed by measuring the amplitude of the scotopic b-wave. The stimulus intensity for scotopic ERG was 0.01 cd · s/m2 for rod response and 3 cd · s/m2 for maximum response.
Ischemia Reperfusion Model
Under anesthesia, the anterior chamber of the right eye of each rabbit was cannulated with a 25-gauge butterfly needle connected to a BSS bottle high enough to raise IOP to 120 mm Hg, as confirmed by intraoperative manometry (Meri-Cal Portable Digital Manometer; Meriam Process Technologies, Cleveland, OH).
The IOP rise was maintained for 90 minutes. This pressure resulted in the cessation of blood flow in the retinal vessels, as confirmed by blanching of the fundus.
After 90 minutes, the bottle was slowly lowered to prevent sudden hypotony aiming at normal IOP. The needle was removed, and the wound was made watertight by a 10/0 nylon stitch. Reperfusion of the fundus was confirmed by fundus examination.
Oxygen-Generating Electrode Implantation
For the oxygenation group, the anterior vitreal cavity of the right eye was oxygenated for 30 minutes immediately after reperfusion, when IOP returned to normal. Oxygen was generated by electrolysis of the water content of the vitreous gel, as described previously.20
For the sham treatment group, after the 90-minute ischemic period, the electrodes were implanted into the vitreous cavity for 30 minutes without turning the pulse stimulator on. For the no treatment group, no electrodes were implanted.
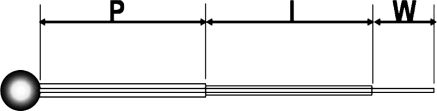
Electrodes were fabricated from high-purity, fine-gauge platinum wire. Platinum spheres were fused at the ends of the wires using an oxy-acetylene torch. The wires were then sealed in thin glass (Pyrex; Corning Inc., Corning, NY) tubing with the spheres projecting immediately beyond the seal. The outer diameter of the platinum terminal segment measured 0.8 mm. The length of the thin glass (Pyrex; Corning Inc.) tubing was 2.5 cm (Fig. 1).
Figure 1.
Schematic drawing shows the design of the oxygen electrode. P, glass (Pyrex) with stiff insulation; I, soft flexible insulation; W, bare flexible platinum wire.
Two electrodes were inserted into the anterior vitreal cavity through sclerotomies 2.5 mm from the limbus in the upper nasal and temporal quadrants of the globe. A pulse stimulator (Isolated Pulse Stimulator, model 2100; A-M Systems, Inc., Sequim, WA) was used to activate the electrodes in the oxygenation group. Repetitive pulse currents of electrolysis resulted in steady state rates of oxygen and hydrogen production in midvitreous.
The settings used for oxygen generation were as follows: pulse duration, 2 ms; interpulse duration, 1 ms; range amplitude, 100 μA. The hydrogen produced at the cathode was metabolically inert. The small hydrogen molecules eventually diffused out of the ocular cavity.
Po2 Measurement
For Po2 measurement, we used a fiberoptic probe connected to a meter (OxyLite 4000 system; Oxford Optronix Ltd., Oxford, UK). The probe has an outer diameter of 250 μm and contains a luminescent dye at the tip for which the signal half-life is inversely proportional to the oxygen concentration. The data-collecting device excites the dye with a light pulse and then measures the signal half-life and converts it to its corresponding Po2.
The probe was introduced into the vitreous cavity of anesthetized rabbits through a sclerotomy 2.5 mm from the limbus in the lower temporal quadrant of the globe. Baseline measurement of the preretinal Po2 in the posterior vitreous, from an area 4 disc diameters below the optic disc, was performed for all animals at the end of the baseline ERG (3–5 days before surgery). For the control groups, Po2 was measured 30 minutes after ischemia. For the oxygenation group, Po2 was measured 30 minutes after the end of oxygenation. For Po2 measurement, the tip of the probe was introduced into the vitreous cavity to <2 mm from the retinal surface, as guided by direct visualization through the surgical microscope; 10 readings over 5 minutes were taken from the retina (4 disc diameters below the optic disc), and the readings were averaged. All measurements were performed in light-adapted conditions. The microscope light was turned off immediately before actual preretinal Po2 measurements because this probe (OxyLite; Oxford Optronix Ltd.) is very sensitive to bright light. The measurement procedure took only a few seconds.
Po2 measurements were repeated on days 3, 6, 9, and 12 after the primary experiment under the same anesthesia and aseptic conditions. No further oxygenation was received by any group during the follow-up period. Gentamicin eye ointment was applied after closure of the sclerotomy used for insertion of the probe (OxyLite; Oxford Optronix Ltd.). During the postoperative follow-up period, we used polymyxin-hydrocortisone eye ointment and tropicamide eyedrops daily to reduce the risk of uveitis.
