Patients with central vision loss showed a substantial improvement in reading speed after six sessions of perceptual learning. Perceptual learning might be an effective way of enhancing visual performance for people with central vision loss.
Abstract
Purpose.
Perceptual learning has been shown to be effective in improving visual functions in the normal adult visual system, as well as in adults with amblyopia. In this study, the feasibility of applying perceptual learning to enhance reading speed in people with long-standing central vision loss was evaluated.
Methods.
Six observers (mean age, 73.8) with long-standing central vision loss practiced an oral sentence-reading task, with words presented sequentially using rapid serial visual presentation (RSVP). A pre-test consisted of measurements of visual acuities, RSVP reading speeds for six print sizes, the location of the preferred retinal locus for fixation (fPRL), and fixation stability. Training consisted of six weekly sessions of RSVP reading, with 300 sentences presented per session. A post-test, identical with the pre-test, followed the training.
Results.
All observers showed improved RSVP reading speed after training. The improvement averaged 53% (range, 34–70%). Comparisons of pre- and post-test measurements revealed little changes in visual acuity, critical print size, location of the fPRL, and fixation stability.
Conclusions.
The specificity of the learning effect, and the lack of changes to the fPRL location and fixation stability suggest that the improvements are not due to observers adopting a retinal location with better visual capability, or an improvement in fixation. Rather, the improvements are likely to represent genuine plasticity of the visual system despite the older ages of the observers, coupled with long-standing sensory deficits. Perceptual learning might be an effective way of enhancing visual performance for people with central vision loss.
Reading is difficult and slow for many low vision patients, especially those with central vision loss who are obligated to use their peripheral retina to read. The leading cause of visual impairment in developed countries is age-related macular degeneration (AMD),1–3 which is also the leading cause of central vision loss. Because reading is the most common clinical complaint as well as the primary goal for patients with central vision loss seeking visual rehabilitation,1,4,5 improving the reading performance for these patients is a key challenge facing low vision rehabilitation.
Previous studies have examined a number of ways to improve reading performance in people with central vision loss. For instance, in low vision clinics, patients are routinely prescribed with magnifiers for reading tasks. However, even with magnification, reading speed in people with central vision loss is still lower than that at the normal fovea.5–8 Substantial effort has been invested to determine the mode of text presentation that offers people with central vision loss the fastest reading speed, including page format, scrolling-text in the horizontal or the vertical direction, and rapid serial visual presentation (RSVP), where words are presented one at a time on a display. Most studies found no significant differences in reading speed for different text presentation modes for people with central vision loss.9–11 A handful of studies found a small advantage of using RSVP,12 especially if the word presentation rate varied with word length13 or when observers were allowed to adjust their own presentation rate.14 Other attempts have explored whether simple manipulation of text typography and typesetting such as increasing letter spacing15,16 and line spacing,17 which presumably reduces the crowding effect among text, could improve reading speed. Unfortunately, none of these simple manipulations of text typography or typesetting improve reading speed for people with central vision loss.16,17
In this study, I explored the feasibility of using perceptual learning, a method that has proven to be effective in improving visual functions in normal and amblyopic visual systems, to improve reading speed for people with central vision loss. Perceptual learning is defined as “any relatively permanent and consistent change in the perception of a stimulus array, after practice or experience with this array”.18 Practically, perceptual learning is synonymous with “training” or “practice.”19 Previous studies have shown that visual performance improves with practice for a variety of tasks,19–25 in younger as well as in older adults,26,27 and in the normal fovea and periphery alike.19,27–31 In addition, perceptual learning has also shown effectiveness in improving visual functions in adults with amblyopia (monocular sensory loss of vision in the absence of an organic origin).32–38 In many cases, adults with amblyopia improved not only on the trained task, but their visual acuities (an untrained task) also improved as a result of training.33–37 Considering the effectiveness of perceptual learning in improving visual functions in the normal visual system and in adults with amblyopia, I asked whether perceptual learning would also be effective in improving reading performance for people with central vision loss. Clearly, there are many challenges facing the use of perceptual learning in improving visual functions in people with central vision loss. Specifically, the most common cause of central vision loss is AMD,1–3 which primarily afflicts people older than 65 years of age. It is well known that even though visual performance of older adults can improve with practice, more training may be required before the improvement reaches a plateau26 and that there may be more day-to-day lapses in improvement, which would lead to an overall reduction in the amount of learning.27 Also, in contrast to amblyopia, the majority of people with central vision loss suffer from bilateral vision loss and their functioning retina may not be healthy; whether these would impact the effectiveness of perceptual learning for people with central vision loss is unknown. Hence, despite the promising benefits that perceptual learning can deliver, it remains unclear if people with central vision loss can benefit from it. To my knowledge, there exists no published paper on using perceptual learning to improve visual functions in people with central vision loss, although previous studies have examined whether or not reading performance could be improved by training comprehension,39 or training patients to use a CCTV or stand magnifier to read.40,41 Comprehension training is a cognitive task, and the use of a CCTV or stand magnifier requires motor skills, making it unclear that any improvement from these training represents genuine improvement in the sensory system, which is the basis of perceptual learning. The goal of this study was to determine the feasibility of using perceptual learning to improve reading speed for people with central vision loss.
