The authors review the evidence that apoptosis is an intrinsic part of the pathology of diabetic retinopathy and present its potential causes. Criteria for the next experimental questions in this important research field are also presented.
Abstract
The most striking features of diabetic retinopathy are the vascular abnormalities that are apparent by fundus examination. There is also strong evidence that diabetes causes apoptosis of neural and vascular cells in the retina. Thus, there is good reason to define diabetic retinopathy as a form of chronic neurovascular degeneration. In keeping with the gradual onset of retinopathy in humans, the rate of cell loss in the animal models is insidious, even in uncontrolled diabetes. This is not surprising given that a sustained high rate of cell loss without regeneration would soon lead to catastrophic tissue destruction. The consequences of ongoing cell death are difficult to detect, and even the quantification of cumulative cell loss requires painstaking histology and microscopy. This subtle cell loss raises the issue of the relevance of the phenomenon to the progression of diabetic retinopathy and the ultimate loss of vision. Neuronal function may be compromised in advance of apoptosis, contributing to an early deterioration of vision. Here we review some of the evidence supporting apoptotic cell death as a contributing mechanism of diabetic retinopathy, explore some of the potential causes, and discuss the potential links between apoptosis and loss of visual function in diabetic retinopathy.
Retinopathy is one of the most common complications of diabetes, afflicting about 20% of adults with diabetes. As of 2008 diabetic retinopathy affected more than 4.4 million Americans age 40 and older (Vision Problems in the U.S., the National Eye Institute and Prevent Blindness America, 2008, available at http://www.preventblindness.org/vpus/). The disease is characterized and diagnosed by visual fundus examination revealing vascular lesions and macular edema. There is plenty of evidence, however, that these visible manifestations of the disease are accompanied by insidious degenerative changes that can only be detected by more invasive histological or biochemical assays. One of the most perplexing of these changes is persistent apoptosis of a variety of vascular and neural cells, which has been observed in retinal tissue from humans with diabetes and diabetic animal models. While the evidence for an appreciable increase in retinal cell apoptosis has become abundant, the underlying mechanisms are still unknown. Furthermore, the relationship between apoptosis of vascular and neural cells is unclear, with the possibility that loss of these different classes of cells occurs over different timeframes and possibly by unrelated mechanisms. Finally, the relatively low rate of chronic cell loss that appears to occur in diabetes confounds our scientific efforts and renders the physiological relevance of apoptosis to vision loss uncertain. In this article, we will discuss the evidence for apoptosis of retina cells in diabetes, and briefly consider some of its potential mechanisms and physiological consequences.
Apoptosis of Vascular Cells
It is well established that diabetes leads to loss of vascular cells in the retina, as indicated by the appearance of acellular capillaries in which basement membrane or remnant endothelial cell cytoplasm remains while the cell nucleus is absent.1 These lesions are best observed in the protease (trypsin or elastase) digest preparation, which has remained the most widely used technique for the histologic inspection of retinal vasculature in fixed specimens.2 Vascular apoptosis was explored using terminal dUTP nick-end labeling (TUNEL) in human and rat retinas treated by trypsin digestion.3 There was a small but significant increase in the number of vascular cells positive for TUNEL in postmortem retinas of seven human donors who had diabetes for an average of 9 ± 4 years compared with retinas from nine donors who did not have diabetes. A similarly modest increase in vascular cell death was found in rats after 31 weeks of streptozotocin-induced (STZ)-diabetes.1 This study was the first to quantify an increase in apoptosis throughout the entire vasculature of the retina. Other studies confirmed the increase in retinal vascular cell apoptosis in diabetic retinas4–8 and in db/db mice.9 The increase in vascular cell apoptosis also suggested a potential mechanism for the appearance of acellular capillaries given that the endothelial cell bodies and nuclei were thought to disappear while leaving their intact basement membranes behind.3
Apoptosis may also account for the appearance of pericyte “ghosts” (pockets within the basement membrane that appear to have once contained a pericyte) frequently noted in trypsin digest samples.5 TUNEL labeling of pericytes in human retinas was increased by diabetes and also localized with Bax immunoreactivity, a proapoptotic Bcl2 family member protein.10 Another study of postmortem retinas noted that pericytes were absent from some blood vessels that still contained intact endothelial cells and that vessels with microaneurysms tended not to contain pericytes, suggesting that their loss permits uncontrolled proliferation of endothelial cells.11 Pericyte dropout has been used as an index of diabetic retinopathy,12,13 but the functional consequences of this finding remain unclear. Given the potential contractile properties of these cells,14 it is conceivable that their loss could alter the way blood flow is regulated in the retinal microvasculature.
Although much has been learned about apoptosis of vascular endothelial cells, it is unclear whether the modest increase in apoptosis is related to other functional consequences of diabetes, such as loss of blood-retinal barrier integrity leading to macular edema.15–17 Increased retinal vascular permeability, which precedes vascular proliferation, is a well-documented consequence of diabetes in humans and animal models.18–24 It is also unclear whether there is a causal link between vascular cell apoptosis and increased vascular permeability. The concept that loss of an endothelial cell leaves a transient hole in the vessel wall seems simplistic. Occasional losses in the vasculature of control animals are likely to occur during the normal turnover of endothelial cells, but this does not appear to increase vascular permeability. Furthermore, a study using STZ-diabetic rats to identify the most permeable regions of blood vessels by in situ fixation of fluorescent concanavalin A showed that permeability did not occur at isolated cells or capillaries.23 Rather, permeability occurred in many vessels simultaneously, first in the larger superficial vessels and then progressing to the capillaries of the outer plexiform layer within 2 months of the onset of diabetes. It therefore seems more likely that vascular permeability increases as a consequence of regulatory changes in tight junction proteins within a broad population of endothelial cells and is less likely the consequence of the apoptosis of small numbers of endothelial cells.24
The long-term functional significance of vascular cell apoptosis is unclear, and it is uncertain whether it is responsible for, or a result of, the development of localized capillary nonperfusion and retinal ischemia. Vascular cell dropout can be a response to reduced metabolic demands from the surrounding neurosensory retina, as in photoreceptor degeneration; hence, it is conceivable that vascular apoptosis represents a final response to localized cell death in the surrounding neural tissue.
