A new method of retinal blood flow measurement using Doppler optical coherence tomography was developed. It showed circulatory abnormalities in retinal and optic nerve diseases that correlated with disease location and severity.
Abstract
Purpose.
To investigate blood flow changes in retinal and optic nerve diseases with Doppler Fourier domain optical coherence tomography (OCT).
Methods.
Sixty-two participants were divided into five groups: normal, glaucoma, nonarteritic ischemic optic neuropathy (NAION), treated proliferative diabetic retinopathy (PDR), and branch retinal vein occlusion (BRVO). Doppler OCT was used to scan concentric circles of 3.4- and 3.75-mm diameters around the optic nerve head. Flow in retinal veins was calculated from the OCT velocity profiles. Arterial and venous diameters were measured from OCT Doppler and reflectance images.
Results.
Total retinal blood flow in normal subjects averaged 47.6 μL/min. The coefficient of variation of repeated measurements was 11% in normal eyes and 14% in diseased eyes. Eyes with glaucoma, NAION, treated PDR, and BRVO had significantly decreased retinal blood flow compared with normal eyes (P < 0.001). In glaucoma patients, the decrease in blood flow was highly correlated with the severity of visual field loss (P = 0.003). In NAION and BRVO patients, the hemisphere with more severe disease also had lower blood flow.
Conclusions.
Doppler OCT retinal blood flow measurements showed good repeatability and excellent correlation with visual field and clinical presentations. This approach could enhance our understanding of retinal and optic nerve diseases and facilitate the development of new therapies.
The development of noninvasive methods such as magnetic resonance angiography1 and functional magnetic resonance imaging (MRI)2 to measure cerebral hemodynamics has greatly enhanced the study of neurologic diseases and functional neuroanatomy. Such methods would also be very helpful in the eye because the leading causes of blindness in the industrialized world—diabetic retinopathy, macular degeneration, and glaucoma—are all related to abnormal retinal3,4 or optic nerve blood flow.5 Unfortunately, MRI resolution is too coarse for quantitative imaging of retinal blood vessels, which have a very fine caliber.
Although several techniques are being used for retinal blood flow evaluation, they all have serious limitations. Ultrasound color Doppler imaging has sufficient resolution to measure only the larger retrobulbar vessels.6 It can measure blood velocity but not vessel diameter; therefore, volumetric blood flow cannot be determined. Several types of laser Doppler techniques are able to measure flow in individual retinal vessels7–9 or capillary beds.10 Although it is possible to measure total retinal blood flow by adding measurements from individual vessels, this requires many measurements over a long session.8 These specialized instruments are generally available only in major research centers because they are expensive. Fluorescein and indocyanine green angiographies are widely used to visualize retinal and choroidal circulations. However, they do not provide quantitative measurements of blood flow and require the intravenous injection of dyes that have potential side effects.11
Optical coherence tomography (OCT)12 is commonly used in the diagnosis and management of retinal diseases.13–16 It has the requisite resolution to image retinal blood vessels.17 Because it is a coherent detection technique, OCT can detect the Doppler frequency shift of back-scattered light, which provides information on blood flow velocity.18,19 With the development of high-speed Fourier-domain OCT,20–22 it has become possible to capture the pulsatile dynamics of blood flow.23,24
Using Doppler Fourier-domain OCT, we developed a double circular scanning pattern (Fig. 1) that measures flow in all the blood vessels around the optic nerve head four to six times per second.25 Total retinal blood flow could be calculated with the data sampled within 2 seconds. We have demonstrated that flow measurements in normal subjects26 and in a patients with diabetic retinopathy27 can be reproducibly obtained. In this study, we used this new technique in a systematic investigation of blood flow abnormalities in retinal and optic nerve diseases.
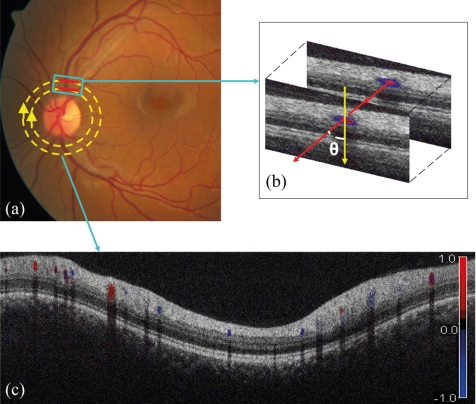
Figure 1.
(a) Fundus photograph showing the double circular pattern of the OCT beam scanning retinal blood vessels emerging from the optic disc. (b) The relative position of a blood vessel in the two OCT cross-sections is used to calculate the Doppler angle θ between the beam and the blood vessel. (c) Color Doppler OCT image showing the unfolded cross-section from a circular scan. Arteries and veins could be distinguished by the direction of flow as determined by the signs (blue or red) of the Doppler shift and the angle θ. Image magnification ratio is 3.39:1.00 (vertical/horizontal).
