This is the first demonstration that mesopic vision is reduced in gene-positive persons with no clinical signs of age-related maculopathy (ARM). These preliminary results have implications for the subclinical detection and monitoring of ARM because risk is modifiable.
Abstract
Purpose.
To determine whether participants with normal visual acuity, no ophthalmoscopically signs of age-related maculopathy (ARM) in both eyes, and who are carriers of the CFH, LOC387715, and HRTA1 high-risk genotypes (gene-positive) have impaired rod- and cone-mediated mesopic visual function compared with persons who do not carry the risk genotypes (gene-negative).
Methods.
Fifty-three Caucasian study participants (mean 55.8 ± 6.1) were genotyped for CFH, LOC387715/ARMS2, and HRTA1 polymorphisms. Single-nucleotide polymorphisms were genotyped in the CFH (rs380390), LOC387715/ARMS2 (rs10490924), and HTRA1 (rs11200638) genes using optimized gene-expression assays. The critical fusion frequency (CFF) mediated by cones alone (long-, middle-, and short-wavelength sensitive cones, LMS) and by the combined activities of cones and rods (LMSR) were determined. The stimuli were generated using a four-primary photostimulator that provides independent control of the photoreceptor excitation under mesopic light levels. Visual function was further assessed using standard clinical tests, flicker perimetry, and microperimetry.
Results.
The mesopic CFF mediated by rods and cones (LMSR) was significantly reduced in gene-positive compared to gene-negative participants after correction for age (P = 0.03). Cone-mediated CFF (LMS) was not significantly different between gene-positive and -negative participants. There were no significant associations between flicker perimetry and microperimetry and genotype.
Conclusions.
This is the first study to relate ARM risk genotypes with mesopic visual function in clinically normal persons. These preliminary results could become of clinical importance because mesopic vision may be used as a biomarker to document subclinical retinal changes in persons with risk genotypes and to determine whether those persons progress into manifest disease.
The pathomechanisms of age-related maculopathy (ARM) involve complex relationships between genetics, oxidative stress, and cardiovascular environmental risk factors.1 The genetic contribution to age-related maculopathy susceptibility (risk) is well known, and numerous risk genotypes have been identified to associate with ARM and its progression.2–5 Potential ARM risk genes involve single-nucleotide polymorphisms (SNPs),6 and although gene carrier status can be used to determine risk, it does not determine whether a person will progress to disease. However, in clinical practice genetic status is not currently used as a marker to predict the need to undertake functional vision assessments in ophthalmoscopically normal eyes, and visual function is quantitatively monitored only after patients show clinical signs of eye disease. Indeed, it is not known how subclinical changes in visual function can be detected in persons with healthy eyes who are genetically at risk of ARM, nor how those with high-risk genes differ in visual function from age-similar healthy persons who do not carry the gene.
In this study, we detect coding variants in the complement factor H region at 1q32 (CFH rs380390) and at 10q26 (LOC387715/ARMS2 rs10490924 and HTRA1 rs11200638) and relate genetic results to mesopic visual function. Mesopic vision occurs under dim light levels where the rod and cone photoreceptors of the human retina simultaneously convey visual information, spanning approximately three to four log units in natural viewing environments. It is known that mesopic vision testing can be sensitive to the early signs of retinal disease.7 Our experimental approach uses a four-primary photostimulator8 to independently control the excitation of the rod and cone photoreceptor classes and determines flicker thresholds under mesopic lighting conditions. The advantage of this approach for the detection of subclinical signs of ARM is that flicker stimulation increases metabolic demand to the neuroretina by inducing higher blood flow,9 and mesopic light conditions increase the activity of all photoreceptor types (rods and cones),10 thereby increasing oxygen demand. Taken together, these conditions could promote hypoxia/ischemia11 in a healthy retina that is susceptible to disease. Losses in flicker sensitivity are highly predictive of early ARM12 and the development of neovascular AMD,13 which is thought to be promoted by ischemia and VEGF release.14 We determine whether persons with ophthalmoscopically normal eyes and normal visual acuity who carry the risk genotypes have poorer mesopic visual function compared with persons who do not carry the ARM risk genotypes.