Previous works have established that reading performance in the normal periphery benefits from perceptual learning based on the following training tasks: identifying random sequences of three letters at various positions across the visual field,19,27,31 performing a lexical decision task,31 and reading.31 The greatest improvement in reading speed was obtained using reading as the training task.31 Consequently, reading was used as the training task in this study.
Methods
Six observers with central vision loss, including four with AMD and two with Stargardt disease, participated in this study. All had long-standing central vision loss (minimum 7 years, see Table 1). Entering visual acuities and other characteristics of the observers are given in Table 1. Although all were avid readers or had the need to read constantly before their vision loss, all observers had stopped reading on a regular basis long before their participation in this study because of their visual deficits. Subjects (S)1 through S3 had previously participated in another study in the laboratory (2–3 hours) in which RSVP was used to measure how reading speed changed with some text manipulation; while S4–S6 had no prior exposure to RSVP reading. All observers gave written informed consent before the commencement of data collection. This research followed the tenets of the Declaration of Helsinki and was approved by the Committee for Protection of Human Subjects at the University of California, Berkeley.
Table 1.
Visual characteristics of the observers
| Observer | Sex | Age (y) | Diagnosis | Acuity (log MAR) |
Years Since Onset | |
|---|---|---|---|---|---|---|
| OD | OS | |||||
| S1 | F | 82 | AMD | 0.50 | 0.52 | 9 |
| S2 | M | 85 | AMD | 0.70 | 0.74 | 11 |
| S3 | M | 84 | AMD | 0.56 | 0.70 | 8 |
| S4 | F | 73 | AMD | 0.66 | 0.48 | 7 |
| S5 | F | 62 | Stargardt | 0.58 | 0.58 | 29 |
| S6 | M | 57 | Stargardt | 1.10 | 1.10 | 40 |
The basic experimental design consisted of a pre-test, six sessions of training, followed by a post-test. The pre-test consisted of measurements of monocular visual acuities, the location of the preferred retinal locus for fixation (fPRL) and fixation stability, and measurements of reading speed as a function of print size using the rapid serial visual presentation (RSVP) paradigm. Details of these procedures are given below. From the reading speed versus print size data obtained at the pre-test, the critical print size, the smallest print size at which maximum reading speed could still be attained, was determined.42 The print size used for training corresponded to 1.4× the critical print size.19 For S1–S5, training consisted of six weekly sessions of RSVP reading. It was shown previously that in the normal periphery, the improvement after perceptual learning was not different whether observers were trained on a daily or a weekly basis,43 justifying my choice of a weekly training schedule in this study, which was more convenient for observers with central vision loss. In each session, observers read a total of 300 sentences, divided into 10 blocks of 30 trials each. Reading speed was determined for each block of 30 trials (average number of words presented per block = 326.3 ± 8.7 [SD]). S6 could complete only seven blocks in the first training session, and eight in each of the subsequent sessions, for a total of 47 blocks over the course of six training sessions. Also, because of his work schedule and illness, he had a three-week gap between training sessions 3 and 4. The post-test, identical with the pre-test except that all measurements were conducted in reversed order, took place a week after the last training session.
Visual Acuity Measurements
Visual acuity was measured monocularly using the Bailey-Lovie high-contrast letter acuity chart.44 Acuities were scored on a letter-by-letter basis, with each letter scoring 0.02 log units.45 All observers were tested at a distance of 10 ft, except for S6 who was tested at 5 ft because of his poorer acuities.
Location of fPRL and Fixation Stability Measurements
The retinal locations used for fixation, and fixation stability of each observer were determined using a scanning laser ophthalmoscope (Rodenstock 101; Rodenstock, Munich, Germany). Observers were asked to look at the center of a fixation cross subtending 1° (2° for observer S6) using their preferred eye. Fundus images were captured continuously for 30 s at a frame rate of 30 Hz. Offline analyses were performed using custom-written software in MATLAB (Mathworks, Natick, MA), and included a frame-by-frame analysis of the retinal locations that the observer used to fixate the cross, and the variability of eye position from frame to frame. To quantify fixation stability, I adopted the conventional method of calculating the bivariate contour ellipse area (BCEA, in deg2)46–49 over each trial of 30 s, which refers to the area of the retinal surface on which the fixation target was imaged 68% of the time. The BCEA value reported in this article, for each observer and for the pre- or post-test, represents the averaged values of two or three trials, collected a few minutes apart.
