The conventional (size III) perimetric stimulus was superior to a frequency-doubling stimulus in preferentially stimulating magnocellular (M) retinal ganglion cells relative to parvocellular (P) cells.
Abstract
Purpose.
Perimetry is used clinically to assess glaucomatous ganglion cell loss. It has been proposed that frequency-doubling stimuli are better than the conventional size III perimetric stimulus in preferentially stimulating magnocellular (M) versus parvocellular (P) ganglion cells. However, little is known about how primate ganglion cells respond to perimetric stimuli. The authors recorded contrast responses of M and P ganglion cells to size III and frequency-doubling stimuli and compared contrast gain of M and P cells to these stimuli to assess the ability of these stimuli to preferentially stimulate M versus P cells.
Methods.
Data were recorded from 69 macaque retinal ganglion cells, by an in vivo preparation, at eccentricities of 5° to 15°. The size III stimulus was a circular luminance increment 26 min arc in diameter, 200 ms in duration. The frequency-doubling stimulus was a sinusoidal grating (0.5 cyc/deg) temporally modulated in counterphase at 13 Hz. A Michaelis-Menten function was fit to each cell's contrast responses to assess contrast gain.
Results.
For both size III and frequency-doubling stimuli, ganglion cell responses increased linearly at low contrasts, and then the increase slowed at high contrasts (saturation). The mean (± SE) difference in estimated log contrast gain between M and P cells for the size III stimulus was significantly higher than that for the frequency-doubling stimulus (1.24 ± 0.09 vs. 0.89 ± 0.13; P < 0.01).
Conclusions.
The size III stimulus was superior to the frequency-doubling stimulus in preferentially stimulating M cells versus P cells.
Perimetry is used clinically to assess visual loss in patients with glaucoma by measuring psychophysical detection thresholds throughout the central visual field. The primary cause of glaucomatous perimetric defects is damage to retinal ganglion cells. Perimetric stimuli were standardized by Goldmann,1 who used a 2.4 log unit range of stimulus sizes and a 2 log unit range of stimulus contrasts. When perimetry was automated, only a single stimulus size was used, which Goldmann termed the size III stimulus, and the range of stimulus contrasts was increased to >3 log units.2
For several decades it has been proposed that detection of glaucomatous defects could be improved by using stimuli that preferentially stimulate the magnocellular (M) ganglion cells relative to parvocellular (P) ganglion cells, based on the notion that M cells are selectively damaged in glaucoma.3–6 This led to the development of a new form of clinical perimetry using frequency-doubling stimuli, which were thought to provide better separation of M and P responses than conventional perimetric stimuli.7,8 The name derives from the illusion that there is an apparent doubling in spatial frequency with contrast reversal of gratings at high temporal frequencies (>10 Hz). For frequency-doubling stimuli, the range of contrasts used in clinical testing is approximately 1.5 log units (3%–100% contrast). Frequency-doubling perimetry was proposed to tap sensitivity in a hypothetical, separate My ganglion cell class.9 In reality it taps the sensitivity of standard M cells.10
Nevertheless, early reports that M cells are preferentially damaged in glaucoma were not confirmed in later studies and might have been an artifact caused by cell shrinkage.11–14 Psychophysical studies that have directly compared responses of M and P pathways have found that visual losses are similar for both pathways.15–19 Clinical studies comparing frequency-doubling perimetry and conventional perimetry have found that glaucomatous losses are comparable for the two types of perimetry.20–31
To better understand how to interpret test results obtained with frequency-doubling and conventional perimetric stimuli, it would be useful to assess the responsiveness of M and P ganglion cells to these stimuli at various contrast levels. We measured contrast responses of primate M and P ganglion cells to the conventional perimetric stimulus and a frequency-doubling stimulus and compared contrast gains of M and P cells to assess the ability of these two types of stimuli to preferentially stimulate M cells.
Methods
Preparation for Ganglion Cell Recordings
Ganglion cell activity was recorded from the retinas of six juvenile macaques (Macaca fascicularis). After initial intramuscular injection of ketamine hydrochloride as a premedication (10 mg/kg), anesthesia was induced with thiopental (10 mg/kg) and was maintained with isoflurane in a 70%:30% N2O-O2 mixture (1%–2% during surgery and 0.2%–1% during recording). Local anesthetic was applied to points of surgical intervention. The electroencephalogram and the electrocardiogram were continuously monitored as a control for anesthetic depth. Muscular relaxation was maintained by intravenous infusion of gallamine triethiodide (5 mg/kg/h) together with approximately 6 mL/h/kg dextrose Ringer. End-tidal CO2 was kept near 4% by adjusting the rate and depth of ventilation, and body temperature was maintained near 37.5°C. Positions of the fovea and the optic disc were ascertained with the aid of a fundus camera. Clarity of the optic media was checked frequently, and, if the smaller retinal vessels could no longer be recognized, recording from that eye was terminated and the second eye was prepared. On completion of recording, the animal was killed with an overdose of barbiturate. All procedures were approved by an on-campus Institutional Animal Care and Use Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Neuronal activity was recorded directly from retinal ganglion cells by an electrode inserted through a cannula entering the eye behind the limbus. The details of the preparation can be found elsewhere.32 A contact lens with the internal radius matched to the corneal curvature was used to focus the eye on a back projection tangent screen that was 226 cm from the animal's tested eye. The screen was used to identify and record the loci of cell receptive–field centers. Classification of M or P cell type was based on the cell's responses to achromatic or chromatic stimuli presented either on a CRT screen or on a Maxwellian view optical system.33 M cells were generally identified by their phasic responses, high responsivity to achromatic contrast, and frequency-doubled temporal responses to red-green chromatic stimuli. P cells were identified by their tonic responses and spectral opponency.