Apoptosis of the Neural Retina
Trypsin digestion isolates the vascular cells of the retina for histologic examination. Studies using TUNEL in histologic sections of retinas of STZ-diabetic rats and postmortem human retinas revealed that diabetes increased apoptosis in neurons, especially in the inner retina, where retinal ganglion cells are located.25,26 When TUNEL labeling was adapted to whole retina, the total number of apoptotic cells could be quantified and was approximately 10-fold greater than the number reported in trypsin digest retinas, suggesting that nonvascular cells also undergo apoptosis in diabetes.27 These findings indicate that significant neural apoptosis is an earlier event than vascular apoptosis and that the rate of neural apoptosis remains constant throughout the duration of diabetes. Similar results were found by other investigators using the same technique,28 and an increase in intraocular pressure further elevated the number of TUNEL-positive cells in diabetic rats.29 Taken together, these data suggest that apoptosis in the neural retina is an early and persistent event in diabetes.
Neurons are unable to proliferate, so apoptosis of these cells will result in a cumulative loss leading to chronic neurodegeneration. The morphology of rat retinas was examined to determine whether the gradual cell loss reduced the overall number of cells remaining in the retina after a long duration of diabetes.27 Paraffin-embedded sections of eyes from STZ-diabetic rats after 30 weeks of diabetes were examined for changes in thickness, and the number of large cell bodies in the retinal ganglion cell layer was counted to determine whether the cumulative cell loss would result in significant differences in the total number of remaining cells in diabetic and control retinas. The total number of cell bodies in the retinal ganglion cell layer was reduced by 10% after 7.5 months of diabetes. This was accompanied by a 22% reduction in the thickness of the inner plexiform layer and a 14% loss in the thickness of the inner nuclear layer. In contrast, there was no change in the thickness of the outer nuclear layer, suggesting that more cells were lost from the inner than from the outer retina. This study attempted to test the origin of cells undergoing apoptosis by pairing TUNEL with immunohistochemistry for von Willebrand factor, an immunohistochemical marker of vascular endothelial cells.30 It was found that, at least in the small number of radial sections sampled, TUNEL labeling did not localize with blood vessels, suggesting that the cells undergoing apoptosis were more likely to be neurons or glia.
Further studies have revealed the loss of multiple subtypes of neurons in mouse retinas.31,32 The number of apoptotic cells in mice that had been diabetic for 4 weeks was measured in whole retinas by counting the number of cells immunoreactive for active caspase-3.31 Again, the number of TUNEL-positive and active caspase-3-immunoreactive cells was increased in the ganglion cell layer, and there was a cumulative loss of retinal ganglion cells33 and amacrine cells in diabetic mice.34 Apoptosis of cells in the retinal ganglion cell layer of STZ-diabetic mice was further confirmed by the appearance of fragmented nuclei in electron micrographs.32 Importantly, the increase in apoptosis occurs soon after the onset of experimental diabetes, corresponding to the earliest changes in vascular permeability.
Other studies have confirmed that diabetes results in the apoptosis of a variety of cells in the retina. The reduction in the number of cell bodies in the retinal ganglion and inner nuclear layers in rats after 1 month and 4 months of STZ-induced diabetes was corroborated by immunohistochemistry for NeuN, a cell-specific marker expressed exclusively in the nuclei of neurons.35 The total number of inner nuclear layer cell bodies with NeuN immunoreactivity was reduced to approximately 50% of controls after 4, 6, and 12 months of diabetes and was accompanied by a 20% reduction in NeuN-positive cells in the retinal ganglion cell layer after 1 month and 4 months. Although this study provides good evidence that retinal ganglion cells are vulnerable in diabetes, it is conceivable that other neurons, such as displaced amacrine cells, would appear positive for NeuN in the retinal ganglion cell layer. A loss of retinal ganglion cell bodies is also indicated by a reduction in the number of axons in the optic nerve of rats after 12 weeks of STZ-diabetes,36 and by a clinical study showing a reduction in the thickness of the retinal nerve fiber layer using scanning laser polarimetry.37 In the latter study of a group of patients who had diabetes for an average of 15 years, the thickness of the nerve fiber layer in the superior polar quadrant of the retina was significantly reduced compared with the control group, indicating a loss of axons in this region and implying an accompanying loss of retinal ganglion cells. Interestingly, an earlier study of 137 patients with type 2 diabetes and 144 subjects without diabetes suggested that nerve fiber layer defects occurred in 20% of the diabetic subjects with no evidence of microaneurysms.38 More recently, OCT studies have demonstrated reductions in the thickness of the inner retinal layers, including the retinal ganglion cell layer, in type 1 diabetes patients with minimal vascular retinopathy39,40
Data from human retinas suggest that there are similar increases in apoptosis of both the vascular and neural components of the retina.3,26,27,41 Therefore, we suggest that current animal models accurately reflect the apoptosis encountered by human retinas in diabetes. In a small number of whole-mounted retinas from human donors, the number of TUNEL-labeled cells was greater than in age-matched nondiabetic donors,27 and apoptosis markers were increased in the inner retinas of humans with diabetes.41 Therefore, it seems likely that diabetes causes apoptosis of retinal neural cells in humans similar to that observed in rodents. It is our experience, however, that the time of onset (duration of diabetes required to significantly elevate apoptosis) was difficult to determine in humans because of a lack of accurate determination of the onset of diabetes in tissue donors. Studies on postmortem tissue are also compromised by the uncertain effects of the variable time span between death and retinal preservation.
Use of multifocal electroretinograms (mfERGs) has provided provocative evidence suggesting a direct link between neural and vascular dysfunctions in diabetic patients. mfERG detected localized defects in neuronal function (implicit time delays) that predicted the appearance of visible vascular dysfunctions in the same regions over the course of 3 years.42 This finding suggests that neuronal apoptosis precedes overt vascular dysfunction. The spatial coincidence might also suggest that neuronal death or dysfunction leads to vascular pathologies; however, subtle undetected vascular defects could have existed before the deficit in ERG response.
