This study was conducted to better characterize the effects of manipulating peripheral retinal defocus on ocular growth in the chick model, using a series of two-zone lens designs that varied in both defocusing power and diameter of the respective zones. The intent was to simulate the bifocal soft contact lenses that have been trialed for myopia control in humans, to promising effect.
Abstract
Purpose.
To characterize the effects on refractive error development and eye growth in young chicks of two-zone concentric lens designs, which differentially affect the defocus experiences of central and peripheral retinal regions.
Methods.
Monocular defocusing lenses were worn for 5 days from 17 days of age. Four two-zone concentric lens designs (overall optical zone diameter, 10 mm) combining plano with either −5- or +5-D power were used. Lens designs were as follows: (1) +5 D center (+5C), (2) +5 D periphery (+5P), (3) −5 D center (−5C), and (4) −5 D peripheral (−5P), with plano in periphery for all C-designs and in the center for P-designs. Five central zone diameters (CZDs) were tested, ranging from 2.5 to 6.5 mm in 1-mm increments. Plano, +5- and −5-D single-vision (SV) lenses were used as the control. A minimum of six birds were included in each lens group.
Results.
For the two-zone lenses, the P designs (i.e., peripheral defocus) had greater effects than the C designs (i.e., central defocus) on both on-axis eye growth and refractions. All but the 6.5-mm CZD +5P lens induced larger changes than the +5SV lens. The +5C lenses with CZD less than 5.5 mm had little effect. The two-zone −5-D lenses had less effect than the −5SV lens, and only the 6.5-mm CZD lens of the −5C series had a significant effect.
Conclusions.
The results demonstrate that peripheral defocus can influence both peripheral and central refractive development. The inhibitory effect on axial eye growth of the +5P lenses opens the possibility that appropriately designed concentric lenses may control the progression of human myopia.
Myopia, once considered a serious public health condition limited to East Asia,1–3 is reaching epidemic levels in North America.4 High myopia is one of the leading causes of vision loss worldwide.5–7 The management of myopia remains largely limited to optical devices such as spectacles and contact lenses prescribed to correct the refractive errors, and ophthalmic surgical procedures for the same purpose. Nonetheless, novel applications of existing contact lens designs for the control of myopia progression have recently been explored, with promising early results.8–10
While the success of optical treatments for myopia rests on the assumption that there are environmental as well as genetic influences on its development and progression, their relative contributions remain the subject of ongoing debate. Results of twin and family studies suggest a strong genetic contribution to myopia,11–13 yet the dramatic increase in myopia's prevalence and severity, reported in recent large-scale population studies, strongly suggests a significant environmental influence, in that the increase is too rapid to be explained by genetic variation alone.3,14,15
Altered emmetropization in response to specific visual manipulations is a consistent finding in a diverse range of animals, including chicken,16,17 tree shrew (Siegwart JT, et al. IOVS 1993;34:ARVO Abstract 2482),18 guinea pig,19,20 and monkey,21,22 with myopia being the product of both spatial form deprivation and imposed hyperopic defocus. The early finding that the eyes of young chicks adjust their growth to compensate for imposed optical defocus has since been generalized to other animal models. With imposed hyperopic defocus (negative lenses), eyes increase in axial length, and with myopia defocus (positive lenses), the elongation slows (Siegwart JT, et al. IOVS 1993;34:ARVO Abstract 2482).17,20,22 Evidence of a role of the peripheral retina in eye growth regulation also comes from early studies in the chick, followed by recent studies in the monkey. In chicks, form deprivation treatments restricted to one half of the visual field cause excessive eye growth only in the affected field,23 and similar field-dependent changes occur when optical defocus is limited in its extent.24 In monkeys, isolated central (foveal) laser lesioning has shown that eyes can recover from induced myopia,25 implying that the peripheral retina alone is capable of guiding emmetropization. In more recent studies, limiting either form deprivation or optical defocus to a hemifield was reported to induce asymmetric eye growth, consistent with appropriately localized responses.26,27 However, despite the emerging evidence of a role for the peripheral retina in guiding eye growth, the nature of the interactions between peripheral and central retinal regions as determinants of eye shape and their respective influences on central (on-axis) refractive errors remains inconclusive.
In human myopia, two examples of hyperopic defocus offer plausible links to the finding of defocus-induced myopia in animals: (1) Relatively prolate eye shapes, which have been linked to myopia, introduce relative hyperopic defocus at peripheral retinal locations28–30; and (2) lags in accommodation introduce hyperopic defocus centrally and are reported to be increased in some studies of myopia.31–33 In the context of myopia control, previously explored bifocal and spectacle lens treatments are based on the premise that lags of accommodation underlie myopia progression. Although clinical trials of these lenses report disappointing efficacy,34–36 it is perhaps noteworthy that their effect on peripheral (off-axis) refractive errors is also likely to be variable, between lens designs and as a function of eye position. On the other hand, two of the contact lens treatments showing promise as myopia control therapies (i.e., corneal reshaping therapy and concentric, distance center multifocal soft contact lenses) are predicted to reduce peripheral hyperopic refractive errors,8–10 as well as accommodative lags.