Apparatus and Stimuli
Visual stimuli were generated (Visual Stimulus Generator; Cambridge Research Systems, Rochester, UK) and were presented on a CRT monitor (Trinitron, frame rate 100 Hz; Sony, Tokyo, Japan) that was 226 cm from the animal's eye. For the conventional size III perimetric stimulus, we used a circular field (26 min arc in diameter) presented as luminance increments (200-ms duration, interstimulus interval 300 ms) on a 10 cd/m2 background. These parameters are the same as the basic parameters of the size III stimulus used in conventional automated perimetry. Stimulus contrast was expressed as Weber contrast, ΔL/L, with L as background luminance (10 cd/m2), and ΔL as the luminance increment of the circular stimulus. The values for ΔL varied from 0.5 cd/m2 to 50 cd/m2, resulting in a Weber contrast range from 5% to 500%. All cells were tested with stimulus contrasts up to 100%; a subset was also tested with contrasts of 200% and 500%. Clinically, perimetric contrast sensitivity (the reciprocal of threshold contrast) for the size III stimulus is expressed in decibel units, where a contrast sensitivity of 1 (Weber contrast of 100%) is referred to as 25 dB on automated perimetry (Humphrey Field Analyzer; Carl Zeiss Ophthalmic Systems, Dublin, CA)34 and a decrease by 1 dB corresponds to an 0.1 log unit increase in stimulus contrast. In these units, our stimuli ranged from 38 dB (5% contrast) to 18 dB (500% contrast).
The frequency-doubling stimulus was a 0.5 cyc/deg sinusoidal grating modulated in counterphase at 13 Hz (mean luminance, 30 cd/m2). These are within the range of spatial and temporal frequencies used for clinical frequency-doubling perimetry. Stimulus contrast was expressed as Michelson contrast (peak − trough)/(peak + trough), which, for sinusoidal gratings, is equivalent to Weber contrast; contrasts ranged from 0.8% to 100%. For frequency-doubling perimetry, contrast sensitivity (the reciprocal of threshold contrast for a frequency-doubling stimulus) is expressed in decibel units, where a contrast sensitivity of 1 (Weber contrast of 100%) is referred to as 0 dB and a decrease by 1 dB corresponds to a 0.05 log unit increase in stimulus contrast. In these units, our stimulus contrasts ranged from 42 dB (0.8% contrast) to 0 dB (100% contrast).
Recording of Ganglion Cell Responses
We recorded spike trains from 69 retinal ganglion cells at eccentricities between 5° and 15°: 41 M cells (24 M-on, 17 M-off) and 28 P cells (11 red-on, 2 red-off, 12 green-on, 3 green-off). The response histogram for each cell and stimulus was averaged across 30 stimulus repetitions, with a 10-ms bin width for the size III stimulus, and was averaged across 100 stimulus cycles with a 2.4-ms bin width for the frequency-doubling stimulus (77.8 ms per cycle). In most experiments, the stimuli were centered on each cell's receptive field. For a subset of 25 cells, further measurements were performed with the size III stimulus positioned at a series of offsets, in steps of 10 to 15 min arc compared with the center of the receptive field.
Data Analysis
To obtain a cell's contrast response functions, mean spike rate was calculated for each stimulus type and each contrast. Mean spike rate for the size III stimulus was computed as the average spike rate across all 30 trials at that contrast. We counted spikes for a 40-ms observation window beginning after a latency of 15 ms after stimulus onset (for on-cells) or offset (for off-cells). The average number of spikes over the 40-ms window was expressed as firing rate (impulses/s). Spike firing rates did not increase when the window was made longer than 40 ms, consistent with a psychophysical critical duration of no more than 40 ms for luminance increments.35 Mean spike rate for the frequency-doubling stimulus was computed as the amplitude of the fundamental component from Fourier analysis of averaged response histograms. We only considered first harmonic amplitude, which is dominant compared with second or higher harmonics for contrast levels near threshold. A model for central detection of sinusoidal stimuli using a window of fixed time width36 yielded results similar to those presented here.