Reading Speed Measurements
Oral reading speed for single sentences was measured binocularly using the RSVP paradigm. The procedures were very similar to those used in previous studies.15,19,30,42 In brief, on each trial, a single sentence was chosen randomly from a pool of 2630 sentences. Each sentence contained between 8 and 14 words (mean, 10.9 ± 1.7 [SD]) and included only words that were among the 5000 most frequently used words in written English, according to word-frequency tables derived from the British National Corpus.50 Words were rendered in Times Roman font and were presented left-justified on the display, one word at a time in rapid succession, each for a fixed exposure duration. For each block of trials, the Method of Constant Stimuli was used to present sentences at five or six word exposure durations (five for all training sessions, five or six for pre-tests and post-tests depending on observers). The number of words read correctly was recorded for each sentence. A cumulative-Gaussian function was used to fit each set of data (based on 30–36 sentences presented) relating the percentage of words read correctly as a function of exposure duration, from which the reading speed based on the word exposure duration that yielded 80% of the words read correctly was derived.
For the pre- and post-tests, reading speeds were determined for six print sizes spanning 0.75 log units in range, for each observer. A two-line fit (on log-log axes) was used to fit each set of reading speed versus print size data,15,19,30,42 with the slope of the second line constrained to zero. The intersection of the two lines represents the critical print size. The height of the second line (slope = 0) gives the maximum reading speed.
Text stimuli were generated using a Visual Stimulus Generator graphics board (VSG 2/5; Cambridge Research Ltd, Rochester, UK) controlled by a workstation (Dell Precision 650; Dell, Austin, TX) and presented on a 24-inch color graphics display monitor (Model# GDM-FW900; Sony, New York, NY). The resolution of the display was 1280 × 960 pixels, at a frame rate of 80 Hz. The temporal dynamics of the display were verified using a photo-detector and an oscilloscope. Stimuli were black text (2 cd/m2) presented on a white background (144 cd/m2).
Results
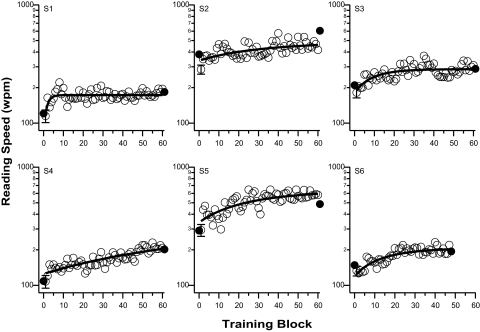
Reading speed (words per minute, wpm) measured using RSVP, is plotted as a function of training block in Figure 1 for each observer. All observers showed improved reading speed with training, although there was substantial individual observer variability. In particular, while the improvement of observer S1 reached a plateau after the first training session, observer S4 continued to improve over the course of the training. The change in reading speed with training can be described by an exponential function of the form (smooth line drawn through each set of data in Fig. 1):
where y0 is the asymptotic reading speed with sufficient training, A is the maximum improvement in reading speed due to training and τ is the time constant. To quantify the improvement, the ratio of reading speed between the last and the first block of training was calculated based on the fitted values. Across the six observers, this ratio ranges between 1.34 and 1.70, with an average of 1.53. In other words, the average improvement in reading speed after six sessions of training was 53% (paired t-test on log reading speed: t(5) = 12.46, P < 0.0001).
Figure 1.
Reading speed (wpm) is plotted as a function of training blocks for the six observers. Filled symbols represent the maximum reading speed obtained at pre- and post-tests (not included in the fitting of the exponential function). In each panel, the solid line represents the best-fit exponential function to all the training blocks. To avoid clutter, only one set of error bars is plotted in each panel (plotted at block 1), representing the averaged SEM across the 60 blocks of trials.