Methods
Participants
Written informed consents were obtained from all participants, and the study was conducted in accordance with the requirements of the Queensland University of Technology Human Research Ethics Committee and the tenets of the Declaration of Helsinki. Fifty-three healthy volunteers (28 female and 25 male) were enrolled through advertisement in university and community newspapers. Participants (mean age, 55.8 ± 6.1 years; range, 46–68 years) had no history of ocular or systemic disease and underwent a complete eye examination by an ophthalmologist (BF) according to the inclusion and exclusion criteria (Table 1). Participants had visual acuity ≥ 20/20, normal color and contrast vision, and no retinal anatomic abnormalities as measured with optical coherence tomography (OCT) and ophthalmoscopy. Crystalline lens and fundus grading was performed according to the Age-Related Eye Disease Study (AREDS) templates, and participants were excluded with any posterior subcapsular cataract, cortical, or nuclear opacities higher than grade 1 or signs of early ARM (level 1).15,16 Perimetry and mesopic vision testing was performed with the right eyes, and practice trials were conducted to familiarize participants with the protocols.
Table 1.
Eligibility and Ineligibility Criteria
| Eligibility Criteria | Ineligibility Criteria |
|---|---|
| Age between 45 and 68 years | Aphakia or pseudophakia |
| Visual acuity ≥20/20 in both eyes | Cataract > grade 1 (AREDS) |
| Glaucoma and/or IOP > 22 mmHg | |
| Normal color vision in both eyes (Lanthony) | Diabetes and diabetic retinopathy |
| No retinal vein or artery occlusion | |
| Normal contrast vision (Pelli Robson) | Uncontrolled hypertension |
| Recent myocardial infarct or stroke | |
| No ARM in both eyes (according to AREDS) | Uncontrolled hypercholesterinaemia |
| Normal OCT in both eyes | Major illness with chronic medication |
| Normal peripheral fundus |
Genotyping
Saliva samples were collected (OraGene DNA Self-Collection kit; DNA Genotek Inc, Kanata, Ontario, Canada), and DNA was manually extracted from the participants' sample using this protocol and genotyped for selected polymorphisms using optimized gene-expression assays (TaqMan Gene Expression Assay; Applied Biosystems, Inc., Foster City, CA) on a real-time thermal cycler (ABI 7300; Applied Biosystems, Inc.).17 DNA was screened for the SNPs in CFH (rs 380,390) of the complement factor H gene on 1q32, LOC387715 (A69S, rs10490924), also known as age-related maculopathy susceptibility 2 gene (ARMS2), and in the high-temperature requirement factor A1, HTRA1 (rs11200638) gene in the region of 10q26. Gene loci 1q32 and 10q26 have been repeatedly linked to age-related maculopathy.2,18 There is controversy as to which SNPs on 10q26 confer risk for ARM, and strong linkage disequilibrium has been demonstrated across this region18,19; we therefore genotyped both SNPs in the ARMS2 and HTRA1 genes.
In total, 53 participants were genotyped and examined ophthalmologically. Ten participants were excluded from mesopic vision and perimetry testing after ophthalmological examination revealed a cataract >AREDS grade 1 (n = 1), early ARM (n = 5), congenital disc disorder (n = 1), protanopia (n = 1), macular gliosis (n = 1), and intraocular lens extraction after cataract (n = 1). Of the remaining 43 participants, 28 carried one or more of the risk genotypes (gene-positive), and 15 had no gene variants (gene-negative). Genotyping confirmed the strong linkage disequilibrium across the 10q26 by showing complete disequilibrium between the LOC387715 and HTRA1. The investigator (BF) was masked to the genetic results as genotyping was performed after the vision testing.
Clinical Testing
We assessed flicker perimetry (Medmont Perimeter M700; Medmont International, Vermont, Australia)20,21 using the standard M700 flicker protocol. The flicker stimuli (800 ms duration) are equivalent to a Goldman size III (0.43°). Landers et al.22 describe formulas to convert between M700 and Humphrey visual field indices.
Standard automated perimetry was evaluated (MP-1 Microperimeter; Nidek Co., Ltd, Gamagori, Aichi, Japan) using the a program for equivalence to the standard Humphrey field test (MP-1 Humphrey 10-2 program). The microperimeter allows visualization of the retinal location of the threshold measurement,23 and an autotracking system corrects the stimulus projection every 40 ms to compensate for eye movements. Standard perimetric indices (mean sensitivity and pattern defect) were used for statistical analysis of all visual field data.
