Ganciclovir and trifluorothymidine are clinically approved topical antiherpetic agents for ophthalmic use. Combination therapy against an acyclovir-resistant HSV isolate was able to produce significant synergistic antiviral activity in vitro with half the concentration required if each drug were used separately.
Abstract
Purpose.
To determine whether trifluorothymidine (TFT) and ganciclovir (GCV) are synergistic against herpes simplex virus type 1 (HSV-1).
Methods.
TFT and GCV activity against 12 strains of HSV-1 (including an acyclovir-resistant strain) was measured by plaque-forming unit (PFU) inhibition. Cellular toxicity was assessed with an MTT dye reduction assay. Synergism was determined by calculating fractional inhibitory concentration (FIC indices) based on PFU reduction.
Results.
Concentrations of TFT resulting in 50% inhibition of PFUs (IC50) of acyclovir-susceptible HSV-1 strains ranged from 3.07 ± 0.36 to 12.52 ± 0.61 μM. GCV IC50 values ranged from 0.40 ± 0.02 to 1.59 ± 0.14 μM. IC50 values of TFT and GCV against the acyclovir-resistant strain were 15.40 ± 3.17 and 93.00 ± 9.64 μM, respectively. Concentrations of TFT or GCV resulting in 50% cell cytotoxicity (CC50) were 0.99 ± 0.01 and 92.91 ± 8.92 μM, respectively. TFT and GCV combined (10:1) were 10 times more potent against all acyclovir-susceptible HSV-1 strains. For 8 of 12 HSV-1 strains, the IC50 of TFT and GCV combined was lower than the CC50 of either drug. For acyclovir-susceptible HSV-1 strains, TFT and GCV combined generated a FIC index of <0.5, suggesting strong synergism between the two drugs. The FIC value for TFT and GCV combined against the acyclovir-resistant HSV-1 strain was 0.84, indicating nonantagonism.
Conclusions.
TFT and GCV are synergistic against acyclovir-susceptible HSV-1 at concentrations significantly less toxic than if each antiviral were used as a sole agent.
Herpes simplex virus type 1 (HSV-1) is a common cause of acute and recurrent ophthalmic disease worldwide1,2 and is the leading cause of infectious blindness in the United States.1,3 HSV-1 infections of the cornea typically result in sight-threatening epithelial (keratitis) or stromal disease.2,4,5 Several antiviral drugs have shown efficacy in the treatment of experimental HSV-1 keratitis, including acyclovir, valacyclovir, cidofovir, trifluorothymidine (TFT), and ganciclovir (GCV).6–8
Until recently, the only antiviral agent approved in the United States to treat acute HSV-1 keratitis was a 1.0% solution of TFT (Viroptic; King Pharmaceuticals, Bristol, TN).9 Topical acyclovir has not been approved in the United States for ophthalmic use. In September 2009, GCV (0.15% gel, Zirgan; Sirion Therapeutics, Tampa, FL) was approved by the US Food and Drug Administration as a second agent to treat HSV-1 corneal disease. This formulation of GCV has been used as an antiherpetic ophthalmic medication in Europe for more than a decade.10
Irreversibly inhibiting cellular thymidylate synthase after being phosphorylated by cellular and viral thymidine kinases (TK), TFT is clinically effective in treating acyclovir-resistant HSV-1 corneal disease.4 An acyclovir-resistant phenotype arises because of a mutation in viral TK or more rarely in the DNA polymerase of HSV-1.11 In contrast with TFT, GCV (which is phosphorylated only with viral TK) is ineffective against acyclovir-resistant HSV-1.11 Topical TFT can produce adverse effects such as a burning sensation on application, corneal edema, and increased intraocular pressure.12 GCV is reported to cause less discomfort (stinging, burning) or blurred vision.12
TFT and GCV are clinically proven antiherpetic agents formulated for ophthalmic use. Combining these two drugs might allow the use of less toxic TFT concentrations without sacrificing antiviral activity. In this study, a 10:1 combination of TFT and GCV had significant antiviral activity against acyclovir-susceptible HSV-1 strains at concentrations less toxic to cells than if each agent had been used alone. Combination therapy against an acyclovir-resistant HSV-1 strain was also successful in that significant antiviral activity was achieved with half the concentration required if each antiviral was used individually.