Euthanatization
Two weeks after the ischemia reperfusion experiment, rabbits were euthanatized by intracardiac injection of pentobarbital (Beuthanasia-D; Schering Plough Animal Health, Omaha, NE), and both eyes were enucleated.
Superoxide Dismutase Activity Assay
Retinal samples (5 × 5 mm) were cut from the retina in a standardized location (4 disc diameters below the optic disc) of the enucleated eyes immediately after euthanatization and were washed with cold 1× phosphate-buffered saline (PBS). The samples were homogenized using a sonicator and were centrifuged to separate the sedimented tissue pellets. The pellets were then washed with cold PBS, diluted with 10 volumes of 1× PBS, and centrifuged at 4°C for 10 minutes at 10,000 rpm. The supernatant was transferred to new Eppendorf tubes and immersed into liquid nitrogen to be stored at −80°C until the time of superoxide dismutase (SOD) assay. Using a SOD kit (Assay Designs, Inc., Ann Arbor, MI), absorbance was read at 450 nm, measuring SOD activity in international units (IU).
Light Microscopic Examination
Enucleated eyes were immersed in Davidson's fixative solution (two eyes from each group) overnight and then dehydrated in a series of graded alcohol solutions over the next 24 to 48 hours before paraffin embedding. Contralateral nonoperated eyes were used for normal controls. Blocks were obtained from cuts through the whole globe oriented perpendicularly to the medullary wings. Sections 5-μm thick were obtained by microtome, stained with hematoxylin and eosin, and examined by light microscopy. Retinal regions 4 disc diameters below the optic disc were photographed at the same magnification; retinal thickness was measured (Photoshop, version CS3; Adobe, Mountain View, CA). The retinal thickness in the operated right eye was compared with that of the left, nonoperated eye.
Immunohistochemistry
Enucleated eyes (2 rabbits from each group) were fixed in 4% paraformaldehyde for 2 hours. After the anterior segment of the globe was removed, the eyecups were fixed further in paraformaldehyde overnight and then transferred to 25% sucrose solution for an additional 24 hours. Blocks were cut from the posterior eyecup 4 disc diameters below the optic nerve head and then embedded in optimal cutting temperature compound with 2-methylbutane and dry ice. Cryosections (5-μm thick) were prepared at −20°C, fixed in cold methanol for 15 minutes, rinsed in 1× PBS for 5 minutes, and incubated with 10% goat serum for 45 minutes at room temperature.
The primary antibodies were anti–glial fibrillary acidic protein (GFAP) antibody (mouse IgG, 1:200; BD PharMingen, Franklin Lakes, NJ) as a marker for glial cells and anti–acrolein antibody (mouse IgG, 1:100; Genox Corporation, Baltimore, MD) as a marker for oxidative protein modification (FDP-lysine derivatives). Incubation with either primary antibody was performed for 1 hour at 37°C. Sections then were washed with 1× PBS three times and incubated with either FITC (anti-mouse IgG, 1:200; Abcam, Cambridge, MA)– or Texas Red (anti-mouse IgG, 1:200; American Qualex, San Clemente, CA)–conjugated secondary antibody at room temperature for 1 hour. Retinal sections were washed for 30 minutes with 0.1 M PBS and coverslipped with mounting medium (Vectashield; Vector Laboratories Inc., Burlingame, CA) containing 4,6-diamidino-2-phenylindole (DAPI). Sections were then analyzed using a confocal microscope (LSM 510; Carl Zeiss Inc., Thornwood, NY). FITC staining was captured using the 488-nm argon laser. Texas Red staining was captured using a 543 nm HeNe laser. DAPI staining was captured using 800 nm titanium sapphire laser. Immunofluorescence images were processed using Zeiss software (LSM-PC; Carl Zeiss Inc.). Corresponding negative controls were prepared by substitution of the primary antibody with 10% normal goat serum in PBS.
Scanning Electron Microscopy
For this purpose, we sectioned 5 × 5-mm retinal samples (4 disc diameters below the optic disc) from the enucleated eyes to be fixed in half-strength Karnovsky's fixative for 2 days. After initial fixation, the specimens were rinsed several times with phosphate buffer then post fixed with 1% osmium tetroxide in 0.1 M PBS for 1 hour. After a rinse with PBS, the specimens were dehydrated using a series of graded ethyl alcohol solutions and then chemically dried using hexamethyldisilazane. After drying, the specimens were mounted on aluminum stubs with adhesive tabs and sputter coated for 3 minutes (Polaron; Energy Beam Sciences, Agawam, MA). Specimens were then viewed under a scanning electron microscope (JSM-6390; JEOL USA., Inc., Peabody, MA).