Animal studies of multifocal lenses have been limited so far to two studies in chicks. Both involved Fresnel lens designs, each incorporating two defocusing powers in alternative narrow rings (zones) (Diether S, et al. IOVS 2003;44:ARVO Abstract B879).37 These studies reported similar outcomes for otherwise normal eyes. When positive lens power was incorporated into the lens design, its effect dominated, whether or not the alternate rings included plano (0 D) or negative lens power. Similar domination of myopic defocus over hyperopic defocus has been reported in other types of experiments. For example, when myopic and hyperopic defocuses were set in competition in a cone-imaging system that also restricted the visual experience to two planes of defocus, the eyes showed reduced elongation, as expected with imposed myopic defocus (Diether S, et al. IOVS 2002;43:ARVO E-Abstract 188). In other studies in which appropriate single-vision lenses with powers in opposite signs were interchanged, myopic defocus was shown to be about five times more effective than hyperopic defocus in inducing axial growth changes.38 Moreover, interrupting hyperopic defocus with several brief periods of myopic defocus, spaced across the day, completely negated the ocular response to hyperopic defocus but not myopic defocus.39
In the present study, we sought to better characterize the effects of manipulating peripheral retinal defocus on ocular growth in the chick model, using a series of two-zone lens designs that varied in both defocusing power and diameter of respective zones. Our intention was to simulate the bifocal soft contact lenses trialed for myopia control in humans, to promising effect.
Methods
Animals
White-Leghorn day-old chicks, obtained from a commercial hatchery (Privett Hatchery, Portales, NM), were used in this study. They were reared in a normal diurnal lighting environment (12 hours on/12 hours off), with food and water freely available. Spectacle lenses were fitted monocularly on 17-day-old chicks and worn for 5 days. A total of 185 birds were used. All animal care and treatments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Experimental protocols were approved by the Animal Care and Use Committee of the University of California Berkeley.
Lens Treatments
All lenses had a total optical zone diameter of 10 mm and overall lens diameter of 12.2 mm. Four two-zone concentric lens designs combining plano with either −5 or +5 D were used to differentially expose the central and peripheral retina to defocus. Lens designs were as follows: (1) +5 D center (+5C), (2) +5 D periphery (+5P), (3) −5 D center (−5C), and (4) −5 D periphery (−5P), with plano in periphery for all C-designs and in the center for P-designs. Five central zone diameters (CZDs) were tested, ranging from 2.5 to 6.5 mm in 1-mm increments. Because of eye movements, only limited regions of the central and peripheral visual fields experience unifocal (single vision) effects with the two-zone lenses; the remaining, paracentral visual field receive optical input from both the central and peripheral lens zones. The dimensions of the multifocal central field and the unifocal peripheral field also depend on the interactions between the CZDs of the lenses, the entrance pupil of the eye, and the vertex distance (VD) of the lenses. As the CZD is increased, the multifocal central field expands, while the unifocal peripheral field narrows. Table 1 summarizes the estimated dimensions of these visual field zones for the various two-zone lens designs, based on a VD of 3.3 mm.40,41 The latter was measured with high frequency A-scan ultrasonography, after filling the space between the lens and the cornea with ultrasound gel; VD was defined as the distance between the peaks corresponding to the back surface of the lens and the front surface of the cornea. Three single vision (SV) lenses (plano, +5 and −5 D) were used as control treatments. These lenses also had optical zone diameters of 10 mm. The chickens were randomly assigned to 1 of 16 treatment groups, with repeated runs of experiments achieving final group sizes of between 6 and 11 chickens. Lenses were cleaned and inspected at least three times daily to ensure that their optical centers remained approximately aligned with the pupil centers of the eyes of the chickens, thereby minimizing the potential confounding effect of lens decentration.
Table 1.
Dimensions of Center and Peripheral Optical Zones (CZD, PZD) of the Five Two-zone Lens Designs Used and Corresponding Dimensions of the Unifocal Central Visual Field and Annular Multifocal Paracentral and Unifocal Peripheral Visual Fields
| CZD (mm) | PZD (mm) | Unifocal Central Field (deg) | Multifocal Paracentral Hemifield (deg) | Unifocal Peripheral Hemifield (deg) |
|---|---|---|---|---|
| 2.5 | 3.75 | — | 34 | 20 |
| 3.5 | 3.25 | — | 38 | 16 |
| 4.5 | 2.75 | 12 | 35 | 13 |
| 5.5 | 2.25 | 34 | 27 | 10 |
| 6.5 | 1.75 | 54 | 19 | 8 |
Calculations based on an anterior chamber depth (ACD) of 1.56 mm and a VD of 3.3 mm (the means for 185 chickens tested) and an estimated entrance pupil diameter of 4 mm, which is 0.2 mm in front of the iris based on an assumed total corneal power of 96 D.42
Measurements
Baseline refractive errors and axial ocular dimensions were measured immediately before the start of lens treatments by using static retinoscopy and high-frequency A-scan ultrasonography respectively, with the chickens under gaseous anesthesia (1.5% isoflurane in oxygen). Ultrasonography measurements were repeated every other day, around the same time of day (early afternoon), and retinoscopy was repeated once, after 5 days of lens treatment. Refractive error measurements were made on-axis (centrally) as well as 30° nasally and temporally. Angular estimations were made with reference to a protractor, fitted to a custom-built head holder used to deliver isoflurane.