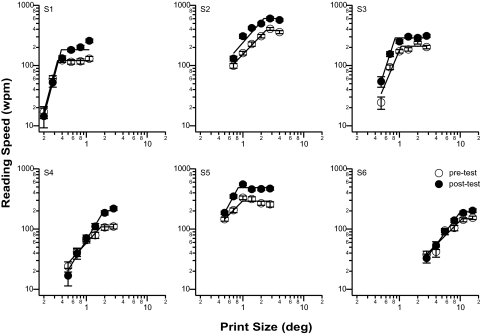
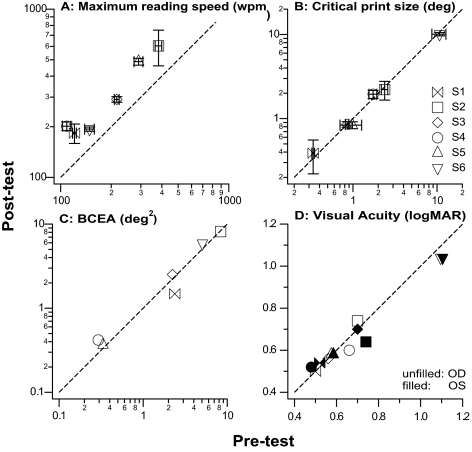
An alternative way to quantify the improvement in reading speed due to training is to compare the maximum reading speed derived from the reading speed versus print size plots before and after training. Such plots are shown in Figure 2 for all six observers. The straight lines in each plot represent the two-line fit. The ratio of the maximum reading speed (the plateau of the two-line fit) after and before training averages 1.55, representing a significant improvement in reading speed (paired t-test on log reading speed: t(5) = 8.24, P = 0.0004). This improvement can also be visualized in Figure 3A, in which the pre- and post-maximum reading speeds are compared for all observers. The dashed line represents the 1:1 line, indicating no change in the maximum reading speed before and after training. All the data points lie above the 1:1 line, implying that the maximum reading speeds improved after training for all observers.
Figure 2.
Reading speed (wpm) is plotted as a function of print size (deg) before (unfilled symbols) and after (filled symbols) training, for each observer. Each set of data was fit with a two-line fit on log-log axes from which the maximum reading speed and the critical print size were derived. Error bars represent ± 1 SEM.
Figure 3.
Post-test values are compared with pre-test values for (A) maximum reading speed (wpm), (B) critical print size (deg), (C) BCEA (deg2), and (D) visual acuity (logMAR). Each symbol represents one observer except for (D), where each symbol represents one eye of each observer. Dashed line is the 1:1 line, representing no change in the value after training. (D) Acuities for the right (unfilled symbols) and left (filled symbols) eyes are plotted separately. For observers S5 and S6, the acuities and the changes after training were identical for both the right and left eyes. To avoid clutter, these data points (upward-pointing and downward-pointing triangles) are slightly shifted along the abscissa.
A characteristic of perceptual learning is its specificity.20,21,23,30 To examine if the training effect transfers to an improvement in critical print size and/or visual acuity, the pre- and post-test critical print size and visual acuities were compared in Figures 3B and 3D were compared. Clearly, all data points fall very close to the 1:1 line, implying that neither the critical print size (averaged post/pre ratio = 1.0; paired t(5) = 0.95, P = 0.38) nor the visual acuity (averaged post/pre ratio = 0.99; paired t(11) = 1.17, P = 0.27) changed after training.
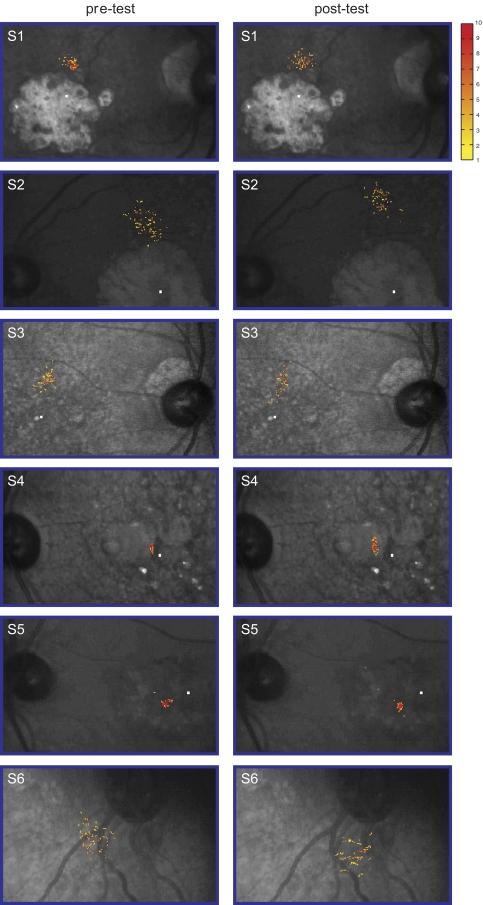
To determine whether the improvement in reading speed was due to observers adopting a different PRL with better visual capabilities, or that observers learned to maintain steadier fixation, the location of the fPRL and fixation stability of each observer before and after training were compared. Figure 4 shows the fixation frequency distribution—the frequency distribution of the retinal locations used for fixation—superimposed on the fundus image, for each observer, before and after training. Clearly, the region over which most of the fixation occurred (roughly representing the fPRL), and the spread of the region, did not change substantially before and after training. To quantify the fixation stability, the conventional measurement of BCEA was adopted.46–49 Figure 3C shows that the BCEA (in deg2) of the observers was practically the same before and after training (paired t(5) = 0.13, P = 0.90), suggesting that the improvement in reading speed after training cannot be attributed to the adoption of a different PRL with better visual capabilities or the oculomotor system becoming more stable.
