The role of capsule in pneumococcal endophthalmitis was tested in vivo using a clinical ocular strain and an isogenic capsule-deficient mutant. Results showed that the presence of capsule was required for full clinical severity and for full bacterial survival in the vitreous.
Abstract
Purpose.
To determine whether Streptococcus pneumoniae capsule was necessary for pathogenesis of pneumococcal endophthalmitis.
Methods.
An isogenic capsule-deficient strain was created using homologous recombination. New Zealand White rabbits were injected intravitreously with 102 colony-forming units (CFU) of the parent strain or the capsule mutant. Slit lamp examination (SLE), electroretinography, and myeloperoxidase activity were performed 24 and 48 hours postinfection (PI). Serial dilutions of vitreous were plated to quantitate CFU, eyes were extracted for histology, and host cytokine mRNA expression was determined.
Results.
Eyes infected with the parent strain had significantly higher SLE scores than eyes infected with the capsule-deficient strain 24 and 48 hours PI (P < 0.001). CFU recovered from eyes infected with the capsule mutant were significantly fewer than CFU recovered from eyes infected with the parent strain 24 and 48 hours PI (P < 0.001). The parent strain caused a significantly greater decrease in retinal function and more retinal destruction than the mutant strain 48 hours PI (P = 0.026). Vitreal IL-1β, IL-6, and TNF-α were upregulated by both the parent and mutant strain 12 hours PI. By 48 hours PI, there was significantly more neutrophil infiltration in the vitreous infected with the parent strain.
Conclusions.
Endophthalmitis caused by the encapsulated strain is more damaging to retinal function and structural integrity. These findings indicate that capsule is an important virulence factor of S. pneumoniae endophthalmitis, in contrast to keratitis, suggesting that the anatomic host site in pneumococcal ocular infections is important.
The bacterium Streptococcus pneumoniae is a major cause of bacterial endophthalmitis, especially after ocular surgery.1–4 Intravitreal vancomycin or ceftazidime is often administered to treat pneumococcal endophthalmitis; however, despite treatment, this disease is particularly devastating to the eye and can result in evisceration and poor visual outcome.2,4 Little is known about the factors responsible for the pathogenesis of pneumococcal endophthalmitis, except that the S. pneumoniae toxin pneumolysin is responsible for some of the retinal damage caused by the bacterium.5,6
The polysaccharide capsule surrounding S. pneumoniae has been well established as a major virulence factor in nonocular pneumococcal infections such as systemic infections, lung infections, and bacteremia.7–12 The function of the capsule in these infections is to allow the bacterium to evade phagocytosis.7,10 The current vaccine used to protect adults against pneumococcal infections is a 23-valent polysaccharide vaccine composed of the most commonly encountered capsule types in pneumonia at the time it was formulated.13,14 For children, a vaccine consisting of 13 different capsule types conjugated to a diphtheria protein is used.15
In sharp contrast to findings in nonocular infections, Reed et al.16 discovered that S. pneumoniae does not require the presence of capsule to cause keratitis. Reed et al.16 compared Avery's strain (an encapsulated serotype 2 strain, also known as D39) with strain R6 (a nonencapsulated derivative of D39) in a rabbit keratitis model and showed that there was no significant difference in ocular pathology caused by the two strains. Therefore, capsule did not appear to be necessary for keratitis caused by S. pneumoniae, a finding opposite that of all the nonocular infections studied to date.
As with keratitis, capsule does not appear to be required for pneumococcal conjunctivitis. Most outbreaks of pneumococcal conjunctivitis are caused by nonencapsulated strains.17–20 Furthermore, a study by Hanage, et al.21 examined 70 nontypeable S. pneumoniae conjunctivitis isolates and discovered that most of these nontypeable strains contained no capsule locus.
The association of capsule with pneumococcal endophthalmitis is different in that most studies have reported the specific capsule types encountered in S. pneumoniae endophthalmitis rather than examining whether the presence of a capsule is necessary. A study by Soriano et al.4 reported 36 cases of endophthalmitis caused by S. pneumoniae and determined that the most common types were 6, 19, 9, 15, and 23 (in decreasing order of occurrence). The current 23-valent vaccine uses polysaccharides from all these serotypes.22 However, the 13-valent vaccine covers all but serotype 15.15 Of the 36 isolates, the serotype of the isolate was determined for 24 of the cases. The study did not mention whether the nontypeable isolates were encapsulated. The observation that at least 66% of the endophthalmitis isolates were encapsulated suggests a possible role for capsule in endophthalmitis. No reports have been identified as to the potential impact of the capsule-based pneumococcal vaccines on endophthalmitis.