In summary, there is now ample evidence that diabetes increases the rate of apoptosis of both neural and vascular cells in the retina. Histologic evidence of neuronal loss has been noted in monkeys with spontaneous type 2 diabetes43 and in humans.44,45 Taken together, these data clearly indicate that apoptosis of retinal vascular and neural cells is a consequence of diabetes in multiple species, including humans. We propose that research must continue to move beyond the characterization of pathology and seek better understanding of the disease mechanisms that are the precursors of diabetic retinopathy.
Potential Causes of Retinal Cell Apoptosis in Diabetes
The potential mechanisms of diabetic retinopathy are too numerous to comprehensively review in this Perspective. We will focus instead on selected mechanisms (illustrated in Fig. 1) that have been implicated as elevators of apoptosis in the retina and that we suggest as important candidates for further study.
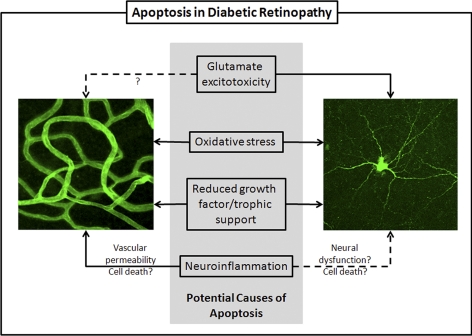
Figure 1.
Summary of potential causes of apoptosis in diabetic retinopathy. Evidence from animal models and postmortem human tissue support an increase in apoptosis in the retinal vasculature (left) and neural cells (including all neurons and photoreceptors; right). Some potential mechanisms considered in this Perspective include general ones such as oxidative stress and reduced growth factor signaling, which could induce cell death in all cells. Also considered are glutamate excitotoxicity, which is known to cause apoptosis in neurons though its potential to kill vascular cells is less clear, and neuroinflammation, which is known to induce vascular permeability and may also cause dysfunction in neural tissue.
Glutamate Excitotoxicity
Neuronal apoptosis in the central nervous system is often associated with increased release of extracellular glutamate, which is thought to play a central role in both chronic and acute neurodegeneration.46,47 A number of studies in rats and humans suggest that the total content of glutamate in the retina and vitreous is elevated by diabetes.48–50 Diabetes also reduces the rate at which explant retinas convert 14C-glutamate to 14C-glutamine, suggesting that the activity of glutamine synthetase is reduced.49 The 35% loss in activity of this Müller cell-specific enzyme was also accompanied by reduced glutamine synthetase protein content measured by Western blot.51 Oxidation of 14C-glutamate to 14CO2 by the TCA cycle was reduced to 62% of the control value in retinas from STZ-diabetic rats.51 Interestingly, the general transaminase inhibitor, aminooxyacetate, blocked the oxidation of glutamate in control retinas but caused no further deficit in the retinas of diabetic rats, suggesting that the reduction in the rate of glutamate oxidation is caused by impaired transamination between glutamate and keto-acids, thus lessening the production of α-ketoglutarate from glutamate and ultimate oxidation of glutamate carbons.52–54 Taken together, these data suggest that diabetes may impair glutamate uptake and metabolism, resulting in a potential accumulation of extracellular glutamate, leading to excitotoxicity in which excess glutamate stimulation causes an uncontrolled intracellular calcium response in postsynaptic neurons.55,56
Changes in the uptake of glutamate by Müller cells may also contribute to excitotoxicity in diabetes by causing an excessive synaptic glutamate accumulation. Aspartate immunoreactivity was increased in Müller cells of diabetic rats, suggesting slower clearance of glutamate,57 whereas excised Müller cells from STZ-rats after 1 month of diabetes demonstrated reduced glutamate transporter activity, measured by patch clamp analysis.58,59 A more recent study of mouse Müller cells showed that high glucose had no effect on the expression and activity of the cystine-glutamate exchanger (xCT), but the induction of oxidative stress in these cells more than doubled xCT mRNA expression and elevated its activity, whereas the activity of the glutamate transporter GLAST was moderately decreased by oxidative stress.60 The results of these studies on Müller cells suggest that diabetes reduces the activity of glutamate transport, potentially elevating the concentrations of extracellular and synaptic glutamate in the retina, which, in turn, could lead to excitotoxic cell death.
In addition to a potential imbalance in the glutamate-glutamine cycle, studies suggest that diabetes may alter the regulation of glutamate receptor expression. For example, an immunohistochemical study compared the content of α-amino-3-hydroxy-5-methyl-isoxazole-4-propionate (AMPA) receptor and the N-methyl-D-aspartate (NMDA) glutamate receptor subunit protein expression in 12 diabetic and 6 control donor eyes. The results showed that the immunoreactivity of GluR2 and NR1 subunits was significantly increased by diabetes.61 Studies in diabetic rats also indicated that immunoreactivity of AMPA and NMDA receptor subunits Glu2/3 and NMDA1 were increased.62 In addition, one of these studies indicated that AMPA receptor subunit GluR2 phosphorylation and cellular distribution were altered during the early stages of diabetes, perhaps reflecting a shift in receptor recycling.63 High glucose also increased the expression of the GluR2 subunit by cultured retinal neurons, whereas the expression of GluR1 and GluR6/7 subunits was decreased and coincided with a decrease in calcium permeability through AMPA receptor-associated channels,64 which may be a compensatory mechanism to reduce chronically elevated glutamate stimulation.
Elevated glutamate levels may be the consequence of altered glutamate metabolism and reduced glial uptake (or vice versa). These may be accompanied by adaptive changes in glutamate receptor content and binding. The combined changes in the glutamate signaling system may ultimately increase apoptosis despite compensatory mechanisms designed to protect neurons, and the resultant imbalance in neurotransmission activity could also lead to dysfunction in visual signal processing in the retina and to apoptosis. Thus, the glutamate excitotoxicity hypothesis suggests a mechanism not only for the increase in cell death but also for the loss of visual function in diabetes.