Optical coherence tomography (OCT) (Stratus III; Carl Zeiss Pty Ltd, OberKochen, Germany) imaging was performed in both eyes using six diagonal fast, low-density 6 mm scans (128 A-scans/diagonal) and six diagonal slow, high-density 6 mm scans (512 A-scans/diagonal) at 30° angles. Visual acuity (Bailey-Lovie charts), contrast vision (Pelli–Robson), and color vision (Lanthony desaturated) were assessed in both eyes in accordance with standard procedures.
Mesopic Vision Testing with the Four-Primary Photostimulator
The photostimulator is a two-channel, Maxwellian view optical system with four narrow bandwidth primary lights for each channel derived from light emitting diode–interference filter combinations with dominant wavelengths of 459 nm (blue), 516 nm (greenish-yellow), 561 nm (green), and 658 nm (red). The design and control of the photostimulator is described in detail elsewhere.8,24–26 Independent control of the excitation of the four photoreceptor types in the human eye8 is achieved using the method of silent substitution.26
To evaluate long-, middle-, and short-wavelength-sensitive cone (L-, M-, S-cone) and rod (R) activity under mesopic light levels, we investigated two types of luminance stimuli, one mediated by cones alone (LMS) and the other mediated by the combined activities of rod and cones (LMSR). The LMS stimulus modulates cone luminance signals to the postreceptoral magnocellular (MC) pathway, and rod excitations remain steady. Because S-cones do not contribute to luminance, modulating cone luminance (L+M) requires a proportional change in S-cone excitation to prevent a change in chromaticity.27,28 The LMSR stimuli measured combined LMS-cone and rod inputs to the postreceptoral MC pathway.
The stimulus was a 2° circular field set within a 13° surround and positioned at 7.5° temporal eccentricity, the approximate locus of equal rod and cone density.29 Any difference in sensitivity to the two stimulus types reflects local rod and cone interactions (within the stimulus area) in the inferred MC pathway.27 This configuration is important for studying persons at risk of ARM because we can evaluate both rod and cone function in the same retinal area; the first photoreceptor losses occur parafoveally in ARM.30 The time-averaged retinal illuminance was 33 photopic Troland with a chromaticity metameric to the equal-energy-spectrum [L/(L+M) = 0.667, S/(L+M) = 1.0]. Participants were dark adapted for 10 minutes because pilot studies indicate this was sufficient to study mesopic vision under our experimental conditions. Testing was performed with natural pupils through a 2 mm artificial pupil; refractive correction was placed on the instrument side of the artificial pupil if required. The critical fusion frequency (CFF) was measured for the two postreceptoral stimulus types modulated at 15% Michelson contrast (LMS and LMSR) using a paradigm developed by the coauthors.27 The stimulus was presented in a 1 second raised cosine envelope and alternated with a 1 second steady field. On each trial, the initial frequency was randomly set between 5 and 30 Hz, and the observer altered the stimulus frequency using a method of adjustment to determine the CFF, that is, the transitional frequency between seeing flicker and no-flicker (steady). Six repeats were performed for each stimulus type.
Statistical Analysis
We first examined the distributions of the variables that did not show major deviations from normality. Therefore, we used parametric tests that allowed for controlling age, an important factor for studying age-related maculopathy. We performed Pearson's correlation to determine the relationship between mesopic CFF, mean sensitivity (MS) and pattern defect (PD) (microperimetry and flicker perimetry), retinal thickness (OCT), and age. ANOVA was performed to evaluate the difference between the CFF measurements, flicker perimetry, microperimetry, and the odds ratio (OR) of the three SNPs, with age controlled. The following odds ratios were assigned to each genotype according to previously published values2,3: CFH rs380390,2 GG OR = 7.4, CC+CG OR = 1; LOC387715/ARMS2 rs10490920,3 TT OR = 6.09, GT OR = 1.35, GG OR = 1; HTRA1 rs11200638,3 AA OR = 6.56, AG OR = 1.85, GG OR = 1.