Materials and Methods
Viruses, Viral Culture, and Antivirals
Three laboratory strains (McKrae, KOS, and TKG7+2G,13 an acyclovir-resistant TK mutant of KOS) and nine clinical isolates (VT7581, VT242, VT4688, VT5227, VT00694, VT7644, VT7632, VT53, and VT1736) were included in this study. Both KOS (acyclovir-susceptible) and its TK mutant (acyclovir-resistant) were obtained from Donald M. Coen (Harvard Medical School, Boston, MA).
All clinical isolates were obtained from Gary H. Cohen (University of Pennsylvania School of Medicine, Philadelphia, PA). The clinical isolates were low passage and of oral origin.14 The reservoir of corneal infection for HSV-1 is most likely the mouth. Therefore, any viruses isolated from corneal lesions were originally inhabitants of the oral mucosa. HSV-1 can reach the cornea directly in droplets of oral secretions or by a “backdoor” mechanism via the trigeminal ganglion.15
All HSV-1 strains were maintained on Vero cell (ATCC CCL81; American Type Culture Collection, Manassas, VA) monolayers grown to near confluence in 24-well plates at 37°C with 5% CO2 in Earle's minimal essential medium (EMEM; Invitrogen, Carlsbad, CA) supplemented with l-glutamine and 10% fetal bovine serum (FBS; Gibco, Invitrogen). All strains were passaged once before being stored at −20°C.
TFT and GCV were purchased from Sigma-Aldrich Corp. (St. Louis, MO), dissolved in 10% dimethyl sulfoxide (Sigma), and stored at −20°C.
Toxicity of TFT and GCV
Vero cells were cultured as described above. Dilutions (1:1) of TFT (800 μM) or GCV (640 μM) in EMEM were added to wells, and after 5 days of incubation, 20 μL of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) was added to each well. Plates were allowed to develop for 2 hours at room temperature in the dark. Plates were washed once with D-PBS (Gibco). The cells were solubilized in 200 μL of DMSO, and the absorbance measured at 560 nm on an ELISA plate reader (Synergy 2; Biotek Instruments, Winooski, VT). Concentrations of TFT or GCV resulting in 50% cell cytotoxicity (CC50) were calculated using special software (CalcuSyn v.2.0; BioSoft, Cambridge, UK). All assessments of toxicity were done in triplicate, and the CC50 values are reported as the mean ± SE.
Antiviral Activity of TFT and GCV
Antiviral activity was assessed by a plaque reduction assay essentially as described by the Clinical and Laboratory Standard Institute (Wayne, PA).16 Vero cells were grown as previously described, washed with D-PBS (Gibco), and absorbed with 200 plaque-forming units (PFUs) of a HSV-1 strain for 45 minutes. The medium was removed and wells filled with 1:1 serial dilutions of TFT (50 μM) or GCV (5 μM for all strains except TKG7+2G where [GCV] = 640 μM). Each 24-well plate contained both negative (cells only) and positive (cells, virus, and no antiviral) control wells.
After 48 hours of incubation, cells were fixed with 10% buffered formalin and stained with 0.8% crystal violet (w/v; Sigma) in 50% ethanol for 5 minutes. Plates were washed and air dried before counting plaques with the aid of a dissecting microscope. The antiviral effect for each treatment was calculated using the following formula: (P[+]control − P[test])/P[+]control, where P[+]control represents the average number of plaques in positive control wells and P[test] represents the average number of plaques in drug-treated wells. The inhibitory concentrations of TFT or GCV that yielded a 50% reduction in the number of plaques (IC50) were calculated (CalcuSyn software; BioSoft). All assessments of antiviral activity were done in triplicate and the IC50 values are reported as the mean ± SE.
Antiviral Activity of TFT and GCV Combined
Combinations of TFT (50 μM) and GCV (5 μM for all strains except TKG7+2G where [GCV] = 640 μM) were tested with a plaque reduction assay, as previously described. These concentrations of TFT and GCV were selected because they represented the minimum amount of drug that resulted in a >99% reduction in PFUs.
To evaluate the efficacy of combinations in reducing PFUs, a fractional inhibitory concentration (FIC) index was calculated using the following formula: FIC = (IC50 of drug A in combination/IC50 of drug A alone) + (IC50 of drug B in combination/ IC50 of drug B alone).17,18 Synergism is defined with a FIC value ≤ 0.5, indifference, or nonantagonism with a FIC > 0.5 but ≤ 4, and antagonism as a FIC > 4.