Statistical Analyses
Both central (C) and peripheral (P) refractive errors are represented as spherical equivalent refraction (SER; averages between the refractions of two principal meridians), as there was no significant increase in astigmatism with increased eccentricity, either before or after the lens treatments. Off-axis refractive errors were normalized with respect to the on-axis values (i.e., P-C), by way of identifying differential effects on these regions induced by the lenses. This parameter is referred to as the relative peripheral refraction (RPR). Although A-scan ultrasonography allowed measurements of all ocular components, including the layers making up the posterior wall of the eye (retina, choroid, and sclera), changes in axial length, calculated as the sum of anterior chamber depth, lens thickness, and vitreous chamber depth, largely accounted for the changes in central refractive error, and thus only these data are presented. Initial analyses found no group-related differences in untreated contralateral (fellow) eyes, in refractive errors or axial lengths. Thus, only changes over the treatment period in both refractive errors and axial dimensions for treated eyes are reported as the primary outcome measures of the treatment effects.
Descriptive analyses were performed, and the normality of the distributions of the changes in refractive error and axial dimensions were verified before statistical modeling. Factorial ANOVAs were performed (Stata; Stata Corp., College Station, TX), with the primary outcome measures of interest being changes in the on-axis refractive error and axial length over the treatment period, as well as the end point RPR, with adjustment for baseline interocular difference. These outcome measures were modeled as a function of lens type and CZD, as well as the interaction between these two factors. Differences between groups were assessed with two-sample t-tests. To keep the family-wise error rate for the entire set of tests equal to 0.05, the Hochberg step-up procedure was used. Thus, P values were adjusted to take into account the number of tests undertaken. This procedure has more power to detect differences than the simpler Bonferroni adjustment. Results are illustrated in the figures as box plots, which show medians and the distribution of the data; whisker lengths represent either 1.5 times the interquartile range or the distance to the extreme, whichever is shorter.
Results
Effects of Lens Design on Central (On-Axis) Refractive Errors
On-axis refractive error changes over the treatment period were generally consistent with the sign of the lens power incorporated in all or part of the lens—that is, eyes wearing positive lenses exhibited hyperopic shifts in refractive error and eyes wearing negative lenses exhibited myopic shifts. These data are summarized by lens type and CZD in Table 2. The effect of lens type was highly significant (F6,22 = 87.83, P < 0.0001), but not the effect of CZD alone (F4,22 = 2.03, P = 0.09), although there was a significant interaction between CZD and lens type (F12,22 = 5.64, P < 0.0001).
Table 2.
Central (On-Axis) Refractive Changes over the Treatment Period
| CZD (mm) | Lens Type |
|||
|---|---|---|---|---|
| +5C | +5P | −5C | −5P | |
| 2.5 | +0.58 ± 0.88 | +4.25 ± 0.35 | +0.11 ± 1.98 | −3.71 ± 3.35 |
| (6) | (6) | (9) | (6) | |
| 3.5 | +0.68 ± 1.09 | +5.35 ± 0.93 | −0.53 ± 1.63 | −2.86 ± 2.24 |
| (10) | (10) | (8) | (7) | |
| 4.5 | +1.77 ± 1.91 | +4.48 ± 1.79 | +0.68 ± 1.14 | −2.05 ± 2.28 |
| (10) | (11) | (10) | (10) | |
| 5.5 | +2.75 ± 1.97 | +4.43 ± 1.25 | −0.25 ± 1.36 | −1.89 ± 1.37 |
| (6) | (11) | (6) | (11) | |
| 6.5 | +4.54 ± 1.11 | +1.71 ± 1.23 | −1.79 ± 1.41 | −0.83 ± 0.92 |
| (6) | (6) | (6) | (6) | |
| 10 | +3.36 ± 0.43 | −5.84 ± 0.50 | ||
| (SV) | (7) | (8) | ||
Equivalent values for the plano lens are +0.19 ± 1.14 D (n = 9). Data are the mean ± SD in diopters with the sample size in parentheses, organized by lens type and CZD.
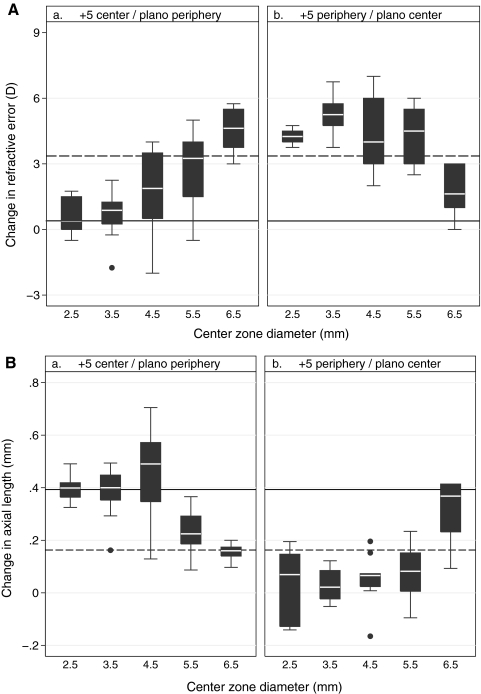
Hyperopic Changes Induced by Two-Zone and SV Positive Lenses (Figs. 1a, 1b, top).
Figure 1.
Boxplots of central refractive error changes (a and b, top), and corresponding axial length changes (a and b, bottom) over the treatment period for (a) the +5C lens designs and (b) the +5P lens designs. Central zone diameters (CZD, on X-axis) ranged from 2.5 to 6.5 mm. Dashed reference lines: mean changes induced by +5SV lens; solid lines: mean change induced by the plano lens. Whisker length denotes the shorter of 1.5 times the interquartile range and the distance to the extreme.