Figure 4.
The frequency distribution of the retinal locations used for fixation is superimposed on the fundus picture of each observer, before (pre-test) and after (post-test) training. The absolute number of fixation for each location is coded by color, with the key given on the right. The frequency distribution in each panel was based on one trial, randomly selected among the three repeated testings for the same condition, for each observer. The small white rectangle in each picture (except for S6) represents the location of the anatomical fovea.
Discussion
After six weekly sessions of repeated training on an RSVP reading task, observers with central vision loss were able to improve their reading speeds by an average of 53%. This improvement did not transfer to visual acuity and critical print size measurements, implying that even though observers were able to read faster, they were not able to read smaller letters on an acuity chart or text of smaller sizes. The improvement cannot be attributed to a change in the location of the fPRL or better oculomotor control (steadier fixation). These results provide evidence for neural plasticity in human adults with long-standing central vision loss.
Neural plasticity in adults with sensory visual deficits is not at all a new concept. For over a decade, many studies have reported that human adults with amblyopia benefit from perceptual learning, in that many visual functions, including visual acuity, can improve through training.32–38 Because of the substantial benefits, perceptual learning has recently been proposed as a treatment for adult amblyopia.37,38 With respect to people with central vision loss, it is well known that after the loss of central vision, many of these individuals eventually adopt a retinal location (sometimes more than one) outside the afflicted macular area to serve as the PRL.51–58 This in itself is strong evidence that even for people with central vision loss, many of whom are elderly suffering from AMD, the visual cortex is still malleable and able to adapt to unfavorable visual experience. Therefore, it is not surprising that these people can benefit from perceptual learning.
Perceptual learning is known for its specificity,20,21,23,30 a characteristic that distinguishes itself from general practicing of a task. In this study, the specific improvements related to reading speed, but not to visual acuity or fixation stability, lend support to the argument that the observed improvements are indeed the consequence of perceptual learning, rather than to general training, the continued adaptation to the vision loss, or recent changes in central vision. Given the specificity of the improvements, an important and practical question that follows is whether the improvement in RSVP reading speed would generalize to the conventional page-reading task. Because page-reading requires more eye movements (inter-word saccades and return sweeps) than RSVP reading, it is possible that improvements in RSVP reading speed may not generalize to page-reading if eye-movement control is the primary limiting factor on page-reading. However, compared with people with intact central vision, patients with central vision loss do not benefit as much from RSVP reading, suggesting that the limitation of eye movements on reading may not be as important for people with central vision loss as for people with intact central fields. Further, there is evidence that RSVP and page-format reading show similar dependence on certain text parameters such as letter spacing.59 Therefore, it would be interesting to test in future studies whether improvements after RSVP training would transfer to page-reading, and whether RSVP training and page-reading training are equally effective in improving reading speed for patients with central vision loss.
Another characteristic of perceptual learning is the substantial individual observer variability with respect to the time course and the amount of improvement. In fact, it has been shown that up to 25–50% of observers failed to show improvement after training.29,60 In the present study, the six observers demonstrated variability in their time course of improvement (Fig. 1), arguing that a tailored amount of training for individual observers may be more appropriate than a one-size-fits-all approach. This is an important issue to resolve, not only from a scientific point of view, but also because the results may directly influence policy-makers in deciding on the number of training sessions that should be covered by health care plans, should perceptual learning be adopted as a rehabilitative option for patients with central vision loss.