Results
The genotype frequencies of all tested SNPs were in Hardy-Weinberg (HWE) equilibrium as assessed using the HWE program.31 Data screening was performed, and seven outliers were identified due to repeated fixation losses on the four-primary photostimulator or because only the 5 Hz adjustment step was used to set CFF. The outliers were removed from subsequent statistical analysis. Of the remaining 36 participants, 11 were classified as normal (low-risk homozygous, gene-negative) and 25 gene-positive (hetero- or homozygous carriers of either one or more than one gene variant tested). Table 2 shows the individual gene variant distributions (e.g., all persons who were heterozygous for the LOC gene variant and not accounting for other coexisting gene variations in these persons). The mean values (±SD) of the CFF for LMSR and LMS for each genotype are given in Tables 3 and 4. Note that LOC and HTRA1 CFF values are the same because of the linkage disequilibrium. Data demonstrate that LMSR CFF decreases in gene-positive compared to gene-negative participants, with the exception of the high-risk homozygote CFH.
Table 2.
The Distribution of Genotypes
| Gene Variant | Low-Risk Homozygotes (n) | Heterozygotes (n) | High-Risk Homozygotes (n) |
|---|---|---|---|
| LOC rs10490924 | 15 (GG) | 19 (GT) | 2 (TT) |
| HRTA1 rs1120038 | 15 (GG) | 19 (AG) | 2 (AA) |
| CFH rs380390 | 5 (GG) | 21 (CG) | 10 (CC) |
Table 3.
Mean (±SD) LMSR CFF for Each of the Gene Variants
| Gene Variant | Low-Risk Homozygotes | Heterozygotes | High-Risk Homozygotes |
|---|---|---|---|
| LOC rs10490924 | 15.3 (±1.25) | 14.4 (±0.96) | 14.9 (±0.95) |
| HRTA1 rs1120038 | 15.3 (±1.25) | 14.4 (±0.96) | 14.9 (±0.95) |
| CFH rs380390 | 14.8 (±1.19) | 14.9 (±1.08) | |
Table 4.
Mean (±SD) LMS CFF for Each of the Gene Variants
| Gene Variant | Low-Risk Homozygotes | Heterozygotes | High-Risk Homozygotes |
|---|---|---|---|
| LOC rs10490924 | 19.8 (±1.07) | 19.4 (±1.43) | 19.7 (±1.27) |
| HRTA1 rs1120038 | 19.8 (±1.07) | 19.4 (±1.43) | 19.7 (±1.27) |
| CFH rs380390 | 19.4 (±1.17) | 20.1 (±1.45) | |
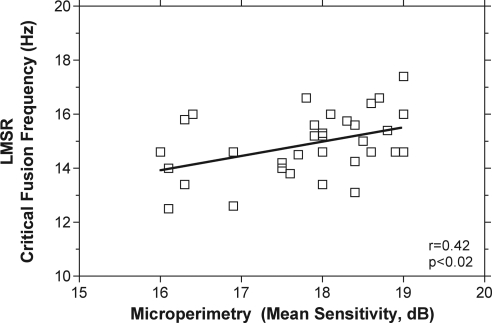
Mean sensitivities and pattern defects were determined for microperimetry and flicker perimetry with four and five exclusions, respectively, because of increased false positive responses. The MS and PD ± SD for the microperimeter (n = 32) were 17.8 dB (±0.93 SD) and −1.6 dB (±0.96 SD), respectively. The mean MS and PD for flicker perimetry (n = 31) were 25.08 dB (±0.7) and 0.9 dB (±0.6), respectively. The values for both flicker and microperimetry were within normal ranges.22,32 Mean central retinal thickness as measured with the OCT (228.6 μm ± 19.6) was within normal limits in all participants.33 Microperimetry mean sensitivity and pattern defect were significantly correlated with age (r = −0.39, P ≤ 0.03) and LMSR (r = 0.42, P < 0.02; Fig. 1). Figure 1 shows that lower LMSR CFF was associated with lower MS on the microperimeter. A significant correlation between central retinal thickness (OCT) and age (r = −0.4, P = 0.02) was found as demonstrated previously.34
Figure 1.
Rod- and cone-mediated (LMSR) critical fusion frequency (CFF) as a function of microperimetry (MP-1) mean sensitivity (MS). There is a significant correlation between LMSR CFF and MS.