Results
Excluding the acyclovir-resistant mutant of HSV-1 constructed in a laboratory, the origin of a strain made little difference in its susceptibility to TFT or GCV, either alone or when combined. The CC50 value for GCV was 92.91 ± 8.92 μM. This concentration of GCV, which elicited a cytotoxic effect in half the Vero cells in a given population, is more than a hundred-fold higher than concentrations of GCV alone or combined with TFT (Table 1) required to reduce PFU formation by half (i.e., the IC50 value) in all clinical and laboratory strains of HSV-1 tested, with the exception of the acyclovir-resistant TK- mutant strain TKG+2G. The IC50 of GCV as a single agent against strain TKG7+2G (93.00 ± 9.64 μM) is essentially identical with its CC50 (92.91 ± 8.92 μM). However, when combined with TFT, the concentration of GCV required to reduce TKG+2G PFU by 50% was practically halved (53.77 ± 7.03 μM).
Table 1.
Inhibitory Concentrations of TFT and GCV, as Single Agents or a Combination, Exibiting a 50% Antiviral Effect (IC50) with FIC Indices
| HSV-1 Strain | IC50 TFT (μM) |
IC50 GCV (μM) |
FIC | ||
|---|---|---|---|---|---|
| Single | Combo* | Single | Combo* | ||
| McKrae | 12.52 ± 0.61 | 0.80 ± 0.04 | 0.58 ± 0.04 | 0.08 ± 0.02 | 0.18 ± 0.01 |
| KOS | 7.10 ± 0.28 | 1.09 ± 0.06 | 0.86 ± 0.04 | 0.11 ± 0.01 | 0.23 ± 0.03 |
| VT7581 | 5.32 ± 0.45 | 0.94 ± 0.02 | 0.49 ± 0.04 | 0.09 ± 0.03 | 0.34 ± 0.04 |
| VT242 | 9.70 ± 0.83 | 1.05 ± 0.03 | 1.02 ± 0.07 | 0.11 ± 0.03 | 0.18 ± 0.02 |
| VT4688 | 3.07 ± 0.36 | 0.52 ± 0.03 | 0.45 ± 0.05 | 0.05 ± 0.01 | 0.28 ± 0.01 |
| VT5227 | 9.64 ± 0.78 | 1.60 ± 0.04 | 1.59 ± 0.14 | 0.16 ± 0.06 | 0.26 ± 0.02 |
| VT00694 | 7.33 ± 0.48 | 0.89 ± 0.02 | 1.03 ± 0.04 | 0.08 ± 0.01 | 0.16 ± 0.03 |
| VT7644 | 8.65 ± 0.41 | 0.68 ± 0.01 | 1.05 ± 0.03 | 0.07 ± 0.01 | 0.18 ± 0.02 |
| VT7632 | 7.19 ± 0.45 | 0.58 ± 0.01 | 0.41 ± 0.04 | 0.06 ± 0.01 | 0.19 ± 0.03 |
| VT53 | 4.39 ± 0.39 | 0.61 ± 0.03 | 0.40 ± 0.02 | 0.05 ± 0.01 | 0.40 ± 0.06 |
| VT1736 | 6.91 ± 0.16 | 0.75 ± 0.01 | 0.75 ± 0.03 | 0.05 ± 0.01 | 0.31 ± 0.05 |
| TKG7+2G† | 15.40 ± 3.17 | 4.20 ± 0.16 | 93.00 ± 9.64 | 53.77 ± 7.03 | 0.84 ± 0.11 |
Synergism defined as a FIC index of ≤0.5, indifferent or nonantagonistic effect as a FIC index of >0.5 but ≤4 and antagonism as a FIC index of >4. Experiments were performed in triplicate. Values represent the mean value ± SEM.
TFT (50 μM) combined with GCV (5 μM) except for TKG7 + 2G, where GCV = 640 μM.
Thymidine kinase negative mutant of KOS.
TFT was 100-fold more toxic to Vero cells on a molar basis when compared with GCV. The concentration of TFT that resulted in 50% Vero cell cytotoxicity (CC50) was 0.99 ± 0.01 μM. In contrast with GCV where the CC50 was less than the IC50 for all HSV-1 strains except the acyclovir-resistant TK-deficient strain, the IC50 values for TFT alone against all 12 strains of HSV-1 tested were more than threefold higher than the CC50 of TFT (Table 1).