Statistical Analysis
Data are shown as the mean ± SD. To compare data obtained from the right (experimental) eye and the left (unoperated) eye in the study groups and to specifically compare baseline and final results in the same eye, independent Student's t-tests were performed. To compare data obtained at various time points among the three study groups, one-way ANOVA tests were performed. P < 0.05 was considered statistically significant. All analyses were conducted using statistical analysis (SPSS V 15.0; SPSS Inc., Chicago, IL).
Results
Po2 Measurements
In initial experiments, we evaluated preretinal Po2 values in the posterior vitreous 4 disc diameters below the disc in naive rabbits 3 to 5 days before the ischemia experiment. In these normal rabbit eyes (n = 20), we found the preretinal Po2 levels ranged from 16 to 22 mm Hg, with a mean of 18 ± 2.56 mm Hg.
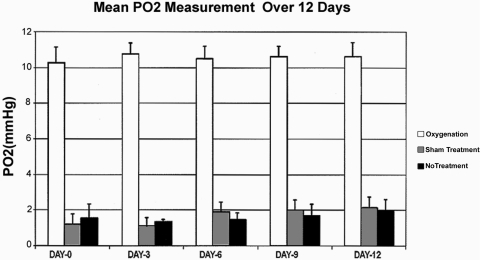
Results of Po2 measurements at the end of the initial surgical procedure to induce IOP elevation and over the 12-day period after surgery (measured on days 0, 3, 6, 9, and 12) revealed a statistically significant difference in the preretinal Po2 between the oxygenation group (n = 10), and the sham treatment (n = 5) and no treatment (n = 5) groups (P < 0.0001; one-way ANOVA). The final preretinal Po2 in the oxygenation group 12 days after surgery was 10.64 ± 0.77 mm Hg compared with 2.14 ± 0.61 mm Hg in the sham treatment group and 1.98 ± 0.63 mm Hg in the no treatment group (Fig. 2).
Figure 2.
Graph shows mean preretinal Po2 measurements (in mm Hg) at days 0, 3, 6, 9, and 12 after surgery. The measurements taken from the oxygenation group (n = 10) were significantly higher than those from the sham treatment (n = 5) and the no treatment (n = 5) groups over the 12-day follow-up period (one-way ANOVA; P < 0.0001). Error bars represent 1 SD.
ERG Changes
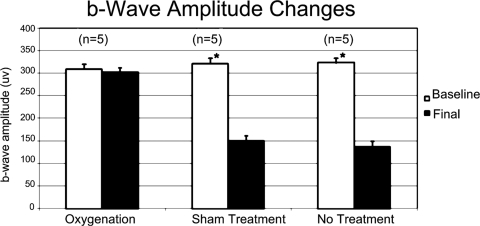
Scotopic b-wave amplitude was depressed in the control groups compared with the oxygenation group. Both the sham treatment and the no treatment groups showed a statistically significant depression of the final scotopic b-wave amplitude at 2 weeks from the baseline recordings (P = 0.02 and P = 0.03, respectively), whereas the oxygenation group showed no statistically significant difference from the baseline recordings (P = 0.18) (Fig. 3).
Figure 3.
Graph shows the amplitude changes of the scotopic b-wave comparing the initial baseline normal readings with the final readings at 2 weeks. Error bars represent 1 SD. Statistically significant difference exists between the baseline and the final values for the sham treatment and the no treatment groups, whereas no statistically significant difference exists for the oxygenation group. *P < 0.05.
SOD Activity Assay
Results of SOD activity in retinal tissues as measured by ELISA assay revealed a statistically significant difference in SOD activity levels in the operated eyes in the sham treatment and the no treatment groups compared with the contralateral nonoperated eyes (P = 0.03 and 0.025, respectively). In the oxygenation group, there was no significant difference between the oxygenated eyes and the contralateral nonoperated eyes (P = 0.12). The mean SOD measurement was 7.13 ± 1.06 IU for the sham treatment group, 8.21 ± 1.22 IU for the no treatment group, and 3.55 ± 0.79 IU for the oxygenation group.