Methods
Doppler imaging was performed with Fourier-domain OCT using a double circular scan pattern around the optic disc. In subjects with optic nerve or retinal pathologies, only the affected eye was scanned. In normal subjects and subjects with bilateral disease, one eye was randomly selected for scanning.
Study Population
The clinical study was performed at the Doheny Eye Institute at the University of Southern California (USC). The research protocols were approved by the USC institutional review board and carried out in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from each subject.
The normal and perimetric glaucoma subjects were part of the Advanced Imaging for Glaucoma study, and their inclusion criteria are reported in a previous publication28 and on www.AIGStudy.net.
Subjects with established nonarteritic ischemic optic neuropathy (NAION) who were past the initial acute phase, having achieved resolution of initial optic disc and retinal edema, were recruited from the clinic of Alfredo A. Sadun. Subjects with retinal diseases were recruited from the clinic of Amani A. Fawzi.
Optical Coherence Tomography
Two spectrometer-based20,23 Fourier-domain OCT systems were used for Doppler imaging. The initial prototype was built by coauthor Joseph A. Izatt, and its operating software was provided by Bioptigen Inc. (Durham, NC). This unit imaged the initial 33 subjects. Subsequent scans used a high-resolution OCT scanner (RTVue; Optovue Inc., Fremont, CA). Both systems operated at a wavelength of 840 nm with an axial resolution of 5 μm and a transverse resolution of 20 μm in tissue. The power incident on the eye was 500 μW for the Izatt system and 750 μW for the RTVue system. The time interval between two sequential axial scans was 56 μs for the Izatt system and 36.7 μs for the RTVue system. The maximum measurable Doppler shift was 8.9 kHz for the Izatt system and 13.6 kHz for the RTVue system at the phase wrapping limit of ±π radian phase shift between sequential axial scans. This corresponds to a maximum measurable axial velocity component in the eye of 2.8 and 4.2 mm/s, respectively. The normal total blood flows measured by the two devices were not significantly different (Izatt system, 46.7 ± 4.1 μL/min; RTVue system, 48.6 ± 6.8 μL/min; P = 0.45).
Doppler Image Acquisition and Processing
After pupil dilation with 1% tropicamide and 2.5% phenylephrine eyedrops, each subject was seated in front of the OCT scanner and instructed to look at the internal fixation target. The subject's head was stabilized by a chin rest. The fundus of the subject eye was visualized on a real-time video display. The double circular scan pattern was centered on the optic disc. The frame rate was 4.2 circles per second for the Izatt prototype and six frames per second for the RTVue system. Scans were recorded over consecutive 2-second intervals. Five scans were performed on each eye.
Retinal blood flow was measured in post-processing by YW according to a previously described method.25 Blood vessels were identified based on Doppler and reflectance images. For the Izatt system, the pixel dimensions are 12.6 μm in the transverse dimension (horizontal in image) and 3.7 μm in the axial dimension (vertical in image). For the RTVue system, the pixel dimensions are 11.2 μm in the horizontal dimension and 3.1 μm in the vertical dimension. Vessel diameter D was measured by computer caliper on the cross-sectional Doppler OCT images and was used to compute lumen area (πD2/4). The arterial and venous cross-sectional areas for all branch vessels around the optic disc were summed separately to obtain the total arterial and venous areas for the eye. The Doppler angle, between the vessel and OCT beam, was measured by the relative position of each vessel in the two concentric OCT images. The effect of eye motion on the calculation of Doppler angle was small because of the short time interval between the inner and outer circular scans (0.17 second for the RTVue system and 0.25 second for the Izatt system). The error was further minimized by averaging the Doppler angle estimates from the four (Izatt system) or six (RTVue) pairs of concentric circular scans. Flow velocity was computed from the Doppler shift and Doppler angle, with steps to account for the effect of background retinal motion and transverse scan step size.25 The Doppler angle in our study was small enough that the axial velocity component for the great majority of veins was within the range of the OCT systems used. When the peak axial Doppler shift was between π and 2π (or −π and −2π) at the center of the vessel, an unwrapping algorithm was applied automatically to allow valid flow measurement.29 Doppler shifts of greater than ±2π did not occur in any vein in this data set. However, faster arterial velocities could cause multiple phase wrapping (>2π or <−2π), and we did not measure arterial flow because of this difficulty. Veins were identified by the flow direction toward the optic disc. Volumetric blood flow rate for each pixel was calculated by multiplying the velocity with pixel area. Flow F within a vein was calculated by summing the flow in the pixels over a rectangular area slightly larger than the lumen cross-section. Flow measurements were averaged over each 2-second recording. Measurements from all valid scans were averaged. Total retinal blood flow was calculated by summing flow from all detectable veins. Retinal blood flow in arteries and veins should have an equal sum because inflow must equal outflow in any steady state system that obeys the law of conservation of mass. This has been confirmed by actual measurements of retinal arterial and venous flows with a number of techniques.9 Thus, measuring total venous flow alone is sufficient to quantify the total retinal blood flow. For each vein, the average velocity was obtained by F/(πD2/4), where D was vessel diameter. Average arterial velocities were obtained by dividing the total retinal flow by the total arterial areas.