Fractional inhibitory concentration indices (Table 1) indicate that 50 μM TFT plus 5 μM GCV acted synergistically (FIC ≤ 0.5) in reducing PFU of all HSV-1 strains except for TKG7+2G. The combination of antivirals had a nonantagonistic effect (FIC = 0.84) on this virus.
Discussion
In this study, TFT and GCV were evaluated for a synergistic antiviral effect in vitro against nine clinical and three laboratory strains of HSV-1. Topical TFT (1.0%) is the standard for treating HSV-1 keratitis in the United States.7,9,19 In patients with HSV-1 dendritic ulcers, a 1.0% TFT solution effectively treated 90% of cases, with activity comparable to antivirals such as vidarabine, bromovinyldeoxyuridine, and idoxuridine.20–22 Recently GCV (0.15%) has been approved in the United States as a topical medication for acute HSV-1 corneal disease. GCV is a nucleoside analog as effective as acyclovir but with less discomfort (stinging, burning) or blurred vision after application of the drug.12
TFT was effective in vitro against all 12 clinical and laboratory strains but at concentrations much higher than GCV (Table 1). The IC50 values of GCV were similar against all HSV-1 strains except for the acyclovir-resistant, TK-deletion mutant of KOS (TKG7+2G), where, not unexpectedly, the IC50 was two magnitudes higher than its parent strain (Table 1). The efficacy of GCV against a HSV-1 isolate is based on the presence of a functional viral TK.23 In contrast, TFT was effective against all HSV-1 strains tested, including strain TKG7+2G.
TFT acts synergistically against HSV with other compounds such as docetaxel,4 docosanol,24 and interferon alpha,25 whereas GCV acts synergistically with cyclocytidine.26 However, these agents are not used to topically treat HSV-1 keratitis in humans. In this study, the antiviral efficacy of a combination of TFT and GCV against HSV-1 was examined using a 10:1 ratio of these drugs, and a significant synergistic interaction was documented (Table 1). The FIC indices of these two antivirals were <0.5. With the exception of the acyclovir-resistant strain TKG7+2G, this synergism was observed against both laboratory and clinical strains of HSV-1. Coincidentally, the 10:1 ratio of antivirals exhibiting synergism in this in vitro study is similar to what would exist if current commercially available preparations of these drugs were used (a 1.0% TFT solution and 0.15% GCV ointment). The most tangible benefit of TFT and GCV acting synergistically against HSV-1 would be the reduction of ocular toxicity associated with the high concentrations of TFT needed to exert an antiviral effect. In this study, the amount of TFT required to reduce PFU counts by 50% was reduced by >70% when the drug was combined with GCV.
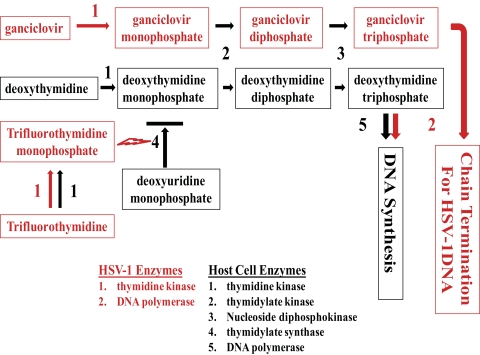
The fates of TFT and GCV once they enter a cell are shown in Figure 1. GCV is phosphorylated to GCV phosphate (GCV-P) by HSV-1 TK. The cellular enzymes thymidylate kinase and nucleoside diphosphokinase further phosphorylate GCV-P into ganciclovir triphosphate (GCV-TP). GCV-TP competitively inhibits incorporation of deoxyguanosine triphosphate (dGTP) into nascent viral DNA by HSV-1 DNA polymerase. Incorporation of GCV-TP into HSV-1 DNA ultimately terminates its elongation.
Figure 1.
The fate of trifluorothymidine (TFT) and ganciclovir (GCV) in a cell. Antiviral agents and HSV-1 enzymes are in red, cellular metabolites and enzymes are in black. Synergism could arise from each antiviral agent targeting a different element in the synthesis of HSV-1 DNA. GCV is phosphorylated to GCV triphosphate (GCV-TP), which competes with cellular deoxyguanosine triphosphate (dGTP) as a substrate for HSV-1 DNA polymerase. Aberrant DNA synthesis occurs when GCV-TP is incorporated into nascent viral DNA by the viral polymerase. TFT is phosphorylated into TFT phosphate which then irreversibly inhibits cellular thymidylate synthase, an enzyme responsible for maintaining adequate concentrations of TTP for DNA synthesis and repair.