For each cell and stimulus, contrast response functions (i.e., mean spike rate, number of impulses per second minus baseline firing versus contrast), were fit with a Michaelis-Menten function:
where R is spike rate at contrast c minus baseline firing rate, G is contrast gain (impulses per second per percentage contrast), and Rmax is maximum spike rate minus baseline firing rate. At low contrasts, the spike rate R increases linearly with contrast c with a slope of G, at high contrasts R asymptotes to Rmax, and when contrast equals the semisaturation contrast (Rmax/G), R reaches half of Rmax. Some of the P cells showed only small amounts of saturation, which means that Rmax is poorly constrained by the data and can become unreasonably high (over 1000 spikes/s). Therefore, a maximum of 500 spikes/s was set for Rmax; for some poorly responsive P cells, this maximum was reached because the semisaturation contrast was higher than the maximum stimulus. In these cases, fits were repeated with Rmax = 300 spikes/s, and in all cases G did not change by more than 0.2 log unit.
For each cell and stimulus, the contrast response function was fitted by minimizing the χ2 value using the Levenberg-Marquardt algorithm, with graphing and data analysis software (Igor Pro, version 6.1.2; Wavemetrics, Portland, OR). The fitted contrast gains (in log units) were then used for statistical analysis. For descriptive analysis, the means and associated standard deviations are reported. Given that some cells were measured with both stimulus types (n = 25; 19 M cells and 6 P cells) while other cells were tested with only one stimulus type, we used a mixed linear model to account for potential correlation among repeated measurements on the same cells. The mixed linear model tested the effects of stimulus type, cell type, and their interaction on log contrast gain. A significant interaction between cell type and stimulus type would indicate a difference between size III and frequency-doubling stimuli in differentiating M cell versus P cell responses. We first conducted mixed linear modeling with all cell data and then with data only from those cells that were measured with both stimulus types. The differences in contrast gains and associated standard errors (SE) estimated from the mixed linear model are reported. The statistical analyses were conducted using statistical software (SAS 9.1.3; SAS Institute, Cary, NC).
Results
We first showed response histograms to size III and frequency-doubling stimuli for both M and P cells. Next we plotted contrast response functions for M and P cells and estimated contrast gain by fitting the Michaelis-Menten function shown in equation 1. Then we compared the contrast gains of M and P cells and the semisaturation contrasts. Finally, we examined the effect of stimulus location for the size III stimulus.
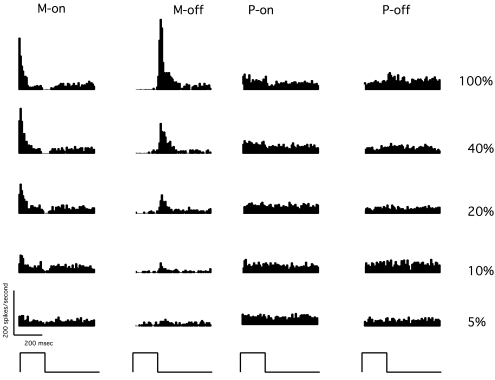
Figure 1 shows response histograms for an M-on, an M-off, a P-on, and a P-off cell to the size III stimulus at contrast levels from 5% to 100% (38–25 dB). At 100% contrast (Fig. 1, top), spike rates for both M and P cells can be seen to be related to the temporal presentation of the stimuli; the M-on cell and M-off cell gave transient responses to the onset and offset of the pulse, respectively, and the P cells gave sustained responses during stimulus duration (P-on cells) or after stimulus offset (P-off cells). M cells were more responsive than P cells, and they showed visible responses at lower contrasts near psychophysical threshold (e.g., 10% contrast, 35 dB), whereas P cells did not.
Figure 1.
Response histograms for the size III stimulus, from four representative cells. Each histogram has 10-ms bin width and is averaged across 30 trials. Bottom: time course of the 200-ms stimulus presentation. Right: stimulus contrasts.
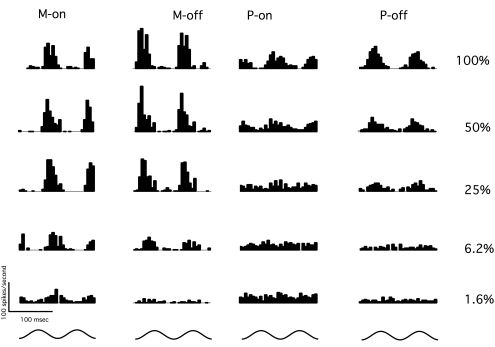
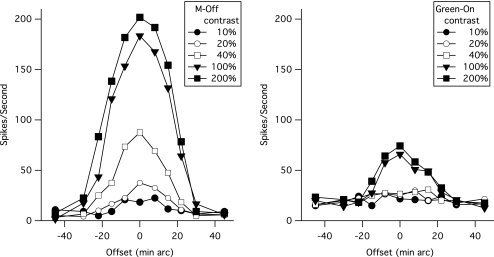
Figure 2 shows response histograms for the same cells shown in Figure 1, for the frequency-doubling stimulus at various contrast levels. Each histogram shows cell responses to two stimulus cycles. When stimulus contrast is at maximum (Fig. 1, top), both M and P cells gave vigorous responses. At lower contrasts near psychophysical threshold (e.g., 6.2%), the response to the stimulus is visible in the M cells' histograms but not the P cells' histograms. In general, M cells were more responsive than P cells and showed more nonlinearity.