Given these results, a logical question to ask is what underlies the improvements. A psychophysical approach to answer this question is to evaluate how observer performance is affected by the presence of different amount of external noise superimposed on the stimulus. The basis of this approach is to attribute the limitation in human performance to (1) the presence of internal noise in the visual system that limits the precision of perceptual responses and (2) the inability of the visual system to make full use of the information available in the stimulus.24,25,29,61,62 The mechanism underlying perceptual learning can then be inferred, by tracking how performance changes with different levels of external noise with time. In this study, because reading performance in the presence of external noise was not measured, the functional mechanism that underlies learning could not be inferred. However, based on previous studies for a variety of training tasks, the improvement after training is most likely due to the visual system being more capable of extracting the crucial information from the stimulus.24,25,29,61,62
A few caveats should be kept in mind while evaluating the interpretations presented here. First, I showed that the location of the fPRL did not change substantially after training. Because people with central vision loss can adopt different PRLs for different tasks,51–58 the data presented in this study cannot convincingly rule out the possibility that observers adopt an alternative retinal location that has better visual capability for the reading task. Currently, a larger-scale perceptual learning study is being designed. It will include the PRL for reading as a pre-post comparison measurement. Note that the measurements of fPRL were made monocularly, while the training was performed binocularly; therefore, it is also plausible that the characteristics of the binocular PRL could have changed as a result of training. Unfortunately, there is currently no known method to measure the binocular PRL. Second, considering that this was a feasibility study, a no-training control group was not included. However, it is easy to envision that a no-training control group is unlikely to show improvement because in real life, patients with central vision loss almost never show improvement in reading performance without practicing the use of their residual vision. A rough estimate of the improvement shown by a no-training control group based on simply performing the RSVP reading task twice (pre- and post-test) was 7–10%, as reported in previous studies.19,31 Considering that observers who receive training spend more time in the laboratory than those who do not receive any training, to control for the time spent in the laboratory, or the amount of interaction with laboratory personnel and the attention received, a better control design is to train another group of observers on a task that is unrelated to the training task being studied. In a previous study,31 three groups of normally sighted observers were trained on three different psychophysical tasks — lexical decision, trigram letter recognition, and RSVP reading, for a similar amount of time. A fourth group of observers did not receive any training. The group that was trained on RSVP reading yielded the largest magnitude of improvement (averaged 72%) while the improvements for the other two training groups averaged 40–50%, with the improvement for the no-training group being 10%. The differential magnitudes of improvement for the different training groups confirm that the improvements after training on an RSVP task are not due to psychological or general improvements because the observers spent long periods of time in the laboratory or because they received lots of attention from the laboratory personnel. Also, the largest magnitude of improvement exhibited by the RSVP training group is consistent with the specificity of perceptual learning,20,21,23,30 rather than just an improvement due to general training on any psychophysical tasks.
Clearly, if perceptual learning is to be used clinically to benefit patients with central vision loss, other issues have to be considered. For example, does the improvement after RSVP training transfer to a real-life page-reading task that involves a higher oculomotor demand? Will other modes of reading such as large-print page-reading be equally effective as a training task? Should the amount of training be tailored to each individual observer instead of giving the same amount of training to all observers? What is the optimal training duration? Does the improvement in reading speed also help improve the comprehension of the reading materials? These are all interesting questions which will be addressed in future studies.
Acknowledgments
The author thanks Jean-Baptiste Bernard for assistance with the presentation of the fixation frequency distribution data.
Footnotes
Supported by NIH Grant R01-EY012810.
Disclosure: S.T.L. Chung, None
References