Loss of Trophic Support/Survival Signaling
Another hypothesis to explain apoptosis is that diabetes reduces the abundance of trophic factors or the effectiveness of growth factor signaling mechanisms that are essential for the survival of neurons, pericytes, and endothelial cells in the retina. Administration of nerve growth factor to STZ-induced diabetic rats protected the retina from apoptosis,25 but another study found that nerve growth factor content and its receptor expression were unchanged by diabetes in the retinas of BioBreeding/Worcester rats, a model of spontaneous insulin-deficient diabetes.65 Other studies showed that diabetes depletes the content of brain-derived neurotrophic factor (BDNF) in both the brain and the retina66,67 and reported that intravitreal replacement of BDNF reduced the loss of dopaminergic amacrine cells, indicated by immunoreactivity for tyrosine hydroxylase, suggesting that this growth factor may be important for amacrine cell survival in the inner retina.67 It has also been demonstrated that insulin acts as a survival factor for retinal neurons.68 The retina has an abundance of insulin receptors with high basal activity that respond to physiological concentrations of insulin (10 nM) with the phosphorylation of Akt.69 Diabetes also reduces the kinase activity of the insulin receptor/phosphoinositide 3-kinase/Akt signaling pathway in the retinas of STZ- diabetic rats,70,71 suggesting that the sensitivity or responsiveness of downstream components of the tyrosine kinase growth factor signaling pathway may be reduced.
Growth factor signaling is also important to the survival of the vasculature. Apoptosis of retinal vascular cells during diabetes may be attributed to lack of platelet-derived growth factor B (PDGF) signaling because mice with endothelial cell-targeted knockout of the PDGF gene exhibit retinal pericyte loss resembling that of diabetic animals.72 Recently, hyperglycemia and diabetes have been shown to cause phosphorylation and inhibition of the PDGF receptor beta in a protein kinase C delta (PKCδ)-dependent fashion, such that PKCδ knockout mice did not exhibit the accumulation of acellular retinal capillaries during diabetes.73
It is likely that diabetes progressively impairs the function of multiple trophic signaling pathways in both vascular and neural components of the retina, thus reducing the strength of survival signals and elevating the chance of apoptosis induced by other disease-related stresses.
Oxidative Stress
Many studies have focused on the possibility that cellular dysfunction in diabetic retinopathy (and other complications of diabetes) is caused by oxidative stress induced by hyperglycemia.74–77 Levels of 8-hydroxydeoxyguanosine (8-OH-dG), an indicator of oxidative DNA damage, are increased in both the urine78 and the vitreous79 of patients with diabetic retinopathy. Vascular cell TUNEL labeling was reduced by the antioxidant drug aminoguanidine in diabetic rats,5 suggesting that endothelial apoptosis may be triggered by oxidative stress. Direct measurement of free radicals in tissue is difficult; however, indirect evidence of oxidative stress in the retinas of diabetic animals has been reported. The intracellular store of reductive capacity, glutathione, was depleted after 2 months of STZ-diabetes in rat retinas, but not brain, suggesting that oxidative stress may be more pronounced in the retina.80 Lipid peroxides measured by thiobarbituric acid reactive substances were also found to be increased.81 Caspase-3 activity was diminished after 2 and 14 months of diabetes in alloxan-injected rats fed with a complex of antioxidants including Trolox, α-tocopherol, acetyl cysteine, ascorbic acid, β-carotene, and selenium.82 When fed to STZ-rats diabetic for 11 months, the antioxidant α-lipoic acid also reduced TUNEL labeling in the trypsin-digested vasculature, possibly by inhibiting the oxidation of DNA and nitrotyrosine.83 Diabetic mice fed lutein exhibited lessened generation of reactive oxygen species in the retina, resulting in diminished caspase-3 activation, fewer TUNEL-positive cells, significant prevention of ganglion cell loss, inner plexiform layer and inner nuclear layer thinning, and improved ERG responses.84 Similar results were observed in diabetic rats fed either lutein or docosahexanoic acid.85 These pharmacologic studies show that reducing oxidative stress may be an effective approach to slow the retinal degeneration in diabetic retinopathy.
Mitochondrial oxidative stress and dysfunction may play a key role in retinal vascular dysfunction during diabetes. Recently, a haplotype of the mitochondrial uncoupling protein 2 (UCP2) gene was found to be associated with significantly increased risks for proliferative diabetic retinopathy in both type 1 and type 2 diabetes.86 UCP2 expression is increased in retinal endothelial cells and pericytes cultured in high glucose,87 presumably as a compensatory mechanism to combat increased reactive oxygen production.88 Mitochondrial dysfunction as a consequence of diabetes has been measured in whole rat retina.89 In this study there was also an increase in release of cytochrome c and a greater association of BAX with mitochondrial membranes, suggesting that the intrinsic mitochondrial pathway plays a role in the initiation or propagation of cell death by apoptosis. However, in vitro results suggest that high glucose may cause mitochondrial oxidative stress indirectly by increasing cytokine production, which in turn affects mitochondrial function.90 Another indirect player may be matrix metalloproteinase 2 (MMP2). MMP2 expression was upregulated in diabetic retinas and retinal endothelial cells cultured in high glucose, and the inhibition of MMP2 function or expression blocked the ability of high glucose to induce retinal endothelial cell mitochondrial dysfunction and apoptosis.91 Thus, though hyperglycemia causes endothelial damage, this may not simply be due to increased glucose metabolism by these cells but rather to indirect induction of mitochondrial oxidative stress and dysfunction leading to apoptotic signaling.