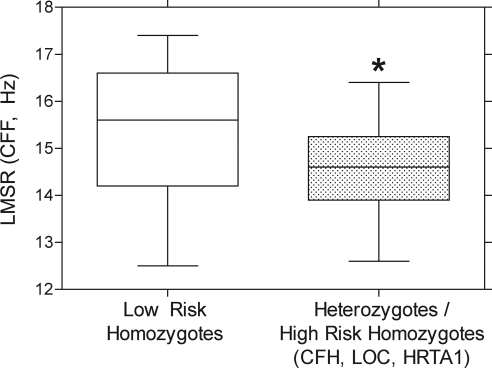
ANOVA demonstrates a significant reduction of LMSR CFF in gene-positive compared with gene-negative participants without correction for age (F1,35 = 4.16, P = 0.05) and after correction for age (F1,35 = 5.18, P = 0.03; Fig. 2). There was no significant difference between gene-positive and gene-negative participants in the other clinical tests (flicker perimetry, microperimetry) as well as in mean retinal thickness (OCT).
Figure 2.
Mean LMSR CFF values for participants who were heterozygous and homozygous for high-risk CFH, LOC, and HRTA1 SNPs are significantly lower compared with the gene-negative participants (asterisk). Box-and-whiskers plot shows the median (50th percentile), interquartile range (25th–75th percentile), and highest and lowest values.
We conducted a subanalysis of the individual gene variant combinations and divided those into five groups according to each persons' genetic combination; group 1 included all gene-negative participants (n = 11), group 2 consisted of all participants who were only heterozygous for LOC/HTRA1 (n = 13), group 3 consisted of participants with only the CFH homo- or heterozygous variant and heterozygous for LOC/HTRA1 (n = 6), group 4 included all participants with only the homo- or heterozygous CFH variant (n = 4), and group 5 consisted of persons only homozygous for the LOC/HTRA1 variant (n = 2; Table 5). Table 5 demonstrates the CFF results for both LMS and LMSR for each group and shows a trend of lower LMSR values in all groups with the risk genes compared with the gene-negative group. A two-sample t-test with equal variances revealed a significant difference for group 2 with lower LMSR CFF in participants heterozygous for the LOC/HTRA1 compared with group 1 (gene-negative) (P = 0.01). Groups 3 to 5 and in particular homozygotes were not significantly different compared with group 1, most likely because of small sample sizes in these remaining subgroups.
Table 5.
The LMSR and LMS CFF for Each of the Gene Combinations
| Gene Combinations (n = 36) | LMSR CFF | LMS CFF |
|---|---|---|
| Group 1 (gene-negative) (n = 11) | 15.4 (±1.38) | 19.8 (±1.13) |
| Group 2 (LOC/HTRA1 heterozygous) (n = 13) | 14.22 (±0.78)* | 19.1 (±1.19) |
| Group 3 (CFH, LOC/HTRA1 heterozygous) (n = 6) | 14.85 (±1.25) | 20.1 (±1.78) |
| Group 4 (CFH only) (n = 4) | 15.1 (±0.92) | 20.2 (±0.96) |
| Group 5 (LOC/HTRA1 homozygous) (n = 2) | 14.9 (±0.95) | 19.7 (±1.27) |
P = 0.01 compared with gene-negative participants (group 1).
Discussion
This is the first demonstration that in a randomly chosen sample with normal visual acuity and central visual fields and no clinical signs of ARM in both eyes, persons with ARM risk genotypes have on average lower mesopic visual function after correction for age compared with those who do not carry the risk genotype (gene-negative). The reduction in rod- and cone-mediated (LMSR) and not cone-mediated only (LMS) mesopic vision is consistent with rod dysfunction or selective rod loss as demonstrated histologically, psychophysically, and electrophysiologically in early ARM and ageing.30,35,36 Previous studies demonstrate reduced rod-mediated neuroretinal function in early ARM sufferers that exceeds those changes associated with normal aging.36,37 Delayed dark adaptation35,38 as well as reduced scotopic sensitivity39 are evident in early ARM. Foveal critical fusion frequency is reduced under photopic light levels in advanced ARM but is not sensitive as a diagnostic tool,40 consistent with our observation that there were no differences in the cone-mediated (LMS) CFF in gene-positive and -negative participants. These preliminary results in persons with no signs of ARM signify rod-mediated mesopic visual function testing as important for the evaluation of older persons with ARM risk genotypes, and that it may become a functional biomarker for disease.