Histopathology
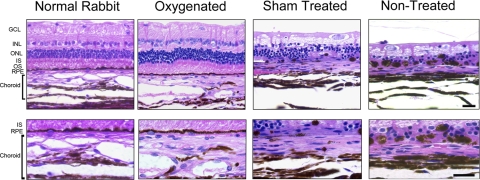
Light microscopic examination revealed that both the oxygenated and the sham/nontreated eyes showed variable degrees of retinal damage compared with the contralateral nonoperated control eyes; however, the extent of retinal layer damage was far more extensive in the sham/nontreated groups (i.e., the ischemic eyes). In evaluation of multiple sections, nearly 80% of the normal retinal thickness was preserved in the oxygenation group, whereas retinal thickness in the sham-treated and nontreated groups was only 20% to 30% that of the contralateral nonoperated eyes. Although sections were always taken from the general region 4 disc diameters below the disc, there was some variability in retinal thickness based on sampling error. The sham operated and nontreated eyes also showed increased vacuolization of the nerve fiber layer and disorganization and extensive cell loss involving the inner and outer nuclear layers with loss of photoreceptor inner and outer segments. Although the retinal pigment epithelium (RPE) layer appeared intact in the oxygenation group, the sham-treated and nontreated retinas showed extensive RPE loss, pigment clumping, and focal RPE migration into the outer retina. Evaluation of the choroid revealed that the sham-treated and nontreated groups showed choroidal thinning with extensive atrophy of the large choroidal vessels and the choriocapillaris. In contrast, the oxygenated groups showed normal choroidal thickness with only mild atrophy of the choroidal vasculature (Fig. 4).
Figure 4.
Hematoxylin and eosin–stained vertical retinal sections from the region 4 disc diameters below the optic disc, comparing the retinal histology 2 weeks after retinal ischemia. Both the sham treatment and the no treatment eyes show reduced retinal thickness, disorganization, and loss of inner and outer nuclear layers (INL, ONL), loss of inner and outer segments (IS, OS) of the photoreceptors, vacuolization of the ganglion cell layer (GCL), and cell loss and pigment clumping of the RPE. In contrast, the oxygenated eye shows good retinal thickness and well-preserved anatomy compared with the contralateral nonoperated normal rabbit eye. The RPE/choroid is shown at higher magnification in the lower panels. There is prominent choroidal atrophy in the sham-treated and nontreated groups, whereas the oxygenated group shows only mild atrophy of the choroidal vasculature. Scale bar, 20 μm.
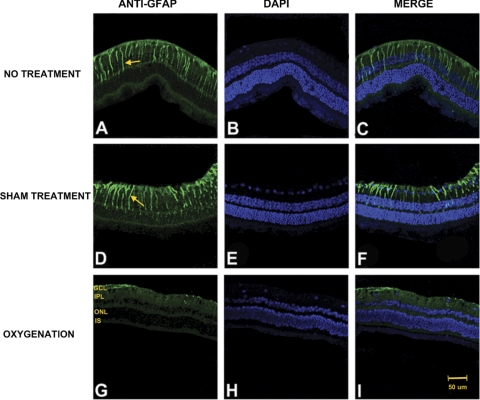
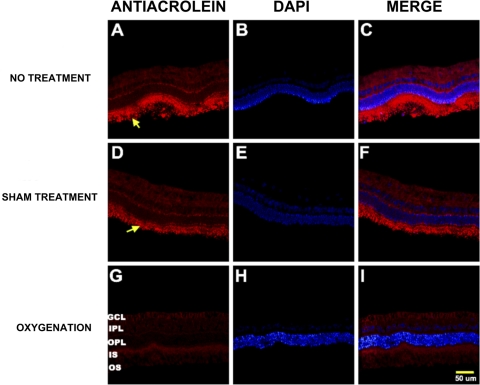
Immunohistochemical studies revealed that both the no treatment and the sham treatment groups showed increased oxidative protein modification, as detected by the anti–acrolein antibody, primarily involving the outer retinal layers and increased GFAP expression, indicating more reactive gliosis mainly in the inner retinal layers. Conversely, the oxygenation group showed less acrolein immunoreactivity and reactive gliosis (Figs. 5, 6).
Figure 5.
Photomicrographs of vertical retinal sections from standardized regions (4 disc diameters below the optic disc) in the retina processed for anti-GFAP immunofluorescence (A, D, G) with DAPI nuclear counterstain shown in images (B, E, H) and merged images (C, F, I). The secondary antibody was FITC-conjugated anti-mouse IgG. High expression of GFAP was shown in both the no treatment (A) and the sham treatment (D) groups compared with the oxygenation group (G). GCL, ganglion cell layer; IPL, inner plexiform layer; ONL, outer nuclear layer; IS, inner segments of the photoreceptor layer. Arrows: areas representative of Müller cells with increased GFAP expression. Scale bar, 50 μm.
Figure 6.