As expected, the +5SV lens induced a significant hyperopic shift in refractive error (+3.36 ± 0.43 D) by the end of the treatment period. Although the endpoint refractive errors (+4.73 ± 1.10 D) closely matched the imposed defocus (+5 D), the mean hyperopic change was less than the imposed defocus, this discrepancy reflecting the low hyperopia at baseline. In contrast, the plano lens-wearing eyes showed minimal change and thus the difference between the two groups was highly significant (P < 0.0001). For the two-zone lenses with positive power in their centers (+5C lenses), those with CZD of less than 4.5 mm had little effect, whereas those with CZDs above this threshold induced increasing hyperopia. Thus, while the lens with a 4.5 mm CZD induced only a small hyperopic shift (+1.77 ± 1.91 D, P = 0.09), the lenses with CZDs of 5.5 mm or larger induced much larger shifts (P = 0.02), with the change induced by the 6.5-mm CZD being slightly greater than that with the +5SV lenses (+4.54 ± 1.11 D; P = 0.06, Fig. 1a, top). The latter trend was also observed with the +5P lenses (Fig. 1b, top). Specifically, all the latter lens designs induced larger hyperopic shifts than the +5SV lens (P < 0.05 for CZD of 2.5, 3.5, and 5.5, P = 0.06 for CZD of 4.5), except for the 6.5-mm CZD, for which the hyperopic shift in refractive error dropped to less than half that with the 5.5-mm CZD (P < 0.01 for both the 5.5-mm CZD +5P and the +5SV lenses).
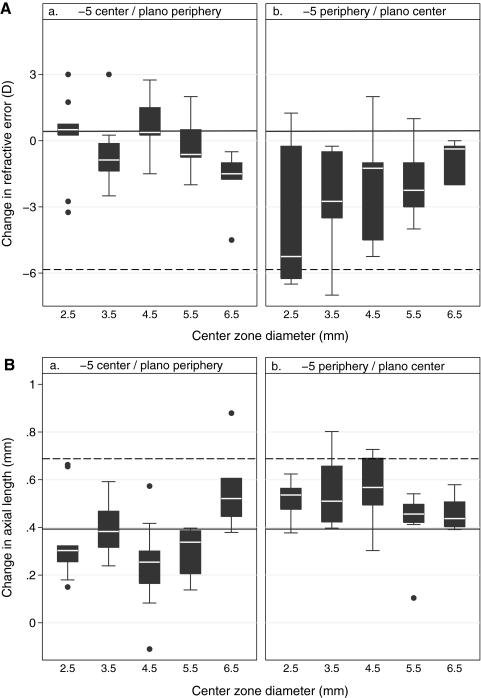
Myopic Changes Induced by Two-Zone and SV Negative Lenses (Figs. 2a, 2b, top).
Figure 2.
Boxplots of central refractive error changes (a and b, top) and axial length changes (a and b, bottom) over the treatment period for (a) the −5C two-zone lens designs and (b) the −5P two-zone lens designs. CZDs ranged from 2.5 to 6.5 mm. Dashed reference line: mean change induced by the −5SV lens; solid lines: mean change induced by the plano lens.
The −5SV lens induced nearly complete compensation over the treatment period, with the observed myopic shift in refractive error being comparable to the imposed defocus and significantly different from the change in the control (plano lens–wearing) eyes (−5.84 ± 0.50 D, P < 0.0001). The myopic shifts in refractive errors induced by the two-zone negative lenses were all much smaller than that recorded with the SV lens, contrasting with the exaggerated responses with some of the two-zone positive lenses. Nonetheless, increasing the CZD for the −5C designs tended to increase the myopic shift in refractive error, with the change reaching statistical significance for the 6.5 mm CZD lens (P = 0.01 for the plano lens, Fig. 2a, top). For the −5P designs, significant myopic changes were seen with all CZDs compared with the plano lens–wearing control (P < 0.05 for all, Fig. 2b, top).
Effect of Lens Design on Ocular Axial Growth
Choroid thickening was significantly associated with the development of hyperopia (R2 = 0.17, P < 0.0001); however, changes in choroidal thickness did not fully account for changes in either the refractive error or the axial length.
The axial length changes in lens-wearing eyes over the treatment period were consistent with observed changes in on-axis refractive errors (R2 = 0.61, P < 0.0001), with the largest changes in negative lens-wearing eyes showing the largest myopic shifts in refractive error and the smallest changes in positive lens-wearing eyes showing the largest hyperopic shifts. These data are summarized by lens type and CZD in Table 3. As with the changes in refractive error, axial length changes were influenced significantly by lens type (F6,22 = 49.60, P < 0.0001) and the interaction between lens type and CZD (F12,22 = 6.47, P < 0.0001). The effect of CZD alone was of borderline significance (F4,22 = 3.22, P = 0.01).
Table 3.