Treatment of cells with TFT results in the production of aberrant host and viral DNA, RNA, and proteins. TFT is phosphorylated to TFT phosphate (TFT-P) by both HSV-1 and cellular TK. TFT-P irreversibly inhibits thymidylate synthase, a key enzyme in supplying the cell with TTP for DNA synthesis and repair.
A combination of TFT and GCV could be synergistic in much the same way that antibacterial agents trimethoprim and sulfamethoxazole are syngergistic. Trimethoprim and sulfamethoxazole inhibit folic acid metabolism in bacteria at different points in the folate pathway. TFT and GCV inhibit HSV-1 DNA production in cells by targeting different elements involved in viral nucleic acid synthesis. There was no synergism (FIC = 0.84) of these two antivirals against strain TKG7+2G, likely because of the reduced effectiveness of GCV. Acyclovir-resistant HSV-1 strains such as TKG7+2G can complicate antiviral therapy.12,27 This resistance is associated with a mutation in the TK gene.11,28 Encountering an acyclovir-resistant corneal HSV-1 isolate is a relatively infrequent event in immunocompetent individuals, but such strains are more common in patients with AIDS.29 Although synergism was not demonstrated against TKG7+2G, combining the two antivirals did reduce the IC50 values approximately 50% compared with the IC50 values obtained with each antiviral agent individually.
In conclusion, GCV and TFT are synergistic against acyclovir-susceptible HSV-1 strains in vitro. Although these in vitro results are very encouraging, these studies must be replicated in vivo using an animal model of HSV-1 before combination therapy can be adopted as an accepted clinical practice. Both of these antivirals are clinically effective as single agents in treating keratitis caused by acyclovir-susceptible HSV-1. However, combination therapy has the potential to overcome TFT-induced ocular toxicity as well as to effectively treat keratitis caused by acyclovir-resistant strains of HSV-1.
Footnotes
Supported, in part, by National Institutes of Health Grants No. EY002622 (HEK), EY006311 (JMH), and EY02377 (LSU Eye Center Core Grant for Vision Research); by an unrestricted research grant from the dean of the School of Medicine of the LSU Health Sciences Center (JMH); by a Research to Prevent Blindness Senior Scientific Investigator Award (JMH); by the Louisiana Vaccine Center and the South Louisiana Institute for Infectious Disease Research sponsored by the Louisiana Board of Regents (JMH); by an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness, New York, NY; and by the Louisiana Lions Eye Foundation, New Orleans, and Lions International.
Disclosure: J.A. Hobden, None; M. Kumar, None; H.E. Kaufman, None; C. Clement, None; E.D. Varnell, None; P.S. Bhattacharjee, None; J.M. Hill, None
References
- 1. Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13 [DOI] [PubMed] [Google Scholar]
- 2. Whitley RJ, Roizman B. Herpes simplex viruses. In: Richman DD, Whitley RJ, Hayden FG. eds. Clinical Virology. Washington, DC: ASM Press; 2009:409–436 [Google Scholar]
- 3. Kaufman HE, Rayfield MA, Gebhardt BM. Herpes simplex infections. In: Kaufman HE, Barron BA, McDonald MB. eds. The Cornea. Boston: Butterworth-Heinemann; 1998:247–277 [Google Scholar]
- 4. Bijnsdorp IV, Kruyt FA, Gokoel S, et al. Synergistic interaction between trifluorothymidine and docetaxel is sequence dependent. Cancer Sci. 2008;99:2302–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM. eds. Fields Virology. 5th ed Baltimore: Lippincott Williams & Wilkins; 2007:2503–2602 [Google Scholar]
- 6. Castela N, Vermerie N, Chast F, et al. Ganciclovir ophthalmic gel in herpes simplex virus rabbit keratitis: intraocular penetration and efficacy. J Ocul Pharmacol. 1994;10:439–451 [DOI] [PubMed] [Google Scholar]
- 7. Kaufman HE, Varnell ED, Thompson HW. Trifluridine, cidofovir, and penciclovir in the treatment of experimental herpetic keratitis. Arch Ophthalmol. 1998;116:777–780 [DOI] [PubMed] [Google Scholar]