Photomicrographs of vertical retinal sections from standardized regions (4 disc diameters below the optic disc) in the retina processed for anti–acrolein immunoreactivity (A, D, G) with the nuclear counterstain DAPI shown in images (B, E, H) and merged images (C, F, I).The secondary antibody was Texas Red-conjugated anti-mouse IgG. High expression of the acrolein was shown in both the no treatment (A) and the sham treatment (D) groups compared with the oxygenation group (G). GCL, ganglion cell layer; IPL, inner plexiform layer; ONL, outer nuclear layer; IS, inner segments of the photoreceptor layer; OS, outer segments of the photoreceptor layer. Arrows: areas of increased acrolein expression in the photoreceptor IS and OS.
RPE flat mounts, evaluated by SEM, revealed that the sham-treated and nontreated groups showed focal RPE loss, whereas the remaining RPE was variable in size and shape and was often rounded up instead of having a normal hexagonal appearance. In contrast, the oxygenated group showed no apparent RPE loss, and cells retained a typical hexagonal shape (Fig. 7).
Figure 7.
Scanning electron micrograph showing the RPE surface in the three groups. RPE flat mounts revealed that the sham-treated and no treatment groups showed focal RPE loss, whereas the remaining RPE was variable in size and shape and often had a rounded rather than a normal hexagonal appearance. In contrast, the oxygenated group showed no apparent RPE loss, and cells retained a typical hexagonal shape.
Discussion
The use of an external source of oxygen to improve retinal oxygenation after exposure to an ischemic hypoxic injury has attracted the attention of researchers for many decades. However, the modalities to date have been complicated.
Hyperbaric oxygen has been tried with variable success in retinal artery occlusion, retinal vein occlusion,21,22 and anterior segment ischemia.23,24 However, many complications have been reported with hyperbaric oxygen therapy, resulting in limited use of this treatment modality in actual clinical ophthalmic practice.
Perfluorocarbon fluids have been reported to decrease retinal ischemic injury, as assessed by both ERG and histology.25 However, it is clear that this procedure requires vitrectomy and leaves the perfluorocarbon liquid in the vitreous cavity with potential retinal toxicity in the long term.26,27
Blair et al.18,28–30 developed vitreoperfusion as a model of oxygenation of the retina after vitrectomy through the perfusion of oxygen-supplemented nutrient fluid into the vitreous cavity. They recorded partial recovery of the ischemic retina by this method. However, this model is complicated and is not easily applicable to clinical work.
Finally, Ben-Nun et al.31 reported the reversal of b-wave changes in ERG in a cat model of retinal arterial occlusion after the use of vitreal oxygenation with catheters connected to an external oxygen source infusing oxygen into the vitreous cavity.
In contrast, vitreal oxygenation through electrolysis of the water content of the vitreous gel has not been tried as a method to manage retinal ischemia/ hypoxia. Initial experiments evaluated whether these oxygen-generating electrodes affected the temperature of retinal tissues. In preliminary safety experiments (n = 10 rabbits), we measured the temperature (over a 50-minute period) of the retina close to the electrodes, the retina away from the insertion site, and the anterior segment (anterior chamber and surface of retina) using a thermocouple (DP460-T; Omega Engineering, Inc., Stamford CT). The sensor has a platinum wire sensor of <1-mm outer diameter and is sensitive to <1°C changes. No significant temperature changes were found (results not shown).
Baseline experiments in the 20 rabbits included in this study revealed that preretinal Po2 levels (4 disc diameters below the optic disc) measured 18 ± 2.56 mm Hg, similar to results reported previously in the normal rabbit eye by Shui et al.32 Our results showed consistently low preretinal Po2 in sham and nontreated animals over the 12 days of observation after IOP elevation, whereas the oxygenation group showed partial restoration of preretinal Po2 to approximately 10 mm Hg, a level about midway between normal nonoperated animals and sham/nontreated animals after ischemia. Moreover, the damaging effects of induced ischemia reperfusion were minimized by intravitreal oxygenation, as proved by the well preserved b-wave amplitude, low levels of SOD enzyme activity, low levels of oxidative protein modification in the inner retinal layers, minimal reactive gliosis, and the well-preserved retinal architecture in the oxygenated eyes compared with the sham treated and no treatment control eyes, as seen in histopathology.