Figure 2.
Response histograms for the frequency-doubling stimulus from the same cells shown in Figure 1. Each histogram has 2.4-ms bin width and is averaged across 100 trials. Bottom: time course of the 13-Hz stimulus presentation over two cycles. Right: stimulus contrasts.
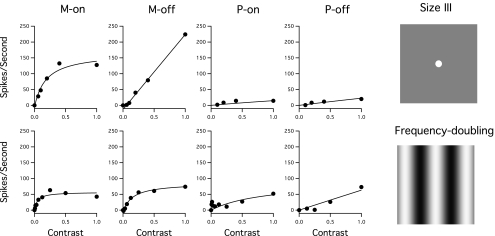
Figure 3 shows contrast response functions for the size III (Fig. 3, top) and frequency-doubling (Fig. 3, bottom) stimuli for the same M and P cells shown in Figures 1 and 2. For both size III and frequency-doubling stimuli, the firing rates for M cells increased linearly at lower contrasts and then showed saturation at higher contrasts, particularly with the frequency-doubling stimulus. For P cells the responses increased more slowly with contrast, and there was little saturation at higher contrasts.
Figure 3.
Contrast responses for the size III stimulus (top) and the frequency-doubling stimulus (bottom) for the same cells illustrated in Figures 1 and 2. Circles: spike rates for different contrasts. Curves: Michaelis-Menten functions fit to the data. For consistency between stimuli, data are shown only up to 100% stimulus contrast. Right: size III and frequency-doubling stimuli.
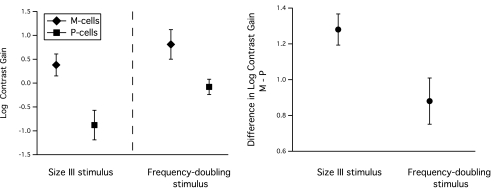
Figure 4 compares log contrast gains from M and P cells for the two stimuli. For both stimuli, mean contrast gain was higher for M cells than for P cells. For both cell classes, sensitivity was higher for the frequency-doubling stimulus than for the size III stimulus. However, this does not mean that the frequency-doubling stimulus was better at preferentially stimulating M cells. The estimated differences (± SE) in log contrast gain between M and P cells are shown (Fig. 4, right); the difference in contrast gain between M and P cells was larger with the size III stimulus. Therefore, the size III stimulus was actually superior to the frequency-doubling stimulus in preferentially stimulating M cells. The mixed linear model for contrast gain indicated a significant interaction effect between stimulus type and cell type (F(1,67) = 14.81; P < 0.001), demonstrating statistical significance for the superiority of the size III stimulus.
Figure 4.
Left: mean contrast gain for size III and frequency-doubling stimuli, for M and P cells. SD (error bars) was similar for all four types of contrast gain. Right: mean (± SE) differences in log contrast gain for M cells minus P cells, for size III and frequency-doubling stimuli. The difference was significantly larger for the size III stimulus than for the frequency-doubling stimulus, and the size III stimulus was superior to the frequency-doubling stimulus in preferentially stimulating M cells.
For some cells the contrast response functions were measured with either the size III stimulus or the frequency-doubling stimulus, but not both stimuli. To exclude variation caused by intercellular differences, we repeated our analysis using contrast gains estimated from the 25 cells that were tested with both stimulus types (Fig. 5). Consistent with the results from all 69 cells, results from these 25 cells also showed a significant interaction effect between stimulus type and cell type (F(1,24) = 11.73; P < 0.01).
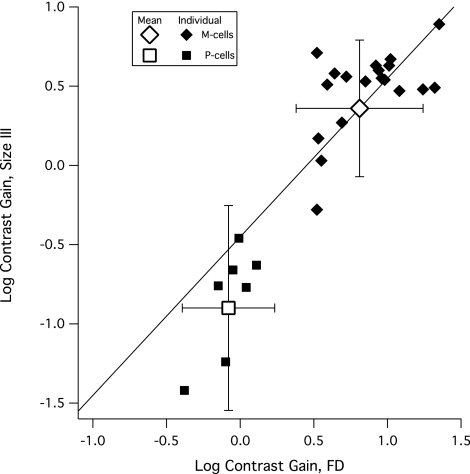
Figure 5.
Scatter plot of contrast gains for the 25 cells tested with both stimuli (small solid symbols) and the means and 95% confidence limits for all 69 cells (large open symbols and error bars). The diagonal line has a slope of 1 and passes through the mean for the M cells; if the frequency-doubling stimulus had been superior to the size III stimulus in stimulating M versus P cells, the contrast gains for P cells would have fallen above this line.
The fits with equation 1 also yielded a semisaturation contrast (Rmax/G) for each ganglion cell and stimulus. For the size III stimulus, the mean ± SD for the semisaturation contrast was 0.06 ± 0.22 log contrast for M cells and 0.97 ± 0.66 log contrast for P cells. For the frequency-doubling stimulus, mean ± SD for the semisaturation contrast was −0.67 ± 0.37 log contrast for M cells and 0.46 ± 0.13 log contrast for P cells. Note that the mean value of the semisaturation contrast for P cells was greater than the maximum stimulus contrast (0.7 log contrast for the size III stimulus, 0.0 log contrast for the frequency-doubling stimulus). Some P cells showed clear signs of saturation at 100% contrast, but many did not. Therefore, the semisaturation contrasts for many P cells could not be accurately assessed, and the mean values give only lower bounds for the estimation.