Visual Field Testing
Visual fields were tested in normal, glaucoma, and NAION subjects using the Humphrey Field Analyzer II (Carl Zeiss Meditec, Inc., Dublin, CA) with the 24–2 threshold test, size III white stimulus, and the Swedish Interactive Threshold Algorithm.
Statistical Analysis
Reproducibility was assessed using pooled standard deviations and coefficients of variation. Two-sided t-tests were used to compare the average values of measurements. Given that we made comparisons for five parameters (flow, venous area, venous velocity, arterial area, arterial velocity), Bonferroni correction was applied to make the level of significance P < 0.01. Linear regression was used to analyze the relationship between visual field and blood flow. All the statistical analyses were performed with statistical software (SAS 9.1.3; SAS Institute, Cary, NC).
Results
We compared the retinal blood flow parameters in human subjects with retinal or optic nerve diseases with those of a normal reference group (Table 1). The repeatability of total retinal blood flow, measured as the coefficient of variation, was 10.9% in the normal group and 14.3% in the diseased eyes. The overall intraclass correlation coefficient (ICC) was 0.85. The blood flow in some eyes could not be determined either because of motion artifacts (poor fixation) or near perpendicular orientation of the vessels relative to the OCT beam, primarily in eyes with tilted discs.
Table 1.
Retinal Blood Flow, Vessel Cross-Sectional Areas, and Average Velocities in Normal and Pathological Eyes
| Group (No. eyes) | Blood Flow (μL/min) | Venous Area* (mm2) | Venous Velocity† (mm/s) | Arterial Area* (mm2) | Arterial Velocity‡ (mm/s) |
|---|---|---|---|---|---|
| Normal (20) | 47.6 ± 5.4 | 0.046 ± 0.008 | 17.7 ± 3.1 | 0.033 ± 0.005 | 24.6 ± 4.0 |
| Glaucoma (16) | 34.1 ± 4.9 | 0.046 ± 0.008 | 12.7 ± 1.7 | 0.034 ± 0.008 | 17.1 ± 3.6 |
| (P <0.001) | (P = 0.977) | (P <0.001) | (P = 0.454) | (P <0.001) | |
| NAION (7) | 28.2 ± 8.2 | 0.030 ± 0.007 | 15.4 ± 3.2 | 0.025 ± 0.006 | 19.0 ± 3.4 |
| (P <0.001) | (P <0.001) | (P = 0.109) | (P = 0.002) | (P = 0.003) | |
| PDR (5) | 15.8 ± 10.1 | 0.024 ± 0.007 | 10.4 ± 3.6 | 0.018 ± 0.011 | 16.0 ± 5.0 |
| (P <0.001) | (P <0.001) | (P = 0.001) | (P <0.001) | (P = 0.001) |
Values are shown as average ± SD. P values are in comparison with the normal average. Blood flow shown is the total venous flow. Arterial flow was not independently measured.
Venous and arterial lumen cross-sectional areas are sums of all detected retinal vessels.
Venous velocity is calculated by dividing the flow by the total venous area in each eye.
Arterial velocity is calculated by dividing the flow by the total arterial area in each eye.
Normal Eyes
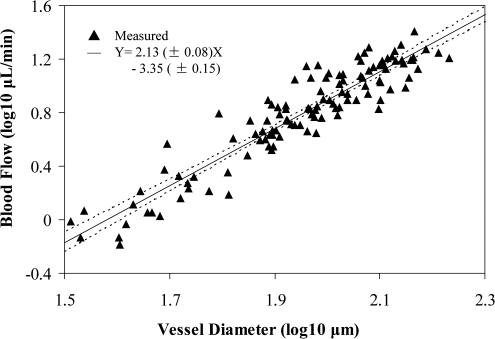
The eyes of 25 normal healthy human subjects (9 men, 16 women; average age, 58.8 years; age range, 35–79 years) were scanned. Retinal blood flow could be determined for 20 of the 25 subjects. Total retinal blood flow ranged from 40.8 to 60.2 μL/min. The measured venous diameters ranged from 27.9 to 183.2 μm. Analysis showed that 66% of veins were larger than 80 μm in diameter, and these larger veins carried approximately 88% of the total flow. There was no statistically significant difference between retinal blood flow in the superior retinal hemisphere (24.2 ± 3.4 μL/min [mean ± SD]) and the inferior hemisphere (23.4 ± 4.1 μL/min). In addition, there was no significant difference in total blood flow between men (46.9 ± 5.5 μL/min) and women (47.7 ± 5.6 μL/min; P = 0.84). For the relationship between blood flow and vessel diameter, linear regression analysis of the combined data from all 20 subjects (Fig. 2) gave a slope of 2.13 ± 0.08 (R = 0.93). This indicated that velocity increased with vessel diameter. There was a weak trend for age-related reduction in blood flow (P = 0.17, linear regression).