In this study, a capsule-deficient isogenic mutant strain of S. pneumoniae was constructed from a clinical ocular strain, and the two were compared in a rabbit endophthalmitis model. The purpose of these experiments was to determine whether capsule was necessary for pathogenesis in pneumococcal endophthalmitis.
Methods
Preparation of Capsule-Deficient Mutant
S. pneumoniae K1544, a clinical ocular strain, was kindly provided by Regis Kowalski of the Charles T. Campbell Eye Microbiology Laboratory (Pittsburgh, PA). The capsule type of K1544 (type 38) was determined using the multiplex PCR method described by Pai et al.23 A capsule-negative isogenic mutant of K1544 was created by homologous recombination in which the capsule locus was replaced with the bicistronic Janus cassette (kindly provided by Larry McDaniel, University of Mississippi Medical Center, Jackson, MS) encoding resistance to kanamycin and sensitivity to streptomycin, as previously described.24–26 The parent strain was grown in competence medium (CM; 0.2% glucose, 0.2% BSA, and 0.02% CaCl2 in Todd Hewitt broth with 0.5% yeast (THY) pH 7.2–7.4) at 37°C to A600 of 0.6. The culture was diluted 1:50 in fresh CM. After 200 ng competence-stimulating peptides 1 and 227 were added, the culture was incubated at 37°C and 5% CO2 for 12 minutes. One microgram of Janus cassette DNA was added, and the mixture continued to incubate for an additional 4 hours. A double-crossover event occurred in which the capsule locus was replaced with the kanamycin-resistance cassette of the donor DNA. Transformants were selected by isolation on 5% sheep blood agar containing 200 μg/mL kanamycin. Chromosomal DNA was purified from the transformants and screened using PCR with primers specific to the Janus cassette24 (Table 1). PCR was also performed using primers specific for the universal capsule sequence (Table 1) to show that the capsule locus had been removed.23 The Quellung test was performed using capsule-specific antisera (type 38; Statens Serum Institut, Copenhagen, Denmark) to verify the bacterium no longer produced a capsule.
Table 1.
Oligonucleotide Primers Specific for cpsA23 and Janus Cassette24
| Primer Name | Sequence 5′→3′ |
|---|---|
| cpsA-123 | GCAGTACAGCAGTTTGTTGGACTGACC |
| cpsA-123 | GAATATTTTCATTATCAGTCCCAGTC |
| Kan524 | CCGTTTGATTTTTAATGGATAATG |
| Kan724 | AGAGACCTGGGCCCCTTTCC |
Bacterial Growth
Bacterial colonies were isolated on 5% sheep blood agar (plates and media for the mutant strain contained 200 μg/mL kanamycin) and were incubated overnight at 37°C and 5% CO2. THY was inoculated with one colony and incubated at 37°C in 5% CO2 overnight. The overnight culture was inoculated into fresh THY at a 1:100 dilution. The bacteria were grown to an optical density (OD) at A600 that corresponded to approximately 108 CFU per milliliter. Accuracy of the bacterial CFU was verified by colony counts of serial dilutions.
Infection
New Zealand White rabbits (Harlan Sprague-Dawley, Inc, Oxford, MI) were used in these studies and maintained according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines of the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center. Each rabbit was anesthetized by an intramuscular injection of a mixture of ketamine hydrochloride (100 mg/mL; Butler Company, Columbus, OH) and xylazine (100 mg/mL; Butler Company). Proparacaine hydrochloride drops were applied to each eye before injection. Bacterial cultures were diluted such that each vitreous humor was infected with approximately 102 CFU in a volume of 10 μL. A 30-gauge needle was used to inoculate the bacteria into the vitreous humor of each eye. In a separate experiment, purified polysaccharide of capsule types 9, 19, and 23 (100 μL of 1 mg/mL; American Type Culture Collection Manassas, VA) was injected into the vitreous of rabbits to determine whether capsule alone elicited any pathologic effects. (Type 38 polysaccharide was commercially unavailable.)
Slit Lamp Examination
Slit lamp examination (SLE) for endophthalmitis was previously described by Callegan.28 In short, eight parameters were used for determining the severity of endophthalmitis: injection, chemosis, corneal inflammation, anterior chamber cell, anterior chamber flare, red reflex, vitreal clarity, and retinal clarity. Each parameter was given a grade from 0 (no inflammation) to 4 (maximal inflammation), resulting in a total score with a theoretical maximum of 32. Each eye was scored by two examiners who were masked to the experiment, and the two scores were averaged.