For the size III stimulus, we systematically assessed the effect of stimulus location on cell responsivity. Figure 6 shows responses of a typical M and a typical P cell to the size III stimulus centered at various locations relative to receptive field center. Spike rates were highest when the stimulus was centered on the receptive field (offset = 0) and were near baseline firing rates when the stimulus was offset by 30 min arc from the receptive field center. It should be noted that the stimulus size (26 min arc) is large relative to ganglion center size, an issue taken up in the Discussion. The same pattern was seen for both M and P cells, indicating little difference in center size for M- and P-cell receptive fields,37 even though peak firing rates were higher for M cells. This result is consistent with data in the literature.32,38,39
Figure 6.
Ganglion cell responses to the size III stimulus when the stimulus was offset from the receptive field center. Symbols: mean spike rate for each stimulus contrast and offset.
Discussion
Clinical Implications
We found that the difference in contrast gain between M and P cells was larger with the size III stimulus than with the frequency-doubling stimulus and concluded that the size III stimulus was superior to the frequency-doubling stimulus at preferentially stimulating M cells relative to P cells. This contradicts the proposal that frequency-doubling stimuli are superior in preferentially stimulating M cells. Frequency-doubling stimuli were proposed to be superior in selectively stimulating M cells because high temporal frequency modulation is potent for M cells.6 However, the temporal presentation for size III has an abrupt onset and offset that give it high temporal frequency components and make it a very effective stimulus for M cells. For both types of stimuli, the lack of a chromatic component causes P-cell responses to be much weaker because of their low achromatic contrast sensitivity,40–42 and P cells only showed substantial responses to the size III and frequency-doubling stimuli at high contrasts.
This study evaluated responses of individual ganglion cells to perimetric stimuli, whereas clinical perimetry measures psychophysical responses of human observers. Our data are consistent with psychophysical studies suggesting that M cells mediate perimetric sensitivity. Harwerth et al.43 measured luminance increment thresholds in macaque monkeys for size III stimuli filtered to narrow bands of wavelengths and found that, throughout much of the visual field, detection at all wavelengths was mediated by nonopponent processes. At those visual field locations at which an opponent process mediated detection at long and short wavelengths, the nonopponent mechanism had sensitivity only slightly lower than the opponent mechanism. Thresholds for the nonopponent mechanism are, therefore, likely mediated by M cells.41,44 This is because P cells are much more responsive to chromatic modulation than to luminance; therefore, if P cells mediated detection for the white luminance increments, the sensitivity difference between opponent and non-opponent mechanisms would be much larger.45 However, this is not the case.
The conclusion that contrast sensitivities for both size III and frequency-doubling stimuli are mediated primarily by the M-cell pathway can account for findings suggesting that glaucomatous losses are comparable for frequency-doubling perimetry and conventional perimetry.20–31 The differences between results obtained with size III and frequency-doubling stimuli cannot be attributed to selective stimulation of the M-cell pathway and instead may reflect the larger stimulus size and smaller contrast range for frequency-doubling stimuli.22,46,47
Ganglion cell responses increase linearly at low contrasts and then saturate at high contrasts, increasing more and more slowly, as shown in Figure 3. Saturation occurs when contrasts reach or exceed the semisaturation contrast, which for M cells averaged 115% for the size III stimulus and 21% for the frequency-doubling stimulus. When converted to the decibel units used in clinical perimetry, these contrasts would be 24.4 dB for the size III stimulus and 13.4 dB for the frequency-doubling stimulus, respectively. This is consistent with our previous prediction22 that ganglion cell saturation occurs when contrast for the size III stimulus is in the 25-dB to 0-dB range and accounts for findings that, when perimetric defects are severe on conventional perimetry, they are often not as deep on frequency-doubling perimetry.20,22
Response saturation can also account for high test-retest variability for the size III stimulus when sensitivity is in the range 25 dB to 0 dB.48–50 A basic assumption for psychophysical measurements is near-threshold linearity; for stimulus contrasts near psychophysical threshold, the internal response is assumed to increase linearly with stimulus contrast. When stimulus contrasts approach the semisaturation contrast, ganglion cell responses show a nonlinear relation with stimulus contrast and near-threshold linearity is no longer a valid assumption for psychophysical algorithms. For example, the contrast response functions we obtained from M cells increase dramatically as stimulus contrast increases from 35 dB to 25 dB, but they increase by a much smaller amount from 15 dB to 0 dB, so the test-retest variability would be much higher at 15 dB than at 30 dB. This may also account for some of the discordance between perimetric and imaging measures of glaucomatous damage in that ganglion cell saturation at high contrasts may cause perimetric algorithms with the size III stimulus to overestimate the amount of visual loss compared with sinusoidal stimuli, which avoid extremely high contrasts.47
For the size III stimulus at contrasts of 25 dB to 0 dB, the effect of increasing contrast is similar to the effect of increasing the diameter of the stimulus; for both types of stimulus manipulation, the primary effect is recruitment of cells farther from the center of the stimulus. Indeed, a recent report on perimetric sensitivity found that, for size III stimulus contrasts of 15 to 0 dB, an increase in stimulus size was approximately equivalent to an increase in stimulus contrast (Goren D, et al., IOVS 2010;51:ARVO E-Abstract 4335).