Figure 2.
Blood volume flow rate versus blood vessel diameter. Results are on a log-log scale. Solid line: best-fit result of linear regression (P < 0.001; R = 0.93). Dotted lines: 95% confidence interval limits to the fitted line (solid). The SD about the mean slope and intercept values of the linear fit are ±0.08 and ±0.15, separately.
For the individual pathologic eyes presented below, flow values were considered abnormally low if they fell below 35 μL/min, which corresponds to the mean minus 2.33 SD of the normal group (1 percentile cutoff for normal distribution).
Glaucoma
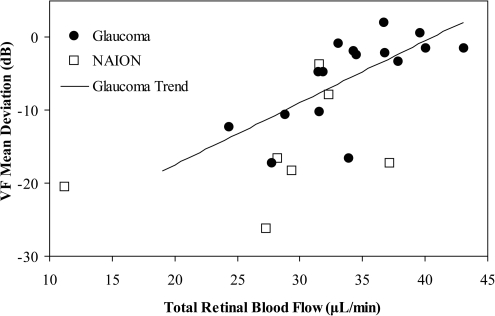
The eyes of 19 perimetric glaucoma subjects (9 men, 10 women; average age, 62.8 years; age range, 49–69 years) were scanned. Retinal blood flow could be determined in 16 subjects; in 10 of them it was abnormally low. As a group, the average flow and arterial and venous velocities were significantly lower than normal, whereas the arterial and venous cross-sectional areas were essentially the same as normal (Table 1). The decrease in blood flow was highly correlated with the severity of glaucoma as measured by mean deviation of the visual field (Fig. 3, black dots).
Figure 3.
Relationship between visual field (VF) loss and retinal blood flow in optic nerve diseases. There was a significant correlation (P = 0.003; Y = 0.85 X − 34.48; R = 0.691) between the VF mean deviation and the total retinal blood flow in the glaucoma group. There were not enough NAION patients to compute the correlation.
Nonarteritic Ischemic Optic Neuropathy
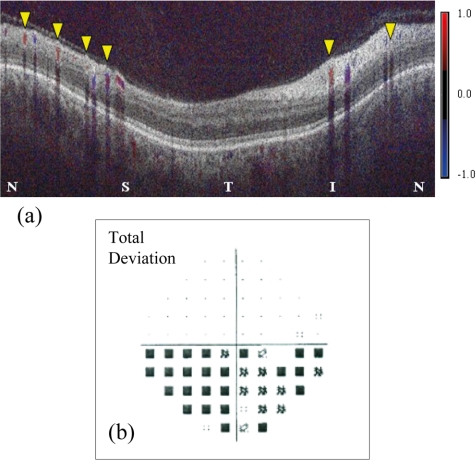
The eyes of nine subjects (7 men, 2 women; average age, 57.0 years; age range, 45–70 years) with NAION were scanned. Retinal blood flow could be measured in seven eyes, and six of these were abnormally low. As a group, the average flow, arterial velocity, venous area, and arterial cross-sectional area were significantly lower than normal, whereas the venous velocity was not significantly lower (Table 1). The blood flow deficit and visual field loss in the NAION group are more severe than in the glaucoma group (Fig. 3). Three NAION eyes had purely altitudinal visual field defects with damage only in the inferior field. All three had lower flow in the corresponding superior retinal hemisphere (one example shown in Fig. 4).
Figure 4.
Color Doppler OCT of an eye with NAION. (a) Superior retinal hemisphere (left half of image) showed lower flow, 13.6 μL/min in 4 veins (yellow arrowheads), compared with the inferior retinal hemisphere (right half of image), where the flow was 18.7 μL/min in two veins (yellow arrowheads). N, nasal; S, superior; T, temporal; I, inferior. Visual field total deviation map (b) showed altitudinal defect in the inferior field (corresponding to the superior retinal hemisphere).