Electroretinography
Electroretinography (ERG) was used to quantify the retinal response to a single, low-intensity flash (one flash per second); resultant B-wave amplitudes in microvolts (μV) were recorded for each eye. Rabbit eyes were pharmacologically dilated, and baseline B-wave amplitudes were obtained using scotopic, bright-flash ERG (Bravo EP OS/2, version 3.0, with Nicolet Ganzfeld GS-2000 System; Nicolet Instrument Corporation, Madison, WI). B-wave amplitudes were also recorded at 24 and 48 hours postinfection (PI). The recorded B-wave amplitude for each time point represented the average of 14 repeated measures. Percentage loss of retinal function was calculated as {[1 − (experimental B-wave amplitude/baseline B-wave amplitude)] × 100}, where ERG results with no demonstrable waveform were assigned a value of 0.00 μV.
CFU Recovery
Rabbits were euthanatized at 24 or 48 hours PI by an overdose of intravenous sodium pentobarbital (Sigma-Aldrich, St. Louis, MO). Vitreous (0.3 mL, nonemulsified) was removed from each eye using a 22-gauge needle. The vitreous samples were serially diluted, cultured in triplicate on blood agar, and incubated in 5% CO2 at 37°C overnight for quantitation of log10 CFU recovered.
Myeloperoxidase Activity Assay
The myeloperoxidase (MPO) activity of neutrophils in infected vitreous was determined using a colorimetric assay, as described previously.29 Uninfected vitreous served as a background control, and purified MPO (Sigma-Aldrich) served as a positive control. MPO activity was expressed as MPO units.
Cytokine Expression
One hundred microliters of vitreous was removed at 3, 6, 9, 12, 24, 36, and 48 hours PI from infected eyes. A mammalian RNA isolation kit (Sigma-Aldrich) was used to isolate host RNA from each vitreous sample. RNA (0.5 μg) was then reverse transcribed into cDNA using a reverse transcriptase kit (MMLV High-Performance Reverse Transcriptase; Promega, Madison, WI). As described by Ramirez et al.,30 primers specific for rabbit cytokines IL-1β, TNF-α, IL-6, IL-10, IL-8, and GAPDH (housekeeping gene) were used in PCR reactions with the cDNA to determine cytokine expression. Two modifications of the protocol were made: 0.5 μg RNA was used as template to generate cDNA, and 28 cycles were used to amplify DNA from cDNA. IL-10 and GAPDH were tested at all time points. All other cytokines were tested at 3, 6, 9, and 12 hours PI.
Histopathology
Whole eyes were removed, and histologic sectioning and staining were performed (Excalibur Pathology, Inc., Moore, OK). Hematoxylin and eosin staining was used to stain eosinophils and general ocular architecture. For neutrophil and macrophage staining, the specimens were fixed and processed to paraffin. Five-micron sections were deparaffinized using xylene and rehydrated through graduated alcohols to water. Sections were then placed in solution (ImmPress Blocking Solution; Vector Laboratories, Burlingame, CA) for 40 minutes and RPN3/57 or MAC387 (Abcam, Cambridge, MA) 1:200 in Dako antibody diluent for 2 hours, rinsed three times with PBS, placed in anti–rabbit immunoglobulin (ImmPress Peroxidase Reagent; Vector Laboratories) for the RPN3/57 antibody or in anti–mouse immunoglobulin (for the MAC387 antibody) for 40 minutes, and rinsed three times with PBS. Chromagen (Vector AEC Chromagen; Vector Laboratories) was applied to the sections for 8 minutes and rinsed three times with distilled water. Hematoxylin (Dilute Gill III; StatLab Medical, McKinney, TX) was applied to the sections for 1 minute, and then the sections were placed in bluing reagent. Mounting medium (ImmunoHistoMount; Immunobioscience Corp, Mukilteo, WA) was applied to cover sections and was allowed to air dry. A coverslip was added using resin. Rabbit polyclonal IgG (Abcam, Cambridge, MA) was used as the isotype control for the RPN3/57 rabbit neutrophil stained sections. A monoclonal antibody to mouse IgG1, CT6 (Abcam), was used as the isotype control for the MAC387 macrophage–stained sections.
Statistical Analysis
Data were analyzed using the SAS (Cary, NC) program for computers. Clinical SLE scores were analyzed using a nonparametric one-way analysis of variance. Bacterial CFUs were analyzed using the general linear models procedure of least squares means. All experiments except cytokine analysis were performed twice, yielding similar results, and the data from the two experiments were combined. Statistical analyses of the MPO and ERG data were performed using a Student's t-test. P < 0.05 was considered significant.