Neuro-inflammation
Inflammation is thought to play a key role in the pathology of several diabetic complications. The role of inflammatory processes in diabetic retinopathy is now generally accepted.92,93 Several studies identified increased concentrations of cytokines, particularly vascular endothelial growth factor (VEGFA), interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α, and monocyte chemoattractant (MCP)-1, CCL2 in the vitreous of patients with proliferative diabetic retinopathy and diabetic macular edema.91,92,94–96 Genomic assessment of whole retinas of diabetic rats identified increased expression of the inflammatory genes CCL2, ICAM-1, STAT3, CCR5, and CD44,97 and Müller cells isolated from diabetic rats had increased expression of several genes associated with immune function and inflammation.98
A causal link between cytokine expression and retinal apoptosis in diabetes has not been well established. However, an inflammatory component was supported by the finding that vascular apoptosis was blocked by systemic administration of an antibody to Fas ligand, preventing leukostasis.6 Injection of IL-1 into the vitreous of normal rats also induced TUNEL labeling and increased numbers of acellular capillaries in trypsin digest retinas, whereas a diet rich in multiple antioxidants reduced the amount of IL-1 and prevented vascular apoptosis in diabetic rats.7 Similarly, a TNF antagonist also reduced the number of apoptotic vascular cells.8
Several studies have observed alterations in the morphology of retinal microglia, the innate immune cells of the nervous system. These changes include shortening and decreased ramification of microglial membrane processes.31,99,100 Altered microglia were associated with the vasculature in human diabetic retinas, leading to the term microglial perivasculitis.99 Diminished ramification of microglia in diabetic animals and human specimens may indicate inflammatory activation of these cells.31,35,101,102 Microglia that have progressed to a fully activated inflammatory state produce numerous cytokines, including IL-6, IL-1β, TNFα, and MCP-1.103 Several animal studies have suggested that these cytokines may contribute to microvascular complications and apoptosis during diabetic retinopathy. For example, 2 months of STZ-diabetes increased IL-1 activity in rat retinas.7 Intravitreal injection of IL-1β in normal rats increased the number of TUNEL-positive vascular cells in trypsin digests and also increased 8-OH-dG content.104 Mice lacking the IL-1RI exhibited greatly diminished retinal caspase activation and acellular capillary formation after 4 to 7 months of STZ-induced diabetes.105 Mice lacking TNFRI or TNFRII expression also exhibited diminished pericyte and endothelial cell loss when fed galactose for 20 months.106 In diabetic rats, inhibition of TNFα with the soluble receptor fusion protein drugs pegsunercept and etanercept reduced caspase activation, endothelium-associated apoptosis, loss of pericytes and endothelial cells, and formation of acellular capillaries.8 In addition, a recent small phase 3 clinical study found that treatment with the anti–TNF monoclonal antibody infliximab improved visual acuity in patients with diabetic macular edema.107 The mechanism by which these cytokines may contribute to retinal vascular and neural apoptosis is not clear but may involve the induction of excitotoxicity, oxidative stress, or mitochondrial dysfunction.108–110 Although a strong association between inflammation and diabetic retinopathy has been established, a precise mechanistic link between inflammatory cytokines and retinal apoptosis during diabetes has not yet been determined.
The Potential for Loss of Neuronal Function in the Absence of Apoptosis
It seems a reasonable assumption that the gradual loss of visual function in diabetic retinopathy is due to a progressive loss of cells, much in the same way that loss of cerebral function occurs in chronic degenerative diseases of the brain (e.g., Alzheimer's and Parkinson's diseases), which are assumed to be the result of a progressive loss of neurons. In diabetic retinopathy, however, the progressive loss of cells is a small effect, and it may not be safe to assume that it is directly responsible for loss of function, especially in the early stages of disease progression. Other changes are known to occur in the retinas of diabetic animals that may affect neuronal function in the absence of apoptosis. Loss of synaptic proteins required for neurotransmission, for instance, has the potential to alter retinal function.111,112 Changes in intracellular calcium signaling as a result of extracellular glutamate activity or changes in the expression of calcium-binding proteins and the activity of calcium-responsive enzymes such as CamKII could also alter the physiological output of the retina.62,113 Early compromise of specific visual functions, such as contrast sensitivity and dark adaptation, are indicators of functional changes within the photoreceptors or other neuronal components of the retina.114–117 Alterations in the biochemistry and physiology of retinal neurons may occur independently or as a precursor to their ultimate demise by apoptosis, making loss of visual function a potential consequence of cell loss by apoptosis and maladaptive dysfunction of surviving cells.
Conclusions
In conclusion, apoptosis of vascular and neural cells in diabetes is clearly established, and these pathologies are well characterized in animal models and postmortem human tissue. Altered glutamate excitation, reduced trophic factor signaling, oxidative stress, and neuro-inflammation are among the many potential causes of the increase in apoptosis and are important candidate mechanisms for further study. The next steps to better understanding of the mechanism of diabetic retinopathy must embrace new challenges that move beyond further characterization of pathology. Given the difficulty in detecting small amounts of apoptosis, even in animal models of diabetes, we must address its physiological relevance to the human disease. We suggest that the next important questions regarding diabetes-induced apoptosis in the retina are: How well do animal models recapitulate what occurs in humans? What are the mechanisms that induce retinal cell apoptosis? What are the vascular and neural functional consequences of apoptosis in the retina? Experiments designed to address these questions will help move the diabetic retinopathy field closer to an understanding of how to reduce the risk for vision impairment in persons afflicted with diabetes.
Footnotes
Supported by the Juvenile Diabetes Research Foundation (AJB, SFA, TWG), American Diabetes Association (AJB, TWG), Animal Models of Diabetes Complications Consortium (AJB, SFA), US Public Health Service Grant RO1EY020582 (SFA, TWG), and The Jack and Nancy Turner Professorship (TWG).