We further report a novel relationship between microperimetry and the four-primary photostimulator where lower MS and PD are significantly correlated with lower LMSR CFF. Although the microperimeter background light level is in the upper mesopic range and the achromatic test stimuli estimate visual sensitivity mediated by rods and cones in different states of relative sensitivity, its inbuilt fixation tracking system may improve the detection of functional vision changes over that of other conventional ophthalmic tests we performed under photopic illuminations (e.g., color vision, flicker perimetry). Although microperimetry results were not significantly reduced in gene-positive compared with gene-negative participants in this cohort, future studies in a larger cohort of older gene-positive participants may have increased statistical power to detect subclinical deficits.
The SNPs identified in this study have been all related to neovascular AMD.2,3,41 Ischemia due to underlying cardiovascular conditions has been suggested as a major factor in the development of ARM and in neovascular AMD.1,11,14,42,43 Moreover, a recent study has identified three other gene variants on chromosomes 15, 16, and 22 with an increased risk of AMD that are related to the cholesterol metabolism and thus cardiovascular risk factors.4 A person with these gene variants may be predisposed to functional deficits because of ischemic insults. Thus a test that increases the oxygen demand by both increased photoreceptor activity (during mesopic conditions) and increased blood flow (during flicker stimulation) as used in our experiment may facilitate the early detection of the first functional deficits.
The demonstrated relationship between a risk genotype and impaired mesopic visual function in persons with clinically normal eyes is important because visual function may be responsive to appropriate environmental manipulation such as changing diet and lifestyle.44 Persons with ARM risk genotypes who smoke or have a poor diet have an approximate doubling of the risk of ARM progression, compared with those who are gene positive but do not have such environmental exposures.45 Moreover, late stages of ARM may be preventable because recent studies indicate that a healthy lifestyle including a healthy diet, physical activity, adequate vitamin D intake, and not smoking can decrease the risk for developing intermediate age-related macular degeneration by about twofold.46 These findings support our approach that the application of sensitive tests as biomarkers for the early detection and appropriate monitoring of disease is vital because risk is modifiable. Monitoring visual function with a quantitative test will further enable the evaluation of the effect of lifestyle changes on a person's health (such as change in a person's environmental exposures) and reduce the economic costs associated with the most common cause of blindness in the western world. The study findings may have future applications in the verification of subclinical ARM without genetic assessment.
Assessing mesopic vision as a biomarker of subclinical ARM may have potential significant implications in determining the pathomechanisms of the subtypes of neovascular ARM such as occult, classic, polypoidal choroidal vasculopathy, or retinal angiomatous proliferations that are poorly understood. It has been demonstrated that different gene variants promote subtypes of ARM.47,48 Further longitudinal clinical investigation is required to study persons with significantly reduced mesopic vision and gene-positive carrier status to determine whether visual function deteriorates faster than those in the low-risk (gene-negative) group and to understand the relationship between genotype and phenotype by quantitatively measuring visual function related to genotypes to determine progression to ARM.
Acknowledgments
The authors thank Anton Haas, Department of Ophthalmology, Medical University Graz, Austria, for critical feedback on a manuscript draft.
Footnotes
Supported by a Queensland University of Technology Vice Chancellor Research Fellowship (BF), Australian Research Council Discovery Projects DP1096354 (AJZ), and NIH National Eye Institute Grant R01-EY019651 (DC).