Changes in Axial Length over the Treatment Period
| CZD (mm) | Lens Type |
|||
|---|---|---|---|---|
| +5C | +5P | −5C | −5P | |
| 2.5 | 0.40 ± 0.056 | 0.035 ± 0.14 | 0.35 ± 0.19 | 0.52 ± 0.086 |
| 3.5 | 0.38 ± 0.095 | 0.027 ± 0.062 | 0.40 ± 0.12 | 0.55 ± 0.14 |
| 4.5 | 0.45 ± 0.19 | 0.053 ± 0.091 | 0.24 ± 0.18 | 0.56 ± 0.14 |
| 5.5 | 0.23 ± 0.096 | 0.071 ± 0.095 | 0.30 ± 0.11 | 0.44 ± 0.12 |
| 6.5 | 0.16 ± 0.036 | 0.32 ± 0.13 | 0.56 ± 0.18 | 0.46 ± 0.071 |
| 10 (SV) | 0.16 ± 0.074 | 0.69 ± 0.061 | ||
Equivalent values for the plano lens are 0.39 ± 0.10 mm. Data are the mean ± SD in millimeters, organized by lens type and CZD.
Axial Length Changes Induced by Two-Zone Positive Lenses (Figs. 1a, 1b, bottom).
Compared with eyes wearing plano lenses, those wearing +5SV lenses showed significantly less axial elongation over the treatment period (0.16 ± 0.074 mm, P = 0.0001), consistent with their increased hyperopia. With the +5C lenses, axial length changes were similar to those of plano lens–wearing eyes for CZDs of less than 5.5 mm, but significantly smaller for both 5.5- and 6.5-mm CZDs (P = 0.009 and P = 0.0001, respectively), these trends being consistent with described CZD-dependent changes in refractive error. Furthermore, the mean change in axial length with the 6.5-mm CZD lens design was similar to and not significantly different from that with the +5 SV lens (P = 0.41). With the +5P lenses, changes in axial length were significantly smaller than that with the plano lens (P < 0.0001 for 2.5 to 5.5 mm CZD), and the change with the 4.5-mm CZD design was also significantly smaller than that with the +5SV lens (P = 0.01, Fig. 1). These results are consistent with the large hyperopic shifts in all but the largest (6.5 mm) CZDs for the +5P design.
Axial Length Changes Induced by Two-Zone Negative Lenses (Figs. 2a, 2b, bottom).
As expected, the myopic shift in refractive error observed with the −5SV lens was linked to a significant increase in axial elongation compared with that seen with the plano lens (0.69 ± 0.061 mm, P < 0.0001). Among the −5C lenses, only the lens with a CZD of 6.5 mm induced greater elongation than the plano lens, and the change was of borderline significance (P = 0.04). In contrast, with the −5P series of lenses, there was a consistent trend of increased axial elongation with all CZDs, with the difference from the plano lens effect reaching statistical significance for the 2.5-, 3.5-, and 4.5-mm CZDs (P < 0.05 for all).
Effect of Lens Design on RPR Errors
Because there was no evidence of nasal–temporal asymmetry in the peripheral refractive errors in either the treatment or control groups, the relative peripheral refractive errors derived for the nasal and temporal fields were averaged for use in the analyses of the influences of lens design and CZD. These data are summarized in Table 4.
Table 4.
RPR Error
| CZD (mm) | Lens Type |
|||
|---|---|---|---|---|
| +5C | +5P | −5C | −5P | |
| 2.5 | −0.40 ± 0.83 | −1.15 ± 0.85 | −0.39 ± 0.90 | +0.79 ± 0.98 |
| 3.5 | −0.45 ± 1.08 | −0.96 ± 0.69 | −0.17 ± 0.25 | 0.00 ± 2.03 |
| 4.5 | −1.08 ± 1.04 | +0.32 ± 1.77 | +0.38 ± 1.36 | −0.80 ± 1.64 |
| 5.5 | −0.85 ± 1.19 | −0.58 ± 1.22 | −0.042 ± 0.30 | +0.32 ± 1.63 |
| 6.5 | −1.98 ± 1.50 | +0.29 ± 1.15 | −0.008 ± 1.46 | +0.083 ± 0.63 |
| 10 (SV) | +0.14 ± 1.12 | +0.83 ± 1.24 | ||
Equivalent values for the plano lens are −0.083 ± 0.63 D. Data are the mean ± SD in diopters, organized by lens type and CZD.
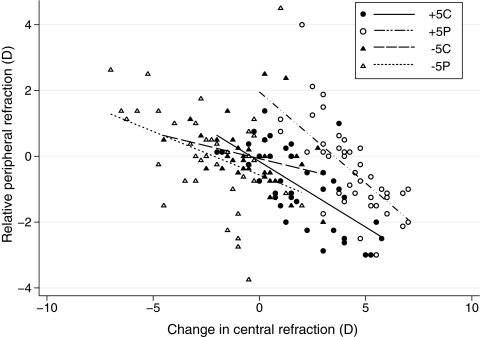
The RPR data were subjected to a factorial ANOVA, with on-axis refractive error change, lens type, and CZD as factors. The main effect of on-axis refractive error change was significant (F1,23 = 50.78, P < 0.0001). The origin of this effect is revealed in Figure 3 in which RPRs are plotted against changes in on-axis refractive error for all four two-zone lens designs; in all cases, increasing on-axis hyperopic changes are associated with increasing relative peripheral myopia and vice versa. Lens design was also found to significantly influence RPR (F6,23 = 5.71, P < 0.0001), with the effect being greater for the positive lens designs, as reflected in the steeper slopes for the +5C and +5P data compared with the −5C and −5P data, after adjustment for all other variables in the model (Fig. 3). Note that despite a moderate level of overlap in the data representing each group, the RPR distributions show design-dependent differences, resulting in similar on-axis refractive error changes being coupled to different RPRs, and implying differences in eye shape. Although the CZD itself had no significant effect on RPR (F4,23 = 0.82, P = 0.51), its interaction with lens design was significant, albeit borderline (F16,23 = 1.76, P = 0.041).