Disclosure: A.J. Barber, None; T.W. Gardner, None; S.F. Abcouwer, None
References
- 1. Engerman RL, Kern TS. Retinopathy in animal models of diabetes. Diabet Metabol Rev. 1995;11:109–120 [DOI] [PubMed] [Google Scholar]
- 2. Bresnick GH, Davis MD, Myers FL, de Venecia G. Clinicopathologic correlations in diabetic retinopathy, II: clinical and histologic appearances of retinal capillary microaneurysms. Arch Ophthalmol. 1977;95:1215–1220 [DOI] [PubMed] [Google Scholar]
- 3. Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sugiyama T, Kobayashi M, Kawamura H, Li Q, Puro DG. Enhancement of P2X(7)-induced pore formation and apoptosis: an early effect of diabetes on the retinal microvasculature. Invest Ophthalmol Vis Sci. 2004;45:1026–1032 [DOI] [PubMed] [Google Scholar]
- 5. Kern TS, Tang J, Mizutani M, et al. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978 [PubMed] [Google Scholar]
- 6. Joussen AM, Poulaki V, Mitsiades N, et al. Suppression of Fas-FasL-induced endothelial cell apoptosis prevents diabetic blood-retinal barrier breakdown in a model of streptozotocin-induced diabetes. FASEB J. 2003;17:76–78 [DOI] [PubMed] [Google Scholar]
- 7. Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci. 2004;45:4161–4166 [DOI] [PubMed] [Google Scholar]
- 8. Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172:1411–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheung AK, Fung MK, Lo AC, et al. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–3125 [DOI] [PubMed] [Google Scholar]
- 10. Podesta F, Romeo G, Liu WH, et al. Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am J Pathol. 2000;156:1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns, IV: Diabetic retinopathy. Arch Ophthalmol. 1961;66:366–378 [DOI] [PubMed] [Google Scholar]
- 12. Kern TS, Engerman RL. Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr Eye Res. 1994;13:863–867 [DOI] [PubMed] [Google Scholar]
- 13. Sima AA, Chakrabarti S, Garcia-Salinas R, Basu PK. The BB-rat—an authentic model of human diabetic retinopathy. Curr Eye Res. 1985;4:1087–1092 [DOI] [PubMed] [Google Scholar]
- 14. Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J Neurocytol. 2001;30:35–44 [DOI] [PubMed] [Google Scholar]
- 15. Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998–1003 [DOI] [PubMed] [Google Scholar]
- 16. Moss SE, Klein R, Klein BE. The incidence of vision loss in a diabetic population. Ophthalmology. 1988;95:1340–1348 [DOI] [PubMed] [Google Scholar]
- 17. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, XV: the long-term incidence of macular edema. Ophthalmology. 1995;102:7–16 [DOI] [PubMed] [Google Scholar]
- 18. Cunha-Vaz J, Faria de Abreu JR, Campos AJ. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975;59:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lobo CL, Bernardes RC, Santos FJ, Cunha-Vaz JG. Mapping retinal fluorescein leakage with confocal scanning laser fluorometry of the human vitreous. Arch Ophthalmol. 1999;117:631–637 [DOI] [PubMed] [Google Scholar]
- 20. Jones CW, Cunha-Vaz JG, Rusin MM. Vitreous fluorophotometry in the alloxan- and streptozocin-treated rat. Arch Ophthalmol. 1982;100:1141–1145 [DOI] [PubMed] [Google Scholar]
- 21. Williamson JR, Chang K, Tilton RG, et al. Increased vascular permeability in spontaneously diabetic BB/W rats and in rats with mild versus severe streptozocin-induced diabetes: prevention by aldose reductase inhibitors and castration. Diabetes. 1987;36:813–821 [DOI] [PubMed] [Google Scholar]
- 22. Do Carmo A, Ramos P, Reis A, Proenca R, Cunha-Vaz JG. Breakdown of the inner and outer blood retinal barrier in streptozotocin-induced diabetes. Exp Eye Res. 1998;67:569–575 [DOI] [PubMed] [Google Scholar]
- 23. Barber AJ, Antonetti DA. Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats. Invest Ophthalmol Vis Sci. 2003;44:5410–5416 [DOI] [PubMed] [Google Scholar]
- 24. Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998;47:1953–1959 [DOI] [PubMed] [Google Scholar]
- 25. Hammes HP, Federoff HJ, Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med. 1995;1:527–534 [PMC free article] [PubMed] [Google Scholar]
- 26. Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035 [DOI] [PubMed] [Google Scholar]
- 27. Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J Clin Invest. 1998;102:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168:235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanamori A, Nakamura M, Mukuno H, Maeda H, Negi A. Diabetes has an additive effect on neural apoptosis in rat retina with chronically elevated intraocular pressure. Curr Eye Res. 2004;28:47–54 [DOI] [PubMed] [Google Scholar]
- 30. Barber AJ, Lieth E. Agrin accumulates in the brain microvascular basal lamina during development of the blood-brain barrier. Dev Dyn. 1997;208:62–74 [DOI] [PubMed] [Google Scholar]
- 31. Barber AJ, Antonetti DA, Kern TS, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218 [DOI] [PubMed] [Google Scholar]
- 32. Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–3336 [DOI] [PubMed] [Google Scholar]
- 33. Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Invest Ophthalmol Vis Sci. 2008;49:2635–2642 [DOI] [PubMed] [Google Scholar]
- 34. Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006;47:3143–3150 [DOI] [PubMed] [Google Scholar]
- 35. Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000;17:463–471 [DOI] [PubMed] [Google Scholar]
- 36. Scott TM, Foote J, Peat B, Galway G. Vascular and neural changes in the rat optic nerve following induction of diabetes with streptozotocin. J Anat. 1986;144:145–152 [PMC free article] [PubMed] [Google Scholar]
- 37. Lopes de Faria JM, Russ H, Costa VP. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol. 2002;86:725–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chihara E, Matsuoka T, Ogura Y, Matsumura M. Retinal nerve fiber layer defect as an early manifestation of diabetic retinopathy. Ophthalmology. 1993;100:1147–1151 [DOI] [PubMed] [Google Scholar]
- 39. van Dijk HW, Kok PHB, Garvin M, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3404–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Dijk HW, Verbraak FD, Kok PH, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:3660–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45:2760–2766 [DOI] [PubMed] [Google Scholar]