Proliferative Diabetic Retinopathy
The eyes of five subjects (2 men, 3 women; average age, 41.6 years; age range, 29–65 years) with proliferative diabetic retinopathy (PDR) were scanned. Each had undergone laser retinal photocoagulation, and the PDR was quiescent. This group had the most severely depressed blood flow, and all eyes had abnormally low values. As a group, the average flows, velocities, and cross-sectional areas of both arteries and veins were all significantly below normal (Table 1).
We also examined retinal blood flow in two diabetic patients (one man, 48 years; 1 woman, 28 years) without retinopathy. Their total retinal blood flow values, 43.26 μL/min and 40.49 μL/min, were within normal limits.
Branch Retinal Vein Occlusion
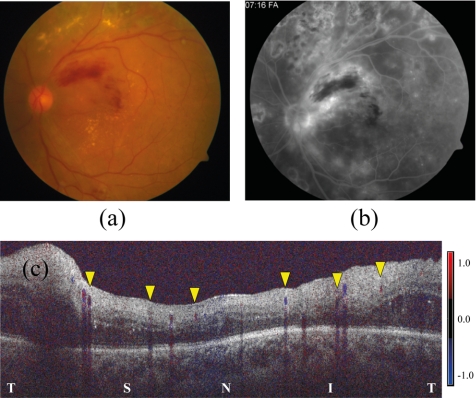
The eyes of two subjects (both women, 42 and 59 years old) with BRVO were scanned. The retinal flow rates of both were abnormally low, 18.6 and 34.8 μL/min. Their average venous velocities, arterial velocities, and arterial areas were below normal, whereas the venous areas were not significantly different from normal. In both cases, the hemisphere with the vein occlusion also had lower flow (one example shown in Fig. 5).
Figure 5.
Doppler OCT of an eye with superotemporal branch retinal vein occlusion. The fundus photograph (a) showed the associated retinal hemorrhage. The fluorescein angiogram (b) showed diffuse dye leakage along the occluded vein and blocked fluorescence associated with the hemorrhage. This eye also had previous panretinal photocoagulation for proliferative diabetic retinopathy; associated exudates, blot hemorrhages, and laser scars were also present. Color Doppler OCT (c) showed flow in three veins of the superior retinal hemisphere (yellow arrowheads, left) was 8.2 μL/min. Flow in the three veins of the inferior retinal hemisphere (yellow arrowheads, right) was 10.4 μL/min.
Discussion
OCT is commonly used for the evaluation of retinal diseases,13 glaucoma,14,28 and other optic neuropathies.30,31 Thus far, OCT has been used to measure the anatomic changes caused by disease. This is the first systematic study that uses OCT to measure a functional change caused by these diseases.
Doppler OCT has previously been used to visualize the two-dimensional17,23 and three-dimensional32 vascular patterns in the retina and choroid. To move beyond visualization and achieve quantitative blood flow measurement, the angle between the flow and the OCT probe beam must be determined. Several methods have been devised to achieve this goal. One approach is to make use of two OCT beams that probe the target from two different angles.33,34 Its drawback is that special hardware is needed; therefore, the method cannot be used with the OCT systems in common clinical use. Another approach is to use a three-dimensional volumetric OCT scan pattern that can be processed to yield the course and orientation of blood vessels.35,36 The limitation of that approach is that a dense volumetric scan of the area around the optic disc requires more than 10 seconds at the speed of current commercially available Fourier-domain OCT systems. This makes measurements susceptible to errors caused by eye motion and variation in flow during the cardiac cycle. To overcome these limitations, we developed the double circular scan pattern that can capture the direction and flow in all retinal vessels six times per second. The method does not require any special hardware and can be implemented on the current generation of Fourier-domain OCT systems.
Our results are largely compatible with previous findings. Retinal blood flow rate and velocity have been shown by several methods to be decreased in glaucoma.37–39 Glaucomatous visual field defects have been correlated with blood flow abnormalities.40 Optic neuropathy is associated with decreased blood flow in the optic nerve head41 and retina,42 as measured by laser Doppler techniques. The PDR subjects in this report had been treated with pan-retinal photocoagulation, which, by destroying retinal tissue, decreases retinal oxygen consumption43 and blood circulation.5 Our method is unique in that vessel flow, velocity, and caliber were rapidly surveyed for the global retinal circulation using a single instrument. In glaucoma, the low flow was associated with low velocity in both arteries and veins, but the vascular cross-sectional areas were comparable to normal. This agreed with previous reports of no differences in retinal vessel diameters between early glaucoma and control subjects.44,45 In NAION and treated PDR, vessel caliber and velocity were both decreased, although the decrease in venous velocity was not statistically significant. The pathophysiological mechanisms for the different patterns of velocity and caliber abnormalities are unclear, and further studies will be needed to validate and explain these findings.