- 1. Elliott DB, Trukolo-Ilic M, Strong JG, Pace R, Plotkin A, Bevers P. Demographic characteristics of the vision-disabled elderly. Invest Ophthalmol Vis Sci. 1997;38:2566–2575 [PubMed] [Google Scholar]
- 2. Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485 [DOI] [PubMed] [Google Scholar]
- 3. Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PTVM, Nemesure B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572 [DOI] [PubMed] [Google Scholar]
- 4. Kleen SR, Levoy RJ. Low-vision care: correlation of patient age, visual goals, and aids prescribed. Am J Optom Physiol Opt. 1981;58:200–205 [PubMed] [Google Scholar]
- 5. Bullimore MA, Bailey IL. Reading and eye movements in age-related maculopathy. Optom Vis Sci. 1995;72:125–138 [DOI] [PubMed] [Google Scholar]
- 6. Legge GE, Rubin GS, Pelli DG, Schleske MM. Psychophysics of reading. II. Low vision. Vision Res. 1985;25:253–265 [DOI] [PubMed] [Google Scholar]
- 7. Whittaker SG, Lovie-Kitchin J. Visual requirements for reading. Optom Vis Sci. 1993;70:54–65 [DOI] [PubMed] [Google Scholar]
- 8. Chung STL. Enhancing visual performance for people with central vision loss. Optom Vis Sci. 2010;87:276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harland S, Legge GE, Luebker A. Psychophysics of reading. XVII. Low-vision performance with four types of electronically magnified text. Optom Vis Sci. 1998;75:183–190 [DOI] [PubMed] [Google Scholar]
- 10. Fine EM, Peli E. Scrolled and rapid serial visual presentation texts are read at similar rates by the visually impaired. J Opt Soc Am A. 1995;12:2286–2292 [DOI] [PubMed] [Google Scholar]
- 11. Bowers AR, Woods RL, Peli E. Preferred retinal locus and reading rate with four dynamic text presentation formats. Optom Vis Sci. 2004;81:205–213 [DOI] [PubMed] [Google Scholar]
- 12. Rubin GS, Turano K. Low vision reading with sequential word presentation. Vision Res. 1994;34:1723–1733 [DOI] [PubMed] [Google Scholar]
- 13. Aquilante K, Yager D, Morris RA, Khmelnitsky F. Low-vision patients with age-related maculopathy read RSVP faster when word duration varies according to word length. Optom Vis Sci. 2001;78:290–296 [DOI] [PubMed] [Google Scholar]
- 14. Arditi A. Elicited sequential presentation for low vision reading. Vision Res. 1999;39:4412–4418 [DOI] [PubMed] [Google Scholar]
- 15. Chung STL. The effect of letter spacing on reading speed in central and peripheral vision. Invest Ophthalmol Vis Sci. 2002;43:1270–1276 [PubMed] [Google Scholar]
- 16. Chung STL. Two approaches to reduce crowding do not lead to improved reading speed in patients with age-related macular degeneration. Presented at VISION, London, England, April 5, 2005 [Google Scholar]
- 17. Chung STL, Jarvis SH, Woo SY, Hanson K, Jose RT. Reading speed does not benefit from increased line spacing in AMD patients. Optom Vis Sci. 2008;85:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gibson EJ. Perceptual learning. Annu Rev Psychol. 1963;14: 29–56 [DOI] [PubMed] [Google Scholar]
- 19. Chung STL, Legge GE, Cheung SH. Letter-recognition and reading speed in peripheral vision benefit from perceptual learning. Vision Res. 2004;44:695–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287:43–44 [DOI] [PubMed] [Google Scholar]
- 21. Ball KK, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–698 [DOI] [PubMed] [Google Scholar]
- 22. Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci USA. 1991;88:4966–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406 [DOI] [PubMed] [Google Scholar]
- 24. Gold J, Bennett PJ, Sekuler AB. Signal but not noise changes with perceptual learning. Nature. 1999;402:176–178 [DOI] [PubMed] [Google Scholar]
- 25. Dosher BA, Lu Z-L. Mechanisms of perceptual learning. Vision Res. 1999;39:3197–3221 [DOI] [PubMed] [Google Scholar]
- 26. Richards E, Bennett PJ, Sekuler AB. Age related differences in learning with the useful field of view. Vision Res. 2006;46:4217–4231 [DOI] [PubMed] [Google Scholar]
- 27. Yu D, Cheung SH, Legge GE, Chung STL. Reading speed in the peripheral visual field of older adults: Does it benefit from perceptual learning? Vision Res. 2010;50:860–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beard BL, Levi DM, Reich LN. Perceptual learning in parafoveal vision. Vision Res. 1995;35:1679–1690 [DOI] [PubMed] [Google Scholar]
- 29. Chung STL, Levi DM, Tjan BS. Learning letter identification in peripheral vision. Vision Res. 2005;45:1399–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chung STL. Learning to identify crowded letters: Does it improve reading speed? Vision Res. 2007;47:3150–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu D, Legge GE, Park H, Gage E, Chung STL. Development of a training protocol to improve reading performance in peripheral vision. Vision Res. 2010;50:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levi DM. Perceptual learning in adults with amblyopia: a reevaluation of critical periods in human vision. Dev Psychobiol. 2005;46:222–232 [DOI] [PubMed] [Google Scholar]