Disclosure: B. Feigl, None; D. Cao, None; C.P. Morris, None; A.J. Zele, None
References
- 1. Feigl B. Age-related maculopathy: linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog Ret Eye Res. 2009;28:63–86 [DOI] [PubMed] [Google Scholar]
- 2. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–993 [DOI] [PubMed] [Google Scholar]
- 4. Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107:7401–7406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seddon JM, Francis PJ, George S, Schultz DW, Rosner B, Klein ML. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297:1793–1800 [DOI] [PubMed] [Google Scholar]
- 6. Ross R, Verma V, Rosenberg K, Chan C-C. Genetic markers and biomarkers for age-related macular degeneration. Exp Rev Ophthalmol. 2007;2:443–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petzold A, Plant GT. Clinical disorders affection mesopic vision. Ophthalmic Physiol Opt. 2006;26:326–341 [DOI] [PubMed] [Google Scholar]
- 8. Pokorny J, Smithson H, Quinlan J. Photostimulator allowing independent control of rods and the three cone types. Vis Neurosci. 2004;21:263–267 [DOI] [PubMed] [Google Scholar]
- 9. Kiryu J, Asrani S, Shahidi M, Mori M, Zeimer R. Local response of the primate retinal microcirculation to increased metabolic demand induced by flicker. Invest Ophthalmol Vis Sci. 1995;36:1240–1246 [PubMed] [Google Scholar]
- 10. Zele AJ, Cao D, Pokorny J. Rod-cone interactions and temporal impulse response of the cone pathway. Vision Res. 2008;48:2593–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arden GB, Sidman RL, Arap W, Schlingemann R. Spare the rod and spoil the eye. Br J Ophthalmol. 2005;89:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phipps JA, Guymer RH, Vingrys AJ. Loss of cone function in age-related maculopathy. Invest Ophthalmol Vis Sci. 2003;44:2277–2283 [DOI] [PubMed] [Google Scholar]
- 13. Mayer MJ, Ward B, Klein R, Talcott JB, Dougherty RF, Glucs A. Flicker sensitivity and fundus appearance in pre-exudative age-related maculopathy. Invest Ophthalmol Vis Sci. 1994;35:1138–1149 [PubMed] [Google Scholar]
- 14. Schlingemann R. Role of growth factors and the wound healing response in age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol. 2004;242:91–101 [DOI] [PubMed] [Google Scholar]
- 15. Age-Related Eye Disease Study Research Group The age-related eye disease study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132:668–681 [DOI] [PubMed] [Google Scholar]
- 16. Age-Related Eye Disease Study Research Group The age-related eye disease study system for classifying cataracts from photographs: AREDS report number 4. Am J Ophthalmol. 2001;131:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawford BR, Young RW, Swagell DD, et al. The C/C genotype of the C957T polymorphism of the dopamine D2 receptor (DRD2) is associated with schizophrenia. Schizophr Res. 2005;73: 31–37 [DOI] [PubMed] [Google Scholar]
- 18. Rivera A, Fisher LG, Fritsche LG, et al. Hyopothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration contributing independently of complement factor H to disease risk. Hum Mol Gen. 2005;14:3227–3236 [DOI] [PubMed] [Google Scholar]
- 19. Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrel lRE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Gen. 2005;77:389–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vingrys AJ, Helfrich KA. The Opticom M-600: a new LED automated perimeter. Clin Exp Optom. 1990;1:10–20 [Google Scholar]
- 21. Zele AJ, Dang TM, O'Loughlin RK, Guymer RH, Harper A, Vingrys AJ. Adaptation mechanisms, eccentricity profiles, and clinical implementation of red-on-white perimetry. Optom Vis Sci. 2008;85:309–317 [DOI] [PubMed] [Google Scholar]
- 22. Landers J, Sharma A, Goldberg I, Graham E. A comparison of global indices between the Medmont Automated Perimeter and the Humphrey Field Analyzer. Br J Ophthalmol. 2007;91:1285–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rohrscheider K, Bueltmann S, Springer C. Use of fundus perimetry (microperimetry) to quantify macular sensitivity. Prog Ret Eye Res. 2008;27:536–548 [DOI] [PubMed] [Google Scholar]
- 24. Puts MJ, Pokorny J, Quinlan J, Glennie L. Audiophile hardware in vision science; the soundcard as a digital to analog converter. J Neurosci Meth 2005;142:77–81 [DOI] [PubMed] [Google Scholar]
- 25. Swanson WH, Uneo T, Smith VC, Pokorny J. Temporal modulation sensitivity and pulse-detection thresholds for chromatic and luminance perturbations. J Opt Soc Am A. 1987;4:1992–2005 [DOI] [PubMed] [Google Scholar]