The average measured total venous flow in our study, 47.6 μL/min for 20 normal subjects, was within the range of previously published values—34.0 μL/min7 to 64.9 μL/min8—determined by laser Doppler velocimetry (LDV). The population SD, 5.4 μL/min, was smaller than that of previous studies, 12.8 μL/min.8 The overall ICC value, 0.85, shows the repeatability is excellent compared with the variation between normal and diseased eyes. We were also encouraged by the high correlation between the Doppler OCT flow measurement and visual field loss in the glaucoma group. These findings may indicate that the Doppler OCT measurements are relatively uncontaminated with measurement errors that could have increased the population SD or obscured correlation with visual function. Accurate measurements could be useful in the diagnosis and staging of glaucoma and in evaluating the effectiveness of new therapies that improve retinal blood flow.
In this study, the volumetric flow rate varied with the vessel diameter with a power coefficient of 2.13 ± 0.08. This is higher than the value of 1.97 in our previous paper,26 though not statistically significant. Our current sample is larger (20 eyes) than our previous study (eight eyes) and likely to be more accurate. This logarithmic slope value is still not as high as the value of 2.8 measured with LDV.7 The lower slope might have resulted from a systematic underestimation of vessel diameter. In a laboratory study, Li et al.46 found that the phase-resolved Doppler OCT algorithm underestimated the diameter of flow profile in a small tube of known diameter. At the edge of the vessel, reflected OCT signal from the stationary vessel wall might have interfered with the Doppler signal from flowing blood within the same beam width. If we added one beam diameter to all our vein diameter measurements, the log-log flow-diameter slope would have been 2.7 and would have agreed better with LDV results and Murray's law.47 We are performing in vitro phantom studies to better characterize these possible measurement biases.
There are several limitations to the double circular scan method. At six circles per second, it is not possible to fully eliminate eye motion and compensate for motion-induced error in the measurement of the Doppler angle. This limits the precision and accuracy of blood flow measurements. Another limitation is that velocity determination is more difficult when the Doppler phase shift is greater than 2π radians between two consecutive axial scans (double phase-wrapping). With the OCT systems used in this study, this can occur in the peak phase of retinal arterial flow, making arterial flow measurements unreliable. Therefore LDV has the advantage of being able to measure both arteries and veins. However, this limitation is not intrinsic. Fourier-domain OCT, with a speed of >200,000 axial scans per second, has been demonstrated using both spectrometric (line-camera)48 and swept-source49 approaches. These ultrahigh-speed OCT systems will be able to measure retinal arterial flow and very high blood velocities at large angles without phase wrapping. For average flow velocity calculation, one limitation was that the vessel cross-sectional area was calculated with the assumption that the vessel was round.
Another limitation occurs when the OCT beam is nearly perpendicular to the blood vessels because of the anatomy around the optic nerve head. Measurement in these eyes is possible if the OCT beam can be repositioned in the pupil, changing the angle of approach to the vessel. We must develop a real-time display of the Doppler angle to help the operator adjust the scan angle and to compensate for anomalous anatomic variations. Other subjects in this study could not be measured because of poor vision and poor fixation. Fortunately, most of the subjects in the present study could be measured using current technique and systems.
A final limitation of this technique was the use of a human expert to delineate vascular outlines. To overcome this limitation, we are developing fully automated computer software to identify, delineate, and measure retinal blood vessels. Automated measurement will be needed for widespread clinical use of the Doppler OCT technology.
In summary, we have demonstrated quantitative measurements of total volumetric flow rate, average arterial velocity, average venous velocity, total arterial area, and total venous area in patients with a variety of optic nerve and retinal diseases. Flow deficit correlated well with visual field loss in the optic nerve diseases and with the site of occlusion in BRVO. This is the first systematic application of OCT in the measurement of functional (blood flow) abnormalities instead of anatomic changes in disease. OCT is already a major imaging modality in ophthalmology and is commonly used for retinal diseases and glaucoma evaluation. Our findings could expand the usefulness of OCT in the evaluation of ocular diseases. Larger studies are needed to validate the clinical application of this new technology.
Footnotes
Supported by National Institutes of Health Grants R01 EY013516 and P30 EY03040 and by a grant from Research to Prevent Blindness.