- 42. Ng JS, Bearse MA, Jr, Schneck ME, Barez S, Adams AJ. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;49:1622–1628 [DOI] [PubMed] [Google Scholar]
- 43. Kim SY, Johnson MA, McLeod DS, et al. Retinopathy in monkeys with spontaneous type 2 diabetes. Invest Ophthalmol Vis Sci. 2004;45:4543–4553 [DOI] [PubMed] [Google Scholar]
- 44. Bloodworth JM., Jr Diabetic retinopathy. Diabetes. 1962;11:1–22 [PubMed] [Google Scholar]
- 45. Wolter JR. Diabetic retinopathy. Am J Ophthalmol. 1961;51:1123–1139 [DOI] [PubMed] [Google Scholar]
- 46. Zorumski CF, Olney JW. Excitotoxic neuronal damage and neuropsychiatric disorders. Pharmacol Ther. 1993;59:145–162 [DOI] [PubMed] [Google Scholar]
- 47. Weiss JH, Sensi SL. Ca2+-Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration. Trends Neurosci. 2000;23:365–371 [DOI] [PubMed] [Google Scholar]
- 48. Ambati J, Chalam KV, Chawla DK, et al. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1997;115:1161–1166 [DOI] [PubMed] [Google Scholar]
- 49. Lieth E, Barber AJ, Xu B, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes. 1998;47:815–820 [DOI] [PubMed] [Google Scholar]
- 50. Kowluru RA, Engerman RL, Case GL, Kern TS. Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int. 2001;38:385–390 [DOI] [PubMed] [Google Scholar]
- 51. Lieth E, LaNoue KF, Antonetti DA, Ratz M. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. Exp Eye Res. 2000;70:723–730 [DOI] [PubMed] [Google Scholar]
- 52. Hutson SM, Lieth E, LaNoue KF. Function of leucine in excitatory neurotransmitter metabolism in the central nervous system. J Nutr. 2001;131:846S–850S [DOI] [PubMed] [Google Scholar]
- 53. LaNoue KF, Berkich DA, Conway M, et al. Role of specific aminotransferases in de novo glutamate synthesis and redox shuttling in the retina. J Neurosci Res. 2001;66:914–922 [DOI] [PubMed] [Google Scholar]
- 54. Lieth E, LaNoue KF, Berkich DA, et al. Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J Neurochem. 2001;76:1712–1723 [DOI] [PubMed] [Google Scholar]
- 55. Laabich A, Cooper NG. Neuroprotective effect of AIP on N-methyl-D-aspartate-induced cell death in retinal neurons. Brain Res Mol Brain Res. 2000;85:32–40 [DOI] [PubMed] [Google Scholar]
- 56. Laabich A, Cooper NG. Regulation of calcium/calmodulin-dependent protein kinase II in the adult rat retina is mediated by ionotropic glutamate receptors. Exp Eye Res. 1999;68:703–713 [DOI] [PubMed] [Google Scholar]
- 57. Ward MM, Jobling AI, Kalloniatis M, Fletcher EL. Glutamate uptake in retinal glial cells during diabetes. Diabetologia. 2005;48:351–360 [DOI] [PubMed] [Google Scholar]
- 58. Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells. Invest Ophthalmol Vis Sci. 2002;43:3109–3116 [PubMed] [Google Scholar]
- 59. Puro DG. Diabetes-induced dysfunction of retinal Muller cells. Trans Am Ophthalmol Soc. 2002;100:339–352 [PMC free article] [PubMed] [Google Scholar]
- 60. Mysona B, Dun Y, Duplantier J, Ganapathy V, Smith SB. Effects of hyperglycemia and oxidative stress on the glutamate transporters GLAST and system xc- in mouse retinal Muller glial cells. Cell Tissue Res. 2009;335:477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Santiago AR, Hughes JM, Kamphuis W, Schlingemann RO, Ambrosio AF. Diabetes changes ionotropic glutamate receptor subunit expression level in the human retina. Brain Res. 2008;1198:153–159 [DOI] [PubMed] [Google Scholar]
- 62. Ng YK, Zeng XX, Ling EA. Expression of glutamate receptors and calcium-binding proteins in the retina of streptozotocin-induced diabetic rats. Brain Res. 2004;1018:66–72 [DOI] [PubMed] [Google Scholar]
- 63. Semkova I, Huemmeke M, Ho MS, et al. Retinal localization of the glutamate receptor GluR2 and GluR2-regulating proteins in diabetic rats. Exp Eye Res. 2010;90:244–253 [DOI] [PubMed] [Google Scholar]
- 64. Santiago AR, Rosa SC, Santos PF, Cristovao AJ, Barber AJ, Ambrosio AF. Elevated glucose changes the expression of ionotropic glutamate receptor subunits and impairs calcium homeostasis in retinal neural cells. Invest Ophthalmol Vis Sci. 2006;47:4130–4137 [DOI] [PubMed] [Google Scholar]
- 65. Chakrabarti S, Sima AA, Lee J, Brachet P, Dicou E. Nerve growth factor (NGF), proNGF and NGF receptor-like immunoreactivity in BB rat retina. Brain Res. 1990;523:11–15 [DOI] [PubMed] [Google Scholar]
- 66. Nitta A, Murai R, Suzuki N, et al. Diabetic neuropathies in brain are induced by deficiency of BDNF. Neurotoxicol Teratol. 2002;24:695–701 [DOI] [PubMed] [Google Scholar]
- 67. Seki M, Tanaka T, Nawa H, et al. Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cells. Diabetes. 2004;53:2412–2419 [DOI] [PubMed] [Google Scholar]
- 68. Reiter CE, Gardner TW. Functions of insulin and insulin receptor signaling in retina: possible implications for diabetic retinopathy. Progr Retinal Eye Res. 2003;22:545–562 [DOI] [PubMed] [Google Scholar]
- 69. Reiter CE, Sandirasegarane L, Wolpert EB, et al. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am J Physiol Endocrinol Metab. 2003;285:E763–E774 [DOI] [PubMed] [Google Scholar]
- 70. Reiter CE, Wu X, Sandirasegarane L, et al. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55:1148–1156 [DOI] [PubMed] [Google Scholar]
- 71. Rajala RV, Wiskur B, Tanito M, Callegan M, Rajala A. Diabetes reduces autophosphorylation of retinal insulin receptor and increases protein-tyrosine phosphatase-1B activity. Invest Ophthalmol Vis Sci. 2009;50:1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Enge M, Bjarnegard M, Gerhardt H, et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790 [DOI] [PubMed] [Google Scholar]
- 75. Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl. 2000;77:S26–S30 [DOI] [PubMed] [Google Scholar]
- 76. Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26:1589–1596 [DOI] [PubMed] [Google Scholar]
- 77. Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nature Med. 2003;9:294–299 [DOI] [PubMed] [Google Scholar]
- 78. Dong QY, Cui Y, Chen L, Song J, Sun L. Urinary 8-hydroxydeoxyguanosine levels in diabetic retinopathy patients. Eur J Ophthalmol. 2008;18:94–98 [DOI] [PubMed] [Google Scholar]
- 79. Wakabayashi Y, Usui Y, Shibauchi Y, Uchino H, Goto H. Increased levels of 8-hydroxydeoxyguanosine in the vitreous of patients with diabetic retinopathy. Diabetes Res Clin Pract. 2010;89:e59–e61 [DOI] [PubMed] [Google Scholar]