Preretinal Po2 is determined by the difference in oxygen supply from the choroid and oxygen consumption by the retinal cells. Clearly, retinas in the sham-treated and nontreated animals were much thinner than the normal or oxygenated retinas, suggesting much lower oxygen consumption in these groups. However, the choroidal vasculature showed marked atrophy in the sham-treated and nontreated groups, whereas the choroidal vessels in the oxygenated group showed only a modest degree of atrophy compared with the contralateral nonoperated control eyes. Similarly, the RPE was essentially normal in the oxygenated group but showed marked degeneration and cell loss in the sham-treated and nontreated groups. Therefore, we conclude that the choroidal oxygen supply was drastically reduced in the untreated groups after the elevation in IOP, resulting in low preretinal Po2 levels. It is interesting that the Po2 levels in the sham and no-treatment groups were low right after surgery (d0) and remained low over the 12 days of evaluation, whereas in the oxygenation group, preretinal Po2 levels remained consistently approximately fivefold higher. This suggests that damage to the choriocapillaris is rapid after IOP elevation and does not recover, whereas anterior vitreal oxygenation inhibits the damage to the choriocapillaris and allows the preretinal Po2 levels to be restored. Histology shows parallel damage to the RPE and choroidal vasculature in the sham-treated and nontreated groups. Although damage to the RPE has been shown to result in secondary atrophy of choriocapillaris,33 we do not know whether IOP elevation results in primary damage to the RPE with secondary choriocapillaris atrophy or is a result of primary damage to both cell types.
Because preretinal Po2 levels were partially restored in eyes with choriocapillaris damage after IOP elevation, we wondered whether preretinal Po2 would also be affected in eyes with normal choroidal vasculature. Therefore, we evaluated the effect of intravitreal oxygenation in normal rabbit eyes (n = 12). No significant alteration in preretinal Po2 was found in normal eyes after oxygenation compared with baseline (results not shown).
Increased SOD activity is a response to oxidative tissue damage; the more oxidative stress tissue faces, the more SOD activity.34,35 We found a statistically significant difference in SOD activity between both the sham treatment and the no-treatment groups compared with their corresponding nonoperated contralateral eyes. In contrast, there was no significant difference in SOD activity in the oxygenated group compared with their contralateral nonoperated eyes. It is thus reasonable to assume that the oxygenated eyes were exposed to less tissue damage and less oxidative stress.
Treatment with intravitreal oxygen provides a direct source of oxygen for the ischemic tissue and may reverse the cascade initiated by ischemic hypoxia that ends in apoptosis. This method of therapy obviously avoids the risks associated with hyperbaric oxygen and obviates the need to ablate the ischemic tissues by laser to avoid the development of neovascularization. Moreover, unlike vitreoperfusion or the use of perfluorocarbon liquid, there is no need for vitrectomy, and there is no risk for transmission of infection between patients. Thus, this method of therapy may ultimately have a place in the treatment of many ocular disorders, be they acute conditions such as central retinal artery and vein occlusion or chronic conditions such as diabetic retinopathy.
The maximum time reported in the literature for retinal tolerance to induced ischemia in the rabbit eye is <75 minutes with an IOP of 160 mm Hg.36 We documented that even 90 minutes of ischemia can be tolerated by the rabbit retina when it is immediately followed by vitreal oxygenation.
One concern in vitreal oxygenation is possible oxygen toxicity in the retina caused by hyperoxic conditions. Previous studies have shown that exposure of neonatal animals to hyperoxia results in the obliteration of retinal vessels,37 but much evidence now indicates that adult retinal vessels are resistant to damage.38 For instance, inhalation of 70% oxygen for 48 hours results in mild reversible constriction of retinal vessels in an adult animal, whereas in a neonate with an immature retina, the result is permanent occlusion of many retinal vessels.39 Retinal vascular development is stimulated by increased vascular endothelial growth factor expression in avascular peripheral retina, which is decreased when vascularization relieves the physiologic hypoxia.40 Hyperoxia results in premature downregulation of this factor expression in the retina and the obliteration of newly formed vessels. Yamada et al.41 have demonstrated a decreased susceptibility of mature versus immature retinal vessels to the damaging effects of hyperoxia that is good for the regression and amelioration of newly formed vessels in proliferative diabetic retinopathy.
One can consider other theoretical difficulties that could prevent or decrease the success of our vitreal oxygenation, among them: that diffusion is essentially the only means of delivering oxygen from the anterior vitreous to the retina, that the diffusion distance is relatively long, and that consumption of oxygen in the inner ocular tissues reduces the Po2 gradient available to the retina. However, our results provide conclusive evidence that our procedure overcomes all these difficulties, especially since the minimal requirement of oxygen for aerobic cellular metabolism is in the order of a few millimeters of mercury.42
In conclusion, in severe total ocular ischemia, anterior vitreal oxygenation supplies enough oxygen to penetrate the retinal thickness, where it can impact the RPE/choriocapillaris. This results in rescue of the RPE/choriocapillaris, which continues to perfuse and, hence, spares the retinal tissue from damage. Our evidence suggests that intravitreal oxygenation could be a useful tool for the treatment of ischemic hypoxic conditions of the retina. Future experiments using less severe increases in IOP resulting in transient ischemia could provide an experimental model with more direct relevance to retinal ischemic disease. Long-term studies are also needed to evaluate both the efficacy and the safety of this procedure. If the procedure is proven safe and effective in the long term, our study may pave the way for fabrication of an implantable intraocular prosthesis for continuous vitreal oxygenation.