The data in this study were recorded from ganglion cells in normal primate eyes; it is possible that ganglion cells in glaucomatous eyes may not behave in the same way. There is a report of dendritic field shrinkage and a decrease in synaptic density in monkeys with experimental glaucoma.51 These changes could lead to decreased contrast gain and increased semisaturation contrast, as we proposed based on contrast discrimination data from patients with glaucoma.14 Further investigation in the glaucomatous retina is warranted.
Physiological Considerations
We presented all our stimuli at the same retinal location over a period of 2 to 15 minutes, whereas clinical perimetry tests more than 50 locations in 7 to 15 minutes and stimulates any given retinal location for only 1 to 3 seconds. However, it is unlikely that retinal adaptation or fatigue effects could have affected our measurements. There has been a report of contrast adaptation in the MC pathway,52 but this consists of a weak fatigue effect that occurs only after a long period of exposure to high-contrast stimuli. Furthermore, since we estimated contrast gains from low-contrast stimuli only, adaptation effects would not be anticipated to affect our findings.
Contrary to the textbook view,53 literature estimates of receptive field center sizes for both M and P cells are similar (see Ref. 37 for review). In the eccentricity range tested here, the receptive center sizes for M and P cells range from 4 to 20 min arc.38 The size III stimulus (26 min arc) is thus somewhat larger than center size. We attempted to estimate center size from such curves as shown in Figure 6 by convolving a Gaussian center profile with the size III stimulus. However, center size estimation was not well constrained because of the large size of the stimulus relative to the receptive field center. Nevertheless, the overall results for center size estimates were consistent with literature estimates.
Estimation of receptive field dimension using area summation or grating measurements is usually performed using contrasts near cell response thresholds; with suprathreshold stimuli (especially the very high contrasts used in perimetry), light scatter and other effects might expand effective center size. We noted an apparent increase in center size with very high contrasts, probably caused by stray light. In glaucomatous eyes, retinal physiology may be compromised but light scatter may be unaffected; hence, a higher contrast stimulus may lead to more scattered light, stimulating ganglion cells over a larger retinal area. This would mean that increasing stimulus contrast would have an effect similar to increasing stimulus diameter.
Based on estimates of ganglion cell density and assuming a proportion of 10% M cells and 60% P cells, and using center estimates from the literature, only four to six centers of M cells would be completely covered by the size III stimulus at an eccentricity of 10°. Neurometric analysis of cell responses indicated that central detection mechanisms (for the normal eye) must therefore be making close-to-optimal use of the size III stimulus. It was remarkable that the difference between psychophysical and neurometric thresholds was similar for frequency-doubling and size III stimuli, despite frequency-doubling stimuli covering a much larger area and hence having a much larger potential spatial summation. In addition, it is striking that the degree of saturation of M cell responses apparent in Figure 3 is much less marked for the size III stimulus than for the frequency-doubling stimulus. We propose that cortical pooling of ganglion cell responses must behave in such a way that a dramatic increase in the number of ganglion cells stimulated by a large stimulus provides only a modest increase in psychophysical sensitivity.46 This has implications for the psychophysical models of detection discussed and for the pulsed-pedestal paradigm that has been used to isolate M- and P-cell function.54 Detailed discussion of these issues is outside the scope of the present study and will be presented elsewhere.
In summary, the size III stimulus was more effective than the frequency-doubling stimulus in preferentially stimulating M cells compared with P cells, contradicting the proposal that frequency-doubling stimuli are more selective for M cells than the conventional perimetric stimulus. The conclusion that M cells mediate the detection of both types of stimulus provides an explanation for findings that defect depth is, on average, similar for perimetric tests with the two stimuli. Saturation of ganglion cell responses at high contrasts may contribute to high test-retest variability in perimetry and to discordance between perimetric and imaging measures of glaucomatous damage.
Footnotes
Supported by National Institutes of Health Grants EY007716 (WHS), EY013112 (BBL), and EY019651 (DC).