Figure 3.
RPRs plotted as a function of changes in on-axis refractive error for each of the four two-zone lens types.
Discussion
We used two-zone lenses to differentially titrate the optical defocus experience of central and peripheral retinal regions. Both our center power (C) and peripheral power (P) designs induced changes in the central (on-axis) refractive errors and axial length of the growing eyes of young chicks. The peripheral power design tended to have greater effects than the central power design, for both positive and negative lenses; and, for the former design, the effect sometimes exceeded that observed with an SV lens of the same power. While we also observed changes in peripheral refractive errors, they cannot be fully explained in terms of local retinal responses to imposed defocus. In this discussion, we consider possible explanations for our results and compare them with a closely related study that involved lenses with central apertures42 instead of plano zones.
Any explanation of our results must take into account the eye movements of our animal subjects. For lenses in which defocusing power was restricted to the lens periphery, even the central retinal region would have experienced defocus, at least intermittently. The defocus experience of midperipheral retinal regions would also have varied over time. Thus, one would expect the peripheral retina and adjacent sclera to be most affected, and the central (axial) regions least affected, with the accumulated dose of defocus experienced by the central region decreasing as the size of the central plano zone increased. The opposite would be true of lenses with power limited to the central zone. However, the effect of CZD did not show a clear dose effect for any of the lens series used in the present study. Except for the largest CZD of 6.5 mm, which reduced the width of the unifocal peripheral field of view to 8°, the P series of lenses induced significant changes in on-axis refractive errors that reflected changes in axial elongation, with these effects being more robust with the +5 than with the −5 series of lenses.
Our finding that the changes induced by the −5C lenses were generally weak, irrespective of the size of the CZD, is consistent with the results of previous studies exploring the temporal integration of defocus-induced signals in the chick, assuming that it is a major determinant of the on-axis response for these lenses. For example, interruption of lens wear with 3 hours of normal vision is sufficient to prevent the development of myopia in response to −10 D lenses in chicks.43 The equivalent experiment with +10-D lenses showed the response to imposed myopic defocus to be more robust. In another study, it was noted that the choroidal thinning induced by short-term exposure to negative lenses was less enduring than the thickening induced by positive lenses.44 With the C series of lenses in the present study, we also found the responses to imposed myopia (+5 D) to be more robust than the responses to imposed hyperopia (−5 D). Lateral interactions between adjacent retinal regions are also likely to influence the response of local retinal regions, either dampening or enhancing the signal generated by the local defocus experience.
The response pattern recorded with the +5P series of lenses is striking in that the induced on-axis refractive error changes are greater than that induced by the +5-D SV lens, with one exception, even though the central retina would have had a more consistent defocus experience with the latter lens. This model cannot fully explain the outcome. A potential alternative explanation relates to interactions between the higher order aberrations (HOAs) introduced by the two-zone lenses and those of the eye, which may alter the target plane of best focus for emmetropization. In studies involving concentric bifocal contact lenses in humans, it has been shown that interactions between lens and ocular aberrations, especially spherical aberration (SA), may alter the position of plane of best focus with respect to the retina.45 SA would also have been most affected by our two-zone lens design. Although we did not measure the optical aberrations of the chicks in the present study, a transition from negative SA at week 2 to positive SA at week 5 was observed in a previous study of the same strain of chicks.46 It is also known from in vivo studies in humans45 and in vitro studies in chicks47 that spherical aberration becomes increasingly negative with accommodation. However, while this explanation seems plausible for the results with the +5P lenses, a similarly exaggerated response was recorded with the largest CZD +5C lens, which would impose the opposite type of SA, implying that other factors are at play.
The inherent complexity of ocular growth regulation is further evidenced by the refractive error profiles of lens-treated eyes. In the chick eye, which has a rigid bi-layered sclera, it is generally assumed that eye shape changes reflect the strength of local retina-derived growth modulatory signals. However, this model does not fully account for the refractive patterns observed with our two-zone lenses. For example, while relative peripheral myopia combined with on-axis hyperopia was recorded with the +5P lens series, the lenses with the smallest CZDs resulted in the largest differences and conversely, for the +5C lenses, those with the largest CZDs induced the largest differences (Table 4). These influences of lens design on refractive error profiles are illustrated in Figure 3. For the two +5-D series of lenses, the regression line fitted to the P series data was displaced to the right of the regression line fitted to the C series data, and was also steeper. Differences in the ability of peripheral retinal regions to decode defocus and/or differences in signal gain for peripheral versus central regions may contribute to, but alone cannot explain, these response patterns. The significant negative correlation between changes in central refractive errors and relative peripheral refractive errors in our data has an intriguing parallel with human refractive error data, where the coupling of relative hyperopic peripheral refractive error with on-axis myopia has led to speculation of a causal link between the two. Follow-up studies are needed to establish whether the response patterns reported here translate to mammals, before any conclusion can be drawn concerning the stimulus for myopic growth in humans.