Acknowledgments
The authors thank Laurie Dustin (Core statistician for the National Eye Institute Core grant) for statistical analysis of data, Zhenhai Chen for performing electroretinography, Larry W. McCollum for electrode fabrication, and Charlene A. Sanders for electrode preparation.
Footnotes
Supported in part by National Eye Institute Core Grant EY03040 and the US Department of Energy Office of Biological and Environmental Research. Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for the US Department of Energy under contract no. DE-AC05–00OR22725.
Disclosure: W. Abdallah, None; H. Ameri, None; E. Barron, None; G.J. Chader, None; E. Greenbaum, P; D.R. Hinton, None; M.S. Humayun, P
References
- 1. Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147 [DOI] [PubMed] [Google Scholar]
- 2. Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786 [PubMed] [Google Scholar]
- 3. Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57 [DOI] [PubMed] [Google Scholar]
- 4. Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035 [DOI] [PubMed] [Google Scholar]
- 5. Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monosodium glutamate. Science. 1969;166:386–388 [DOI] [PubMed] [Google Scholar]
- 6. Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493 [DOI] [PubMed] [Google Scholar]
- 7. Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29 [DOI] [PubMed] [Google Scholar]
- 8. Alm A, Bill A. Blood flow and oxygen extraction in the cat uvea at normal and high intraocular pressures. Acta Physiol Scand. 1970;80:19–28 [DOI] [PubMed] [Google Scholar]
- 9. Ernest JT, Goldstick TK. Response of choroidal vascular resistance to hyperglycemia. Int Ophthalmol. 1983;6:119–124 [DOI] [PubMed] [Google Scholar]
- 10. Yancey CM, Linsenmeier RA. Oxygen distribution and consumption in the cat retina at increased intraocular pressure. Invest Ophthalmol Vis Sci. 1989;30:600–611 [PubMed] [Google Scholar]
- 11. Nork TM, Ver Hoeve JN, Poulsen GL, et al. Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch Ophthalmol. 2000;118:235–245 [DOI] [PubMed] [Google Scholar]
- 12. Fitzgerald ME, Vana BA, Reiner A. Evidence for retinal pathology following interruption of neural regulation of choroidal blood flow: Muller cells express GFAP following lesions of the nucleus of Edinger-Westphal in pigeons. Curr Eye Res. 1990;9:583–598 [DOI] [PubMed] [Google Scholar]
- 13. Braun RD, Linsenmeier RA. Retinal oxygen tension and the electroretinogram during arterial occlusion in the cat. Invest Ophthalmol Vis Sci. 1995;36:523–541 [PubMed] [Google Scholar]
- 14. Haugh-Scheidt LM, Griff ER, Linsenmeier RA. Light-evoked oxygen responses in the isolated toad retina. Exp Eye Res. 1995;61:73–81 [DOI] [PubMed] [Google Scholar]
- 15. Patz A. Oxygen inhalation in retinal arterial occlusion: a preliminary report. Am J Ophthalmol. 1955;40:789–795 [DOI] [PubMed] [Google Scholar]
- 16. Landers MB., 3rd Retinal oxygenation via the choroidal circulation. Trans Am Ophthalmol Soc. 1978;76:528–556 [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson B, Jr, Saltzman HA. Retinal oxygen utilization measured by hyperbaric blackout. Arch Ophthalmol. 1964;72:792–795 [DOI] [PubMed] [Google Scholar]
- 18. Blair NP, Baker DS, Rhode JP, Solomon M. Vitreoperfusion: a new approach to ocular ischemia. Arch Ophthalmol. 1989;107:417–423 [DOI] [PubMed] [Google Scholar]
- 19. Perkins SA, Magargal LE, Augsburger JJ, Sanborn GE. The idling retina: reversible visual loss in central retinal artery obstruction. Ann Ophthalmol. 1987;19:3–6 [PubMed] [Google Scholar]
- 20. Greenbaum E, Humayun MS, Sanders CA, Close D, O'Neill HM, Evans BR. Metabolic prosthesis for oxygenation of ischemic tissue. IEEE Trans Biomed Eng. 2009;56:528–531 [DOI] [PubMed] [Google Scholar]
- 21. Miyamoto H, Ogura Y, Wakano Y, Honda Y. The long term results of hyperbaric oxygen treatment for macular edema with retinal vein occlusion. Nippon Ganka Gakkai Zasshi. 1993;97:1065–1069 [PubMed] [Google Scholar]