Disclosure: W.H. Swanson, Zeiss-Meditec (C); H. Sun, None; B.B. Lee, None; D. Cao, None
References
- 1. Goldmann H. Fundamentals of exact perimetry. Optom Vis Sci. 1999;76:599–604 [DOI] [PubMed] [Google Scholar]
- 2. Anderson DR, Patella VM. Automated Static Perimetry. 2nd ed. St. Louis: Mosby-Year Book; 1999 [Google Scholar]
- 3. Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–464 [DOI] [PubMed] [Google Scholar]
- 4. Glovinsky Y, Quigley HA, Pease ME. Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1993;34:395–400 [PubMed] [Google Scholar]
- 5. Vickers JC, Schumer RA, Podos SMW, RF Riederer BM, Morrison JH. Differential vulnerability of neurochemically identified subpopulations of retinal neurons in a monkey model of glaucoma. Brain Res. 1995;680:23–35 [DOI] [PubMed] [Google Scholar]
- 6. Johnson CA. Early losses of visual function in glaucoma. Optom Vis Sci. 1995;72:359–370 [DOI] [PubMed] [Google Scholar]
- 7. Johnson CA, Samuels SJ. Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest Ophthalmol Vis Sci. 1997;38:413–425 [PubMed] [Google Scholar]
- 8. Maddess T, Severt WL. Testing for glaucoma with the frequency-doubling illusion in the whole, macular and eccentric visual fields. Aust N Z J Ophthalmol. 1999;27:194–196 [DOI] [PubMed] [Google Scholar]
- 9. Maddess T, Hemmi JM, James AC. Evidence for spatial aliasing effects in the Y-like cells of the magnocellular visual pathway. Vision Res. 1998;38:1843–1859 [DOI] [PubMed] [Google Scholar]
- 10. White AJ, Sun H, Swanson WH, Lee BB. An examination of physiological mechanisms underlying the frequency- doubling illusion. Invest Ophthalmol Vis Sci. 2002;43:3590–3599 [PubMed] [Google Scholar]
- 11. Morgan JE, Uchida H, Caprioli J. Retinal ganglion cell death in experimental glaucoma. Br J Ophthalmol. 2000;84:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yucel YH, Zhang Q, Gupta N, Kaufman PL, Weinreb RN. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000;118:378–384 [DOI] [PubMed] [Google Scholar]
- 13. Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002;11:365–370 [DOI] [PubMed] [Google Scholar]
- 14. Sun H, Swanson WH, Arvidson B, Dul MW. Assessment of contrast gain signature in inferred magnocellular and parvocellular pathways in patients with glaucoma. Vision Res. 2008;48:2633–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenstein VC, Halevy D, Zaidi Q, Koenig KL, Ritch RH. Chromatic and luminance system deficits in glaucoma. Vision Res. 1996;36:621–629 [DOI] [PubMed] [Google Scholar]
- 16. Alvarez S, Pierce G, Vingrys A, Benes S, Weber P, King-Smith P. Comparison of red-green, blue-yellow and achromatic losses in glaucoma. Vision Res. 1997;37:2295–2301 [DOI] [PubMed] [Google Scholar]
- 17. Pearson P, Swanson WH, Fellman RL. Chromatic and achromatic defects in patients with progressing glaucoma. Vision Res. 2001;41:1215–1227 [DOI] [PubMed] [Google Scholar]
- 18. Martin L, Wanger P, Vancea L, Gothlin B. Concordance of high-pass resolution perimetry and frequency-doubling technology perimetry results in glaucoma: no support for selective ganglion cell damage. J Glaucoma. 2003;12:40–44 [DOI] [PubMed] [Google Scholar]
- 19. McKendrick AM, Badcock DR, Morgan WH. Psychophysical measurement of neural adaptation abnormalities in magnocellular and parvocellular pathways in glaucoma. Invest Ophthalmol Vis Sci. 2004;45:1846–1853 [DOI] [PubMed] [Google Scholar]
- 20. Artes PH, Hutchison DM, Nicolela MT, LeBlanc RP, Chauhan BC. Threshold and variability properties of matrix frequency-doubling technology and standard automated perimetry in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:2451–2457 [DOI] [PubMed] [Google Scholar]
- 21. Spry PG, Hussin HM, Sparrow JM. Clinical evaluation of frequency doubling technology perimetry using the Humphrey Matrix 24–2 threshold strategy. Br J Ophthalmol. 2005;89:1031–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun H, Dul MW, Swanson WH. Linearity can account for the similarity among conventional, frequency-doubling, and gabor-based perimetric tests in the glaucomatous macula. Optom Vis Sci. 2006;83:455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sample PA, Medeiros FA, Racette L, et al. Identifying glaucomatous vision loss with visual-function-specific perimetry in the diagnostic innovations in glaucoma study. Invest Ophthalmol Vis Sci. 2006;47:3381–3389 [DOI] [PubMed] [Google Scholar]
- 24. Burgansky-Eliash Z, Wollstein G, Patel A, et al. Glaucoma detection with matrix and standard achromatic perimetry. Br J Ophthalmol. 2007;91:933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel A, Wollstein G, Ishikawa H, Schuman JS. Comparison of visual field defects using matrix perimetry and standard achromatic perimetry. Ophthalmology. 2007;114:480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zarkovic A, Mora J, McKelvie J, Gamble G. Relationship between second-generation frequency doubling technology and standard automated perimetry in patients with glaucoma. Clin Exp Ophthalmol. 2007;35:808–811 [DOI] [PubMed] [Google Scholar]