- 26. Shapiro AG, Pokorny J, Smith VC. Cone-rod receptor spaces with illustrations that use CRT phosphor and light-emitting-diode spectra. J Opt Soc Am A Opt Image Sci Vis. 1996;13:2319–2328 [DOI] [PubMed] [Google Scholar]
- 27. Cao D, Zele AJ, Pokorny J. Dark-adapted rod suppression of cone flicker detection: evaluation of receptoral and postreceptoral interactions. Vis Neurosci. 2006;23:531–537 [DOI] [PubMed] [Google Scholar]
- 28. MacLeod DI, Boynton RM. Chromaticity diagram showing cone excitation by stimuli of equal luminance. J Opt Soc Am. 1979;69:1183–1186 [DOI] [PubMed] [Google Scholar]
- 29. Osterberg GA. Topography of the layer of rods and cones in the human retina. Acta Ophthalmol. 1935;13:1–103 [Google Scholar]
- 30. Curcio CA, Owsley C, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000;41:2015–2018 [PubMed] [Google Scholar]
- 31. Ott J. Utility programs for analysis of genetic linkage. Program, HWE version 1.10 New York: Columbia University; 1988 [Google Scholar]
- 32. Springer C, Bueltmann S, Voelker HE, Rohrscheider K. Fundus perimetry with the micro perimeter 1 in normal individuals. Ophthalmology. 2005;112:848–854 [DOI] [PubMed] [Google Scholar]
- 33. Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–332 [DOI] [PubMed] [Google Scholar]
- 34. Neuville J, Bronson-Castein K, Bearse MAJ, et al. OCT reveals regional differences in macular thickness with age. Optom Vis Sci. 2009;86:E810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Owsley C, Jackson GR, White M, Edwards D. Delays in rod-mediated dark-adaptation in early age-related maculopathy. Ophthalmology. 2001;108:1196–1202 [DOI] [PubMed] [Google Scholar]
- 36. Feigl B, Brown B, Lovie-Kitchin J, Swann PG. The rod-mediated multifocal electroretinogram in aging and in early age-related maculopathy eyes. Curr Eye Res. 2006;31:635–644 [DOI] [PubMed] [Google Scholar]
- 37. Feigl B, Brown B, Lovie-Kitchin J, Swann PG. Cone- and rod-mediated mfERG in early age-related maculopathy. Eye. 2005;19:431–441 [DOI] [PubMed] [Google Scholar]
- 38. Dimitrov PN, Guymer RH, Zele AJ, Anderson AJ, Vingrys AJ. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:55–65 [DOI] [PubMed] [Google Scholar]
- 39. Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–273 [PubMed] [Google Scholar]
- 40. Maier M, Groneberg T, Specht H, Lohmann CP. Critical flicker-fusion frequency in age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol. 2010;248:409–413 [DOI] [PubMed] [Google Scholar]
- 41. Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular diseases share common antecedents? Ophthal Epidemiol. 1999;6:125–143 [DOI] [PubMed] [Google Scholar]
- 42. Grunwald JE, Hariprasad SM, DuPont J, et al. Foveal choroidal blood flow in age-related macular degeneration (AMD). Invest Ophthalmol Vis Sci. 1998;39:385–390 [PubMed] [Google Scholar]
- 43. Tan JS, Mitchell P, Smith W, Wang JJ. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2007;114:1143–1150 [DOI] [PubMed] [Google Scholar]
- 44. Hogg RE, Dimitrov PN, Dirani M, et al. Gene-environment interactions and aging visual function: a classical twin study. Ophthalmology. 2009;116:263–269 [DOI] [PubMed] [Google Scholar]
- 45. Wang JJ, Rochtchina E, Smith W, et al. Combined effects of complement factor H genotypes, fish consumption, and inflammatory markers on long-term risk for age-related macular degeneration in a cohort. Am J Epidemiol. 2009;169:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sondel SA, Voland R, Millen AE, et al. Relationship of healthy lifestyles to the prevalence of intermediate age-related macular degeneration (AMD) in the Carotenoids in Age-Related Diseases Study (CAREDS), an ancillary study of the Women's Health Initiative Observational Study (WHIOS). Annual meeting of the Association for Research in Vision and Ophthalmology (ARVO), Fort Lauderdale 2010:E-abstract 4529 [Google Scholar]
- 47. Wegscheider BJ, Weger M, Renner W, et al. Association of complement factor H Y402H gene polymorphism with different subtypes of exudative age-related macular degeneration. Ophthalmology. 2007;114:738–742 [DOI] [PubMed] [Google Scholar]
- 48. Sakurada Y, Kubota T, Imasawa M, et al. Angiographic lesion size associated with LOC387715 A69S genotype in subfoveal polypoidal choroidal vasculopathy. Retina. 2009;29:1522-1526 [DOI] [PubMed] [Google Scholar]