Disclosure: Y. Wang, Optovue (F), P; A.A. Fawzi, None; R. Varma, None; A.A. Sadun, None; X. Zhang, None; O. Tan, Optovue (F); J.A. Izatt, Bioptigen (F); D. Huang, Optovue (F), Carl Zeiss Meditec (F), P
References
- 1. Dumoulin CL, Hart HR., Jr Magnetic resonance angiography. Radiology. 1986;161:717–720 [DOI] [PubMed] [Google Scholar]
- 2. Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173 [DOI] [PubMed] [Google Scholar]
- 3. Ciulla TA, Harris A, Latkany P, et al. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002;80:468–477 [DOI] [PubMed] [Google Scholar]
- 4. Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330 [DOI] [PubMed] [Google Scholar]
- 5. Harris A, Rechtman E, Siesky B, Jonescu-Cuypers C, McCranor L, Garzozi HJ. The role of optic nerve blood flow in the pathogenesis of glaucoma. Ophthalmol Clin North Am. 2005;18:345–353 [DOI] [PubMed] [Google Scholar]
- 6. Goebel W, Lieb WE, Ho A, Sergott RC, Farhoumand R, Grehn F. Color Doppler imaging: a new technique to assess orbital blood flow in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1995;36:864–870 [PubMed] [Google Scholar]
- 7. Riva CE, Grunwald JE, Sinclair SH, Petrig BL. Blood Velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci. 1985;26:1124–1132 [PubMed] [Google Scholar]
- 8. Garcia JPS, Jr, Garcia PT, Rosen RB. Retinal blood flow in the normal human eye using the canon laser blood flowmeter. Ophthalmic Res. 2002;34:295–299 [DOI] [PubMed] [Google Scholar]
- 9. Feke GT, Tagawa H, Deupree DM, Goger DG, Sebag J, Weiter JJ. Blood flow in the normal human retina. Invest Ophthalmol Vis Sci. 1989;30:58–65 [PubMed] [Google Scholar]
- 10. Nicolela MT, Hnik P, Schulzer M, Drance SM. Reproducibility of retinal and optic nerve head blood flow measurements with scanning laser Doppler flowmetry. J Glaucoma. 1997;6:157–164 [PubMed] [Google Scholar]
- 11. Yannuzzi LA, Rohrer KT, Tindel LJ, et al. Fluorescein angiography complication survey. Ophthalmology. 1986;93:611–617 [DOI] [PubMed] [Google Scholar]
- 12. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–332 [DOI] [PubMed] [Google Scholar]
- 14. Schuman JS, Pedut-Kloizman T, Hertzmark E, et al. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology. 1996;103:1889–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583 [DOI] [PubMed] [Google Scholar]
- 16. Bolz M, Kriechbaum K, Simader C, et al. In vivo retinal morphology after grid laser treatment in diabetic macular edema. Ophthalmology. 2010;117:538–544 [DOI] [PubMed] [Google Scholar]
- 17. Yazdanfa S, Rollins AM, Izatt JA. In vivo imaging of human retinal flow dynamics by color Doppler optical coherence tomography. Arch Ophthalmol. 2003;121:235–239 [DOI] [PubMed] [Google Scholar]
- 18. Wang XJ, Milner TE, Nelson JS. Characterization of fluid flow velocity by optical Doppler tomography. Opt Lett. 1995;20:1337–1339 [DOI] [PubMed] [Google Scholar]
- 19. Izatt JA, Kulkarni MD, Yazdanfar S, Barton JK, Welch AJ. In vivo bidirectional color Doppler flow imaging of picoliter blood volumes using optical coherence tomography. Opt Lett. 1997;22:1439–1441 [DOI] [PubMed] [Google Scholar]
- 20. Wojtkowski M, Leitgeb R, Kowalczyk A, Bajraszewski T, Fercher AF. In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002;7:457–463 [DOI] [PubMed] [Google Scholar]
- 21. Choma MA, Sarunic MV, Yang C, Izatt JA. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11:2183–2189 [DOI] [PubMed] [Google Scholar]
- 22. de Boer JF, Cense B, Park BH, Pierce MC, Tearney GJ, Bouma BE. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt Lett. 2003;28:2067–2069 [DOI] [PubMed] [Google Scholar]
- 23. White BR, Pierce MC, Nassif N, et al. In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical Doppler tomography. Opt Express. 2003;11:3490–3497 [DOI] [PubMed] [Google Scholar]
- 24. Leitgeb RA, Schmetterer L, Hitzenberger CK, et al. Real-time measurement of in vitro flow by Fourier-domain color Doppler optical coherence tomography. Opt Lett. 2004;29:171–173 [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Bower BA, Izatt JA, Tan O, Huang D. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence. J Biomed Optics. 2008;13:064003–1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y, Lu A, Gil-Flamer J, Tan O, Izatt JA, Huang D. Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br J Ophthalmol. 2009;93:634–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Fawzi A, Tan O, Gil-Flamer J, Huang D. Retinal blood flow detection in diabetic patients by Doppler Fourier domain optical coherence tomography. Opt Express. 2009;17:4061–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan O, Chopra V, Lu ATH, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghiglia DC, Mastin GA, Romero LA. Cellular-automata method for phase unwrapping. J Opt Soc Am A. 1987;4:267–280 [Google Scholar]