In the present study, changes in relative peripheral refractive errors were used as a surrogate for eye shape. However, we cannot eliminate the potential confounding influences related to the unique properties of the peripheral optics of the eye, which have not been characterized in the chick in any study to date. Off-axis measurements of axial length with an ocular biometer (IOLMaster; Carl Zeiss Meditec, Inc., Dublin, CA), as recently has been popularized human studies, would be an appropriate follow-up study. Nonetheless, our study demonstrates that concentric two-zone spectacle lenses can be used to modify both on- and off-axis refractive development. Note that although there has been no comparable study in primates or humans, the possible causal relationship between myopia progression and relative peripheral hyperopia, evident when myopes are corrected with standard single-vision spectacle lenses, has led to the recent development of a radial refractive gradient spectacle lens, which is reported to reduce such peripheral errors.48
Our observation of a significant influence of peripheral defocus on central refractive development contrasts with the previously reported lack of effect on the latter of spectacle defocusing lenses with central apertures.42 In this study, the chicks wore lenses with central apertures of various diameters, limiting defocus to peripheral retinal regions. Several factors may have contributed to the discrepancy in the results of our two studies. First, when lenses with central apertures were used, as in the earlier study, the chicks may have artificially restricted their eye movements, as in a similarly designed study involving young monkeys, which were observed to limit their eye movements, to allow viewing through the central apertures of attached lenses.41 Modified eye movements are likely with such lenses if not regularly cleaned, as the presence of a central aperture will allow dust to accumulate on the inside of the lenses, turning them into light diffusers. This potential problem was avoided with our two-zone lens design. Second, the previous study used younger chicks than in the present study although one might expect an exaggerated response in the younger animals (i.e., larger changes in on-axis refractive errors), rather than the converse, based on previously reported age-dependent differences in the rates of response to imposed defocus.49 On the other hand, due to the smaller eyes of younger chicks combined with possible shorter lens VDs (range given as 2 to 3 mm), the unifocal peripheral visual field introduced with the central aperture lenses are likely to have been much smaller than those imposed with the two-zone P lenses with corresponding CZDs in the present study, offering a partial explanation for this discrepancy in study outcomes. Finally, it is possible that estimates of the visual angular subtense of the peripheral optical zone of the lenses were inaccurate in the earlier study. Such estimates are directly dependent on the lens VD and axial parameters of the experimental subjects and are prone to error, due to the small distances involved. Our estimates are based on highly accurate measurements made with high-frequency ultrasonography in both cases, whereas approximated parameters from model eyes were used in the earlier study.
In summary, in chicks wearing two-zone concentric spectacle lenses, responses varied according to the sign of the defocusing power and whether it was limited to either the central or peripheral zone. Lens designs with optical power limited to the periphery elicited greater responses than the opposite design, and the responses to lenses incorporating positive powers were more robust than the responses to lenses incorporating negative power. The observed inhibitory effect on axial eye growth of the +5P designs has particular salience for human myopia and is consistent with isolated reports of effective control of myopia progression with some concentric multifocal contact lens designs.10,50 Contact lenses, being in contact with the cornea, are likely to afford better control over the imposed optical conditions and as a consequence, may offer better control of myopia progression than novel spectacle lens designs that introduce centration and eye movement–related confounders. Nonetheless, further animal-based studies of multifocal contact lenses may provide additional insights into how to best optimize inhibitory effects on eye growth.
Acknowledgments
The authors thank Maureen Lahiff for technical advice on the statistical analyses.
Footnotes
Supported by National Institutes of Health (NIH) Grants K12 EY 017296 (YL) and NIH R01 EY12392 (CW).
Disclosure: Y. Liu, None; C. Wildsoet, None.
References
- 1. Lam CS, Goldschmidt E, Edwards MH. Prevalence of myopia in local and international schools in Hong Kong. Optom Vis Sci. 2004;81:317–322 [DOI] [PubMed] [Google Scholar]
- 2. Lin LL, Shih YF, Hsiao CK, Chen CJ, Lee LA, Hung PT. Epidemiologic study of the prevalence and severity of myopia among schoolchildren in Taiwan in 2000. J Formos Med Assoc. 2001;100:684–691 [PubMed] [Google Scholar]
- 3. Saw SM, Hong RZ, Zhang MZ, et al. Near-work activity and myopia in rural and urban schoolchildren in China. J Pediatr Ophthalmol Strabismus. 2001;38:149–155 [DOI] [PubMed] [Google Scholar]
- 4. Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–1639 [DOI] [PubMed] [Google Scholar]
- 5. Saw SM, Katz J, Schein OD, Chew SJ, Chan TK. Epidemiology of myopia. Epidemiol Rev. 1996;18:175–187 [DOI] [PubMed] [Google Scholar]
- 6. McCarty CA, Taylor HR. Myopia and vision 2020. Am J Ophthalmol. 2000;129:525–527 [DOI] [PubMed] [Google Scholar]
- 7. Pierro L, Camesasca FI, Mischi M, Brancato R. Peripheral retinal changes and axial myopia. Retina. 1992;12:12–17 [DOI] [PubMed] [Google Scholar]
- 8. Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30:71–80 [DOI] [PubMed] [Google Scholar]
- 9. Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009;93:1181–1185 [DOI] [PubMed] [Google Scholar]