- 8. Sanitato JJ, Asbell PA, Varnell ED, et al. Acyclovir in the treatment of herpetic stromal disease. Am J Ophthalmol. 1984;98:537–547 [DOI] [PubMed] [Google Scholar]
- 9. Wellings PC, Awdry PN, Bors FH, et al. Clinical evaluation of trifluorothymidine in the treatment of herpes simplex corneal ulcers. Am J Ophthalmol. 1972;73:932–942 [DOI] [PubMed] [Google Scholar]
- 10. Colin J, Hoh HB, Easty DL, et al. Ganciclovir ophthalmic gel (Zirgan; 0.15%) in the treatment of herpes simplex keratitis. Cornea. 1997;16:393–399 [PubMed] [Google Scholar]
- 11. Duan R, de Vries RD, Osterhaus AD, et al. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198:659–663 [DOI] [PubMed] [Google Scholar]
- 12. Yin MT, Brust JCM, Tieu HV, et al. Anti-herpes virus, anti-hepatitis virus, and anti-respiratory virus agents. In: Richman DD, Whitley RJ, Hayden FG. eds. Clinical Virology. Washington, DC: ASM Press; 2009:409–436 [Google Scholar]
- 13. Griffiths A, Coen DM. High-frequency phenotypic reversion and pathogenicity of an acyclovir-resistant herpes simplex virus mutant. J Virol. 2003;77:2282–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Nat Acad Sci U S A. 2006;103:17343–17348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaye S, Choudhary A. Herpes simplex virus. Prog Ret Eye Res. 2006;25:355–380 [DOI] [PubMed] [Google Scholar]
- 16. National Committee for Clinical Laboratory Standards Antiviral Susceptibility Testing: Herpes Simplex Virus by Plaque Reduction Assay; Approved Standard, M33-A. Wayne, PA: NCCLS; 2004 [Google Scholar]
- 17. Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385 [PubMed] [Google Scholar]
- 18. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. [DOI] [PubMed] [Google Scholar]
- 19. Kaufman HE, Varnell ED, Gebhardt BM, et al. Efficacy of a helicase-primase inhibitor in animal models of ocular herpes simplex virus type 1 infection. J Ocul Pharmacol Ther. 2008;24:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carmine AA, Brogden RN, Heel RC, et al. Trifluridine: a review of its antiviral activity and therapeutic use in the topical treatment of viral eye infections. Drugs. 1982;23:329–353 [DOI] [PubMed] [Google Scholar]
- 21. Power WJ, Benedict-Smith A, Hillery M, et al. Randomised double-blind trial of bromovinyldeoxyuridine (BVDU) and trifluorothymidine (TFT) in dendritic corneal ulceration. Br J Ophthalmol. 1991;75:649–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Bijsterveld OP, Post H. Trifluorothymidine versus adenine arabinoside in the treatment of herpes simplex keratitis. Br J Ophthalmol. 1980;64:33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayden FG. Antiviral agents (non-retroviral). In: Brunton LL, Lazo JS, Parker KL. eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2006:1243–1272 [Google Scholar]
- 24. Marcelletti JF. Synergistic inhibition of herpesvirus replication by docosanol and antiviral nucleoside analogs. Antiviral Res. 2002;56:153–166 [DOI] [PubMed] [Google Scholar]
- 25. Birch CJ, Tyssen DP, Tachedjian G, et al. Clinical effects and in vitro studies of trifluorothymidine combined with interferon-alpha for treatment of drug-resistant and -sensitive herpes simplex virus infections. J Infect Dis. 1992;166:108–112 [DOI] [PubMed] [Google Scholar]
- 26. Chen Z, Song J, Chen K. Anti-herpes simplex virus action of combined therapy with cyclocytidine and ganciclovir [in Chinese]. Zhonghua Yan Ke Za Zhi. 1996;32:25–28 [PubMed] [Google Scholar]
- 27. Morfin F, Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J Clin Virol. 2003;26:29–37 [DOI] [PubMed] [Google Scholar]
- 28. Duan R, de Vries RD, van Dun, et al. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis. 2009;200:1402–1414 [DOI] [PubMed] [Google Scholar]
- 29. Ziyaeyan M, Alborzi A, Japoni A, et al. Frequency of acyclovir-resistant herpes simplex viruses isolated from the general immunocompetent population and patients with acquired immunodeficiency syndrome. Int J Dermatol. 2007;1263–1266 [DOI] [PubMed] [Google Scholar]