- 27. Bozkurt B, Yilmaz PT, Irkec M. Relationship between Humphrey 30–2 SITA Standard Test, Matrix 30–2 threshold test, and Heidelberg retina tomograph in ocular hypertensive and glaucoma patients. J Glaucoma. 2008;17:203–210 [DOI] [PubMed] [Google Scholar]
- 28. Tafreshi A, Sample PA, Liebmann JM, et al. Visual function-specific perimetry to identify glaucomatous visual loss using three different definitions of visual field abnormality. Invest Ophthalmol Vis Sci. 2009;50:1234–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clement CI, Goldberg I, Healey PR, Graham S. Humphrey matrix frequency doubling perimetry for detection of visual-field defects in open-angle glaucoma. Br J Ophthalmol. 2009;93:582–588 [DOI] [PubMed] [Google Scholar]
- 30. Nam YP, Park SB, Kang SY, Sung KR, Kook MS. Glaucoma diagnostic performance of Humphrey matrix and standard automated perimetry. Jpn J Ophthalmol. 2009;53:482–485 [DOI] [PubMed] [Google Scholar]
- 31. Prema R, George R, Hemamalini A, Sathyamangalam Ve R, Baskaran M, Vijaya L. Comparison of Humphrey MATRIX and Swedish interactive threshold algorithm standard strategy in detecting early glaucomatous visual field loss. Indian J Ophthalmol. 2009;57:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crook JM, Lange-Malecki B, Lee BB, Valberg A. Visual resolution of macaque retinal ganglion cells. J Physiol. 1988;396:205–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee BB, Martin PR, Valberg A. Sensitivity of macaque ganglion cells to luminance and chromatic flicker. J Physiol. 1989;414:223–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brenton RS, Argus WA. Fluctuations on the Humphrey and Octopus perimeters. Invest Ophthalmol Vis Sci. 1987;28:767–771 [PubMed] [Google Scholar]
- 35. Swanson WH, Pan F, Lee BB. Chromatic temporal integration and retinal eccentricity: psychophysics, neurometric analysis and cortical pooling. Vision Res. 2008;48:2657–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee BB, Sun H, Zucchini W. The temporal properties of the response of macaque ganglion cells and central mechanisms of flicker detection. J Vis. 2007;7:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee BB. Paths to colour in the retina. Clin Exp Optom. 2004;87:239–248 [DOI] [PubMed] [Google Scholar]
- 38. Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984;357:219–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee BB, Kremers J, Yeh T. Receptive fields of primate retinal ganglion cells studied with a novel technique. Vis Neurosci. 1998;15:161–175 [DOI] [PubMed] [Google Scholar]
- 40. Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells with high and low contrast sensitivity. Proc Natl Acad Sci U S A. 1986;83:2755–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crook JM, Lee BB, Tigwell DA, Valberg A. Thresholds to chromatic spots of cells in the macaque geniculate nucleus as compared to detection sensitivity in man. J Physiol. 1987;392:193–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee BB, Pokorny J, Smith VC, Martin PR, Valberg A. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. J Opt Soc Am A. 1990;7:2223–2236 [DOI] [PubMed] [Google Scholar]
- 43. Harwerth RS, Smith EL, 3rd, DeSantis L. Mechanisms mediating visual detection in static perimetry. Invest Ophthalmol Vis Sci. 1993;34:3011–3023 [PubMed] [Google Scholar]
- 44. Swanson WH. Time, color and phase. In: Kelly DH. ed. Visual Science and Engineering: Models and Applications. New York: Marcel Dekker, Inc.; 1993:191–225 [Google Scholar]
- 45. Pearson PM, Swanson WH. Chromatic contrast sensitivity: the role of absolute threshold and gain constant in differences between the fovea and the periphery. J Opt Soc Am A. 2000;17:232–243 [DOI] [PubMed] [Google Scholar]
- 46. Pan F, Swanson WH. A cortical pooling model of spatial summation for perimetric stimuli. J Vis. 2006;6:1159–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hot A, Dul MW, Swanson WH. Development and evaluation of a contrast sensitivity perimetry test for patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49:3049–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987;105:1544–1549 [DOI] [PubMed] [Google Scholar]
- 49. Piltz JR, Starita RJ. Test-retest variability in glaucomatous visual fields (Letter). Am J Ophthalmol. 1990;109:109–111 [PubMed] [Google Scholar]
- 50. Henson DB, Chaudry S, Artes PH, Faragher EB, Ansons A. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Invest Ophthalmol Vis Sci. 2000;41:417–421 [PubMed] [Google Scholar]
- 51. Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005;46:3197–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Solomon SG, Peirce JW, Dhruv NT, Lennie P. Profound contrast adaptation early in the visual pathway. Neuron. 2004;42:155–162 [DOI] [PubMed] [Google Scholar]
- 53. Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4th ed. New York: McGraw-Hill; 2000 [Google Scholar]
- 54. Pokorny J, Smith VC. Psychophysical signatures associated with magnocellular and parvocellular pathway contrast gain. J Opt Soc Am A. 1997;14:2477–2486 [DOI] [PubMed] [Google Scholar]