- 30. Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–969 [DOI] [PubMed] [Google Scholar]
- 31. Bellusci C, Savini G, Carbonelli M, Carelli V, Sadun AA, Barboni P. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch Clin Exp Ophthalmol. 2008;246:641–647 [DOI] [PubMed] [Google Scholar]
- 32. Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Opt Express. 2007;15:4083–4097 [DOI] [PubMed] [Google Scholar]
- 33. Pedersen CJ, Huang D, Shure MA, Rollins AM. Measurement of absolute flow velocity vector using dual-angle, delay-encoded Doppler optical coherence tomography. Opt Lett. 2007;32:506–508 [DOI] [PubMed] [Google Scholar]
- 34. Werkmeister RM, Dragostinoff N, Pircher M, et al. Bidirectional Doppler Fourier-domain optical coherence tomography for measurement of absolute flow velocities in human retinal vessels. Opt Lett. 2008;33:2967–2969 [DOI] [PubMed] [Google Scholar]
- 35. Wehbe HM, Ruggeri M, Jiao S, Gregori G, Puliafito CA, Zhao W. Automatic retinal blood flow calculation using spectral domain optical coherence tomography. Opt Express. 2007;15:15193–15206 [DOI] [PubMed] [Google Scholar]
- 36. Makita S, Fabritius T, Yasuno Y. Quantitative retinal-blood flow measurement with three dimensional vessel geometry determination using ultrahigh-resolution Doppler optical coherence angiography. Opt Lett. 2008;33:836–838 [DOI] [PubMed] [Google Scholar]
- 37. Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393 [DOI] [PubMed] [Google Scholar]
- 38. Mitchell P, Leung H, Wang JJ, et al. Retinal vessel diameter and open-angle glaucoma: the Blue Mountains Eye Study. Ophthalmology. 2005;112:245–250 [DOI] [PubMed] [Google Scholar]
- 39. Nicolela MT, Hnik P, Drance SM. Scanning laser Doppler flowmeter study of retinal and optic disk blood flow in glaucomatous patients. Am J Ophthalmol. 1996;122:775–783 [DOI] [PubMed] [Google Scholar]
- 40. Nicolela MT, Drance SM, Rankin SJ, Buckley AR, Walman BE. Color Doppler imaging in patients with asymmetric glaucoma and unilateral visual field loss. Am J Ophthalmol. 1996;121:502–510 [DOI] [PubMed] [Google Scholar]
- 41. Collignon-Robe NJ, Feke GT, Rizzo JF., III Optic nerve head circulation in nonarteritic anterior ischemic optic neuropathy and optic neuritis. Ophthalmology. 2004;111:1663–1672 [DOI] [PubMed] [Google Scholar]
- 42. Sebag J, Delori FC, Feke GT, Weiter JJ. Effects of optic atrophy on retinal blood flow and oxygen saturation in humans. Arch Ophthalmol. 1989;107:222–226 [DOI] [PubMed] [Google Scholar]
- 43. Stefansson E, Hatchell DL, Fisher BL, Sutherland FS, Machemer R. Panretinal photocoagulation and retinal oxygenation in normal and diabetic cats. Am J Ophthalmol. 1986;101:657–664 [DOI] [PubMed] [Google Scholar]
- 44. Arend O, Remky A, Plange N, Martin BJ, Harris A. Capillary density and retinal diameter measurements and their impact on altered retinal circulation in glaucoma: a digital fluorescein angiographic study. Br J Ophthalmol. 2002;86:429–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berisha F, Feke GT, Hirose T, McMeel JW, Pasquale LR. Retinal blood flow and nerve fiber layer measurements in early-stage open-angle glaucoma. Am J Ophthalmol. 2008;146:466–472 [DOI] [PubMed] [Google Scholar]
- 46. Ren H, Sun T, MacDonald DJ, Cobb MJ, Li X. Real-time in vivo blood-flow imaging by moving scatterer-sensitive spectral-domain optical Doppler tomography. Opt Lett. 2006;31:927–929 [DOI] [PubMed] [Google Scholar]
- 47. Sherman TF. On connecting large vessels to small the meaning of Murray's law. J Gen Physiol. 1981;78:431–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Potsaid B, Gorczynska I, Srinivasan VJ, et al. Ultrahigh speed spectral/Fourier domain OCT ophthalmic imaging at 70,000 to 312,500 axial scans per second. Opt Express. 2008;16:15149–15169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang S, Aguirre AD, Huber RA, Adler DC, Fujimoto JG. Swept source optical coherence microscopy using a Fourier domain mode-locked laser. Opt Express. 2007;15:6210–6217 [DOI] [PubMed] [Google Scholar]