- 22. Roy M, Bartow W, Ambrus J, Fauci A, Collier B, Titus J. Retinal leakage in retinal vein occlusion: reduction after hyperbaric oxygen. Ophthalmologica. 1989;198:78–83 [DOI] [PubMed] [Google Scholar]
- 23. de Smet MD, Carruthers J, Lepawsky M. Anterior segment ischemia treated with hyperbaric oxygen. Can J Ophthalmol. 1987;22:381–383 [PubMed] [Google Scholar]
- 24. Recupero SM, Cruciani F, Picardo V, Sposato PA, Tamanti N, Abdolrahimzadeh S. Hyperbaric oxygen therapy in the treatment of secondary keratoendotheliosis. Ann Ophthalmol. 1992;24:448–452 [PubMed] [Google Scholar]
- 25. Wilson CA, Berkowitz BA, Srebro R. Perfluorinated organic liquid as an intraocular oxygen reservoir for the ischemic retina. Invest Ophthalmol Vis Sci. 1995;36:131–141 [PubMed] [Google Scholar]
- 26. Orzalesi N, Migliavacca L, Bottoni F, Miglior S. Experimental short-term tolerance to perfluorodecalin in the rabbit eye: a histopathological study. Curr Eye Res. 1998;17:828–835 [DOI] [PubMed] [Google Scholar]
- 27. Stolba U, Krepler K, Velikay-Parel M, Binder S. The effect of specific gravity of perfluorocarbon liquid on the retina after experimental vitreous substitution. Graefes Arch Clin Exp Ophthalmol. 2004;242:931–936 [DOI] [PubMed] [Google Scholar]
- 28. Blair NP. Ocular oxygen consumption during vitreoperfusion in the cat. Trans Am Ophthalmol Soc. 2000;98:305–329 [PMC free article] [PubMed] [Google Scholar]
- 29. Blair NP, Liu T, Warren KA, et al. Ocular oxygen consumption: estimates using vitreoperfusion in the cat. Retina. 2004;24:120–131 [DOI] [PubMed] [Google Scholar]
- 30. Blair NP, Shaw WE, Dunn R, Jr, Tsukarhara Y, Floro C, Rusin MM. Limitation of retinal injury by vitreoperfusion initiated after onset of ischemia. Arch Ophthalmol. 1991;109:113–118 [DOI] [PubMed] [Google Scholar]
- 31. Ben-Nun J, Alder VA, Cringle SJ, Constable IJ. A new method for oxygen supply to acute ischemic retina. Invest Ophthalmol Vis Sci. 1988;29:298–304 [PubMed] [Google Scholar]
- 32. Shui YB, Fu JJ, Garcia C, et al. Oxygen distribution in the rabbit eye and oxygen consumption by the eye. Invest Ophthalmol Vis Sci. 2006;47:1571–1580 [DOI] [PubMed] [Google Scholar]
- 33. Korte GE, Reppucci V, Henkind P. RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci. 1984;25:1135–1145 [PubMed] [Google Scholar]
- 34. Yen HC, Oberley TD, Gairola CG, Szweda LI, St Clair DK. Manganese superoxide dismutase protects mitochondrial complex I against adriamycin-induced cardiomyopathy in transgenic mice. Arch Biochem Biophys. 1999;362:59–66 [DOI] [PubMed] [Google Scholar]
- 35. Ardestani A, Yazdanparast R, Jamshidi S. Therapeutic effects of Teucrium polium extract on oxidative stress in pancreas of streptozotocin-induced diabetic rats. J Med Food. 2008;11:525–532 [DOI] [PubMed] [Google Scholar]
- 36. Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina, IV: retinal tolerance time to acute ischaemia. Br J Ophthalmol. 1980;64:818–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. Br J Ophthalmol. 1953;37:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ashton N. Donders Lecture, 1967: some aspects of the comparative pathology of oxygen toxicity in the retina. Br J Ophthalmol. 1968;52:505–531 [PubMed] [Google Scholar]
- 39. Patz A. The role of oxygen in retrolental fibroplasia. Trans Am Ophthalmol Soc. 1968;66:940–985 [PMC free article] [PubMed] [Google Scholar]
- 40. Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamada H, Yamada E, Hackett SF, Ozaki H, Okamoto N, Campochiaro PA. Hyperoxia causes decreased expression of vascular endothelial growth factor and endothelial cell apoptosis in adult retina. J Cell Physiol. 1999;179:149–156 [DOI] [PubMed] [Google Scholar]
- 42. Jobsis FF. Basic process in cellular respiration. In: Handbook of Physiology. Washington, DC: American Physiological Society; 1964:63–125 [Google Scholar]