Results
Preparation of Capsule-Deficient Mutant
A capsule-deficient mutant that was isogenic with the parent strain was constructed by homologous recombination with the Janus cassette.26 PCR amplification using total DNA from the parent strain as template and primers complementary to portions of the cpsA gene gave rise to a 160-bp product; however, no product was observed when using DNA from the mutant strain as template. When parent template was combined with primers complementary to portions of the Janus cassette, no product was produced. Amplification using DNA from the mutant strain as template and primers complementary to portions of the Janus cassette was performed, and a 3195-bp product was observed. Parent and mutant bacteria were then examined by the Quellung reaction. A halo was visible on microscopic examination around the parent bacteria, but not around the mutant bacteria, when exposed to capsule specific antisera (data not shown).
Rabbit Endophthalmitis Model
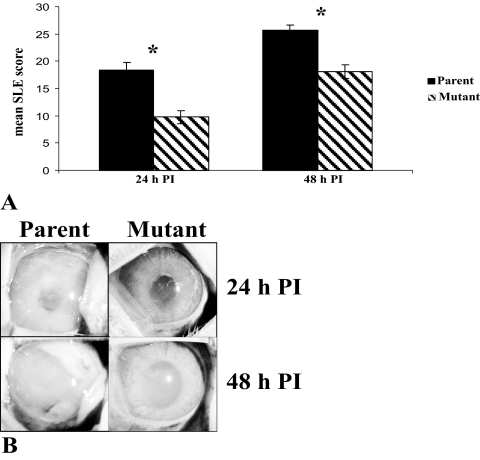
The parent strain and the capsule-deficient isogenic mutant strain were compared in a rabbit endophthalmitis model. Actual bacterial CFU for all the inoculations ranged from 430 to 760 for the mutant strain and 340 to 540 for the parent strain, as determined by enumeration of serial dilutions of the inocula. Two independent experiments were performed. For the first experiment, the inoculum of the mutant strain was higher than that of the parent strain. For the second experiment, the inoculum of the parent strain was higher than that of the mutant strain. Despite the differences in inocula, the results were similar for both experiments. The eyes of rabbits infected with the parent strain (n = 10 eyes) had an average SLE score ± SEM of 18.31 ± 1.51 at 24 hours PI, and the eyes infected with the mutant strain (n = 12 eyes) had a mean score of 9.78 ± 1.16 (P < 0.01). At 48 hours PI, the average SLE scores for eyes infected with the parent and mutant strains were 25.69 ± 0.92 and 18.10 ± 1.25, respectively (P < 0.01; Fig. 1A).
Figure 1.
(A) Average SLE scores 24 and 48 hours PI. (B) Endophthalmitis caused by the parent strain or capsule-deficient mutant strain at 24 and 48 hours PI. Parent, parent strain; mutant, capsule-deficient isogenic mutant strain. *P < 0.01.
Figure 1B shows representative eyes infected with the parent and mutant strains. The parent strain caused a more severe pathology at 24 and 48 hours PI. More infiltration of inflammatory components was observed in the eyes infected with the parent strain than in the eyes infected with the mutant strain at both time points. In contrast, injection of pure polysaccharide caused no clinical symptoms. All eyes infected with pure polysaccharide had clinical scores of 0 at 24 and 48 hours PI (data not shown).
Electroretinography
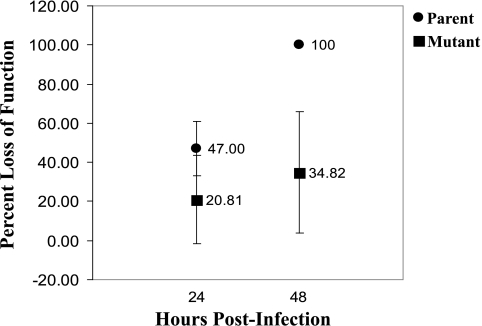
ERG of eyes infected with the parent strain (n = 4) revealed a higher mean baseline B-wave amplitude than did that of eyes infected with the mutant strain (n = 4): 215.22 μV and 188.59 μV, respectively. No significant difference in percentage loss of retinal function was observed at 24 hours PI between eyes infected with the parent (47.00 ± [95% confidence interval] 14.05) or the mutant (20.81 ± 24.74) strain (P = 0.11). However, by 48 hours PI, eyes infected with the parent strain had a significantly higher percentage loss of retinal function (100.00 ± 0.00) compared with eyes infected with the mutant strain (34.82 ± 31.71; P = 0.03; Fig. 2).