- 80. Kowluru R, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes or galactosemia, II: comparison of gamma-glutamyl transpeptidase in retina and cerebral cortex, and effects of antioxidant therapy. Curr Eye Res. 1994;13:891–896 [DOI] [PubMed] [Google Scholar]
- 81. Kowluru RA, Kern TS, Engerman RL, Armstrong D. Abnormalities of retinal metabolism in diabetes or experimental galactosemia, III: effects of antioxidants. Diabetes. 1996;45:1233–1237 [DOI] [PubMed] [Google Scholar]
- 82. Kowluru RA, Koppolu P. Diabetes-induced activation of caspase-3 in retina: effect of antioxidant therapy. Free Radic Res. 2002;36:993–999 [DOI] [PubMed] [Google Scholar]
- 83. Kowluru RA, Odenbach S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53:3233–3238 [DOI] [PubMed] [Google Scholar]
- 84. Sasaki M, Ozawa Y, Kurihara T, et al. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Arnal E, Miranda M, Johnsen-Soriano S, et al. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr Eye Res. 2009;34:928–938 [DOI] [PubMed] [Google Scholar]
- 86. Crispim D, Fagundes NJ, dos Santos KG, et al. Polymorphisms of the UCP2 gene are associated with proliferative diabetic retinopathy in patients with diabetes mellitus. Clin Endocrinol. 2010;72:612–619 [DOI] [PubMed] [Google Scholar]
- 87. Cui Y, Xu X, Bi H, et al. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res. 2006;83:807–816 [DOI] [PubMed] [Google Scholar]
- 88. Barnstable CJ. Mitochondria and the regulation of free radical damage in the eye. J Ocul Biol Dis Informatics. 2009;2:145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44:5327–5334 [DOI] [PubMed] [Google Scholar]
- 90. Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mohammad G, Kowluru RA. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab Invest. 2010;90:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Silva PS, Sun JK, Aiello LP. Role of steroids in the management of diabetic macular edema and proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:93–99 [DOI] [PubMed] [Google Scholar]
- 93. Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86:363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wakabayashi Y, Usui Y, Okunuki Y, et al. Correlation of vascular endothelial growth factor with chemokines in the vitreous in diabetic retinopathy. Retina. 2010;30:339–344 [DOI] [PubMed] [Google Scholar]
- 95. Hernandez C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simo R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabetes Med. 2005;22:719–722 [DOI] [PubMed] [Google Scholar]
- 96. Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye. 2006;20:1366–1369 [DOI] [PubMed] [Google Scholar]
- 97. Brucklacher RM, Patel KM, Vanguilder HD, et al. Whole genome assessment of the retinal response to diabetes reveals a progressive neurovascular inflammatory response. BMC Med Genom. 2008;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P. Expression of acute-phase response proteins in retinal Muller cells in diabetes. Invest Ophthalmol Vis Sci. 2005;46:349–357 [DOI] [PubMed] [Google Scholar]
- 99. Zeng HY, Green WR, Tso MO, Zeng H-y, Green WR, Tso MOM. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–232 [DOI] [PubMed] [Google Scholar]
- 100. Gaucher D, Chiappore J-A, Paques M, et al. Microglial changes occur without neural cell death in diabetic retinopathy. Vision Res. 2007;47:612–623 [DOI] [PubMed] [Google Scholar]
- 101. Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980 [PubMed] [Google Scholar]
- 102. Krady JK, Basu A, Allen CM, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565 [DOI] [PubMed] [Google Scholar]
- 103. Mertsch K, Hanisch UK, Kettenmann H, Schnitzer J. Characterization of microglial cells and their response to stimulation in an organotypic retinal culture system. J Comp Neurol. 2001;431:217–227 [PubMed] [Google Scholar]
- 104. Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88:1343–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–230 [DOI] [PubMed] [Google Scholar]
- 106. Joussen AM, Doehmen S, Le ML, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vision. 2009;15:1418–1428 [PMC free article] [PubMed] [Google Scholar]
- 107. Sfikakis PP, Grigoropoulos V, Emfietzoglou I, et al. Infliximab for diabetic macular edema refractory to laser photocoagulation: a randomized, double-blind, placebo-controlled, crossover, 32-week study. Diabetes Care. 2010;33:1523–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fogal B, Hewett SJ. Interleukin-1beta: a bridge between inflammation and excitotoxicity? J Neurochem. 2008;106:1–23 [DOI] [PubMed] [Google Scholar]
- 109. Tezel G. TNF-alpha signaling in glaucomatous neurodegeneration. Progr Brain Res. 2008;173:409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Abcouwer SF, Shanmugam S, Gomez PF, et al. Effect of IL-1beta on survival and energy metabolism of R28 and RGC-5 retinal neurons. Invest Ophthalmol Vis Sci. 2008;49:5581–5592 [DOI] [PubMed] [Google Scholar]
- 111. Vanguilder HD, Brucklacher RM, Patel K, Ellis RW, Freeman WM, Barber AJ. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur J Neurosci. 2008;28:1–11 [DOI] [PubMed] [Google Scholar]
- 112. Kurihara T, Ozawa Y, Nagai N, et al. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes. 2008;57:2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kim, Kim, Kang, Cho, Choi Resveratrol inhibits neuronal apoptosis and elevated Ca2+/calmodulin-dependent protein kinase II activity in diabetic mouse retina. Diabetes. 2010;59:1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sokol S, Moskowitz A, Skarf B, Evans R, Molitch M, Senior B. Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol. 1985;103:51–54 [DOI] [PubMed] [Google Scholar]
- 115. Di Leo MA, Caputo S, Falsini B, et al. Nonselective loss of contrast sensitivity in visual system testing in early type I diabetes. Diabetes Care. 1992;15:620–625 [DOI] [PubMed] [Google Scholar]
- 116. Ismail GM, Whitaker D. Early detection of changes in visual function in diabetes mellitus. Ophthalmic Physiol Opt. 1998;18:3–12 [PubMed] [Google Scholar]
- 117. Lopes de Faria JM, Katsumi O, Cagliero E, Nathan D, Hirose T. Neurovisual abnormalities preceding the retinopathy in patients with long-term type 1 diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2001;239:643–648 [DOI] [PubMed] [Google Scholar]