- 10. Aller TA, Wildsoet C. Bifocal soft contact lenses as a possible myopia control treatment: a case report involving identical twins. Clin Exp Optom. 2008;91:394–399 [DOI] [PubMed] [Google Scholar]
- 11. Sorsby A, Leary GA, Fraser GR. Family studies on ocular refraction and its components. J Med Genet. 1966;3:269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyhne N, Sjolie AK, Kyvik KO, Green A. The importance of genes and environment for ocular refraction and its determiners: a population based study among 20–45 year old twins. Br J Ophthalmol. 2001;85:1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stambolian D, Ibay G, Reider L, et al. Genomewide linkage scan for myopia susceptibility loci among Ashkenazi Jewish families shows evidence of linkage on chromosome 22q12. Am J Hum Genet. 2004;75:448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sapkota YD, Adhikari BN, Pokharel GP, Poudyal BK, Ellwein LB. The prevalence of visual impairment in school children of upper-middle socioeconomic status in Kathmandu. Ophthalmic Epidemiol. 2008;15:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alward WL, Bender TR, Demske JA, Hall DB. High prevalence of myopia among young adult Yupik Eskimos. Can J Ophthalmol. 1985;20:241–245 [PubMed] [Google Scholar]
- 16. Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163 [DOI] [PubMed] [Google Scholar]
- 17. Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657 [DOI] [PubMed] [Google Scholar]
- 18. Marsh-Tootle WL, Norton TT. Refractive and structural measures of lid-suture myopia in tree shrew. Invest Ophthalmol Vis Sci. 1989;30:2245–2257 [PubMed] [Google Scholar]
- 19. Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Res. 2006;46:267–283 [DOI] [PubMed] [Google Scholar]
- 20. Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vis Res. 2009;49:219–227 [DOI] [PubMed] [Google Scholar]
- 21. Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68 [DOI] [PubMed] [Google Scholar]
- 22. Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–765 [DOI] [PubMed] [Google Scholar]
- 23. Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77 [DOI] [PubMed] [Google Scholar]
- 24. Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37:659–668 [DOI] [PubMed] [Google Scholar]
- 25. Smith EL, 3rd, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith EL, 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51:3864–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith EL, 3rd, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50:5057–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoogerheide J, Rempt F, Hoogenboom WP. Acquired myopia in young pilots. Ophthalmologica. 1971;163:209–215 [DOI] [PubMed] [Google Scholar]
- 29. Mutti DO, Sholtz RI, Friedman NE, Zadnik K. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41:1022–1030 [PubMed] [Google Scholar]
- 30. Atchison DA, Pritchard N, Schmid KL. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vision Res. 2006;46:1450–1458 [DOI] [PubMed] [Google Scholar]
- 31. Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34:690–694 [PubMed] [Google Scholar]
- 32. Abbott ML, Schmid KL, Strang NC. Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic Physiol Opt. 1998;18:13–20 [PubMed] [Google Scholar]
- 33. McBrien NA, Millodot M. The effect of refractive error on the accommodative response gradient. Ophthalmic Physiol Opt. 1986;6:145–149 [PubMed] [Google Scholar]
- 34. Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77:395–401 [DOI] [PubMed] [Google Scholar]
- 35. Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500 [DOI] [PubMed] [Google Scholar]
- 36. Parssinen O, Hemminki E, Klemetti A. Effect of spectacle use and accommodation on myopic progression: final results of a three-year randomised clinical trial among schoolchildren. Br J Ophthalmol. 1989;73:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tse DY, Lam CS, Guggenheim JA, et al. Simultaneous defocus integration during refractive development. Invest Ophthalmol Vis Sci. 2007;48:5352–5359 [DOI] [PubMed] [Google Scholar]
- 38. Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002;42:2651–2668 [DOI] [PubMed] [Google Scholar]
- 39. Zhu X, Winawer JA, Wallman J. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44:2818–2827 [DOI] [PubMed] [Google Scholar]
- 40. Carkeet A. Field restriction and vignetting in contact lenses with opaque peripheries. Clin Exp Optom. 1998;81:151–158 [DOI] [PubMed] [Google Scholar]
- 41. Smith EL, 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schippert R, Schaeffel F. Peripheral defocus does not necessarily affect central refractive development. Vision Res. 2006;46:3935–3940 [DOI] [PubMed] [Google Scholar]
- 43. Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036 [DOI] [PubMed] [Google Scholar]
- 44. Zhu X, Park TW, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46:2238–2241 [DOI] [PubMed] [Google Scholar]
- 45. Tarrant J, Severson H, Wildsoet CF. Accommodation in emmetropic and myopic young adults wearing bifocal soft contact lenses. Ophthalmic Physiol Opt. 2008;28:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tian Y, Wildsoet CF. Diurnal fluctuations and developmental changes in ocular dimensions and optical aberrations in young chicks. Invest Ophthalmol Vis Sci. 2006;47:4168–4178 [DOI] [PubMed] [Google Scholar]
- 47. Choh V, Sivak JG. Lenticular accommodation in relation to ametropia: the chick model. J Vis. 2005;5:165–176 [DOI] [PubMed] [Google Scholar]
- 48. Tabernero J, Vazquez D, Seidemann A, Uttenweiler D, Schaeffel F. Effects of myopic spectacle correction and radial refractive gradient spectacles on peripheral refraction. Vision Res. 2009;49:2176–2186 [DOI] [PubMed] [Google Scholar]
- 49. Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12:448–456 [PubMed] [Google Scholar]
- 50. Aller TA, Wildsoet C. Results of a one-year prospective clinical trial (CONTROL) of the use of bifocal soft contact lenses to control myopia progression. Ophthalmic Physiol Opt. 2006;26(suppl 1):8–9 [Google Scholar]