- 33. Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proc Natl Acad Sci USA. 1996;93:6830–6834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci USA. 2004;101:6692–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou Y, Huang C, Xu P, Tao L, Qiu Z, Li X, Lu ZL. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Res. 2006;46:739–750 [DOI] [PubMed] [Google Scholar]
- 36. Huang CB, Zhou Y, Lu ZL. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc Natl Acad Sci USA. 2008;105:4068–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: a mini-review. Vision Res. 2009;49:2535–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Polat U. Making perceptual learning practical to improve visual functions. Vision Res. 2009;49:2566–2573 [DOI] [PubMed] [Google Scholar]
- 39. Watson GR, Wright V, De l'Aune W. The efficacy of comprehension training and reading practice for print readers with macular loss. J Visual Impair Blin. 1992;86:37–43 [Google Scholar]
- 40. Goodrich GL, Mehr EB, Quillman RD, Shaw HK, Wiley JK. Training and practice effects in performance with low-vision aids: a preliminary study. Am J Optom Physiol Opt. 1977;54:312–318 [DOI] [PubMed] [Google Scholar]
- 41. Cheong AMY, Lovie-Kitchin JE, Bowers AR, Brown B. Short-term in-office practice improves reading performance with stand magnifiers for people with AMD. Optom Vis Sci. 2005;82:114–127 [DOI] [PubMed] [Google Scholar]
- 42. Chung STL, Mansfield JS, Legge GE. Psychophysics of reading. XVIII. The effect of print size on reading speed in normal peripheral vision. Vision Res. 1998;38:2949–2962 [DOI] [PubMed] [Google Scholar]
- 43. Truong S, Arunkumar A, Yu D, Chung STL. A comparison of the effectiveness of perceptual learning on a daily vs. weekly basis Optom Vis Sci. 2009;86:E–abstract 90842 [Google Scholar]
- 44. Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt. 1976;53:740–745 [DOI] [PubMed] [Google Scholar]
- 45. Ferris FL, Kassoff A, Bresnick GH, Bailey IL. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96 [PubMed] [Google Scholar]
- 46. Timberlake GT, Sharma MK, Grose SA, Gobert DV, Gauch JM, Maino JH. Retinal location of the preferred retinal locus relative to the fovea in scanning laser ophthalmoscope images. Optom Vis Sci. 2005;82:177–185 [DOI] [PubMed] [Google Scholar]
- 47. Steinman RM. Effects of target size, luminance, and color on monocular fixation. J Opt Soc Am. 1965;55:1158–1165 [Google Scholar]
- 48. Crossland MD, Sims M, Galbraith RF, Rubin GS. Evaluation of a new quantitative technique to assess the number and extent of preferred retinal loci in macular disease. Vision Res. 2004;44:1537–1546 [DOI] [PubMed] [Google Scholar]
- 49. Crossland MD, Culham LE, Rubin GS. Fixation stability and reading speed in patients with newly developed macular disease. Ophthal Physiol Opt. 2004;24:327–333 [DOI] [PubMed] [Google Scholar]
- 50. Kilgarriff A. Putting frequencies in the dictionary. Int J Lexicogr. 1997;10:135–155 [Google Scholar]
- 51. Cummings R, Whittaker S, Watson G, Budd J. Scanning characters and reading with a central scotoma. Am J Optom Physiol Opt. 1985;62:833–843 [DOI] [PubMed] [Google Scholar]
- 52. Timberlake GT, Mainster MA, Peli E, Augliere RA, Essok EA, Arend LE. Reading with a macular scotoma I. Retinal location of scotoma and fixation area. Invest Ophthalmol Vis Sci. 1986;27:1137–1147 [PubMed] [Google Scholar]
- 53. Timberlake GT, Peli E, Essok EA, Augliere RA. Reading with a macular scotoma II. Retinal locus for scanning text. Invest Ophthalmol Vis Sci. 1987;28:1268–1274 [PubMed] [Google Scholar]
- 54. Whittaker SG, Budd JM, Cummings RW. Eccentric fixation with macular scotoma. Invest Ophthalmol Vis Sci. 1988;29:268–278 [PubMed] [Google Scholar]
- 55. White JM, Bedell HE. The oculomotor reference for humans with bilateral macular disease. Invest Ophthalmol Vis Sci. 1990;31:1149–1161 [PubMed] [Google Scholar]
- 56. Guez J-E, Le Gargasson J-F, Rigaudiere F, O'Regan JK. Is there a systematic location for the pseudo-fovea in patients with central scotoma? Vision Res. 1993;33:1271–1279 [DOI] [PubMed] [Google Scholar]
- 57. Fletcher DC, Schuchard RA. Preferred retinal loci relationship to macular scotomas in a low-vision population. Ophthalmol. 1997;104:632–638 [DOI] [PubMed] [Google Scholar]
- 58. Schuchard RA. Preferred retinal loci and macular scotoma characteristics in patients with age-related macular degeneration. Can J Ophthalmol. 2005;40:303–312 [DOI] [PubMed] [Google Scholar]
- 59. Yu D, Cheung SH, Legge GE, Chung STL. Effect of letter spacing on visual span and reading speed. J Vis. 2007;7(2):2,1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fahle M, Henke-Fahle S. Interobserver variance in perceptual performance and learning. Invest Ophthalmol Vis Sci. 1996;37:869–877 [PubMed] [Google Scholar]
- 61. Lu Z-L, Dosher BA. Characterizing observers using external noise and observer models: Assessing internal representations with external noise. Psych Rev. 2008;115:44–82 [DOI] [PubMed] [Google Scholar]
- 62. Lu Z-L, Dosher BA. Mechanisms of perceptual learning. Learn Percept. 2009;1:19–36 [DOI] [PMC free article] [PubMed] [Google Scholar]