Figure 2.
Percentage loss of retinal function at 24 and 48 hours PI for eyes infected intravitreously with the parent strain or the mutant strain. At 48 hours PI, eyes infected with the parent strain had a significantly higher percentage loss of retinal function than eyes infected with the mutant strain. Error bars represent 95% confidence intervals.
Vitreal CFU Recovery
There was a significant difference between the parent and the mutant strains in CFU recovered from the vitreous of infected rabbit eyes. The average log10 CFU ± SEM recovered from the vitreous of the eyes infected with the parent strain (n = 10) was 8.91 ± 0.26 at 24 hours PI. In contrast, the vitreous of the eyes infected with the mutant strain (n = 12) had a log10 CFU of 6.86 ± 0.32 at 24 hours PI (P < 0.01). At 48 hours PI, 6.52 ± 0.66 and 2.15 ± 0.69 log CFU were recovered from the vitreous of the eyes infected with the parent (n = 9) and the mutant (n = 11) strains, respectively (P < 0.01; Table 2).
Table 2.
Log10 CFU/mL Recovered from Vitreous Infected with S. pneumoniae
| Log10 CFU ± SEM (n)* |
||
|---|---|---|
| 24 Hours | 48 Hours | |
| Parent | 8.91 ± 0.26 (10) | 6.52 ± 0.66 (9) |
| Mutant | 6.86 ± 0.32 (12) | 2.15 ± 0.69 (11) |
Parent, parent strain; Mutant, capsule-deficient isogenic mutant strain.
P < 0.01 at 24 and 48 hours PI.
MPO Activity
At 24 hours PI (n = 6 eyes per group), the MPO units from the vitreous of eyes infected with either the mutant or the parent strain, respectively, were calculated to be 0.17 ± 0.09 and 0.08 ± 0.06 (P = 0.44). However, at 48 hours PI, the vitreous of eyes infected with the mutant strain (0.10 ± 0.07) had significantly lower MPO activity compared with the vitreous infected with the parent strain (4.38 ± 1.77; P = 0.04; Table 3).
Table 3.
MPO Units from Vitreous Infected with S. pneumoniae
| MPO Units (n)* |
||
|---|---|---|
| 24 Hours | 48 Hours | |
| Parent | 0.08 ± 0.06 (6) | 4.38 ± 1.77 (6) |
| Mutant | 0.17 ± 0.09 (6) | 0.10 ± 0.07 (6) |
Parent, parent strain; Mutant, capsule-deficient isogenic mutant strain.
P = 0.44 at 24 hours and P = 0.04 at 48 hours PI.
Cytokine Expression
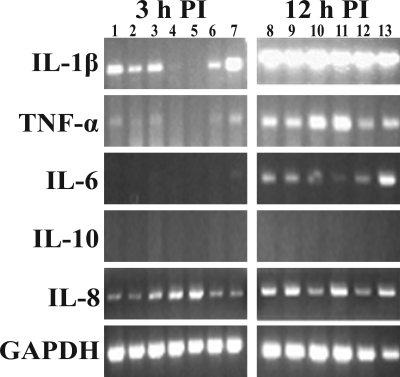
RT-PCR of infected vitreous showed trends in host cytokine expression. Interleukin-1β, TNF-α, and IL-6 expression trended toward upregulation from 3 to 12 hours PI for vitreous infected with both the parent strain and the capsule-deficient mutant strain (Fig. 3). However, IL-10 and IL-8 expression did not appear to change (Fig. 3). Expression of IL-10 was not observed at any time point (3, 12, 24, 36, or 48 hours PI; 24, 36, and 48 hours not shown) for vitreous infected with the parent or the mutant strain. No obvious differences in cytokine expression between vitreous infected with the parent strain or the capsule-deficient mutant were observed at any of the time points tested (6 and 9 hours; not shown).
Figure 3.
Cytokine mRNA expression at 3 and 12 hours PI for eyes infected intravitreously with the parent strain (lanes 1–4, 8–11) or the mutant strain (lanes 5–7, 12, 13). IL-1β, TNF-α, and IL-6 were upregulated in the vitreous by both strains. IL-10 was not expressed by 48 hours PI (12 hours PI shown), and IL-8 was expressed at all time points in both infections. GAPDH was the housekeeping gene. Each lane represents vitreous from an individual eye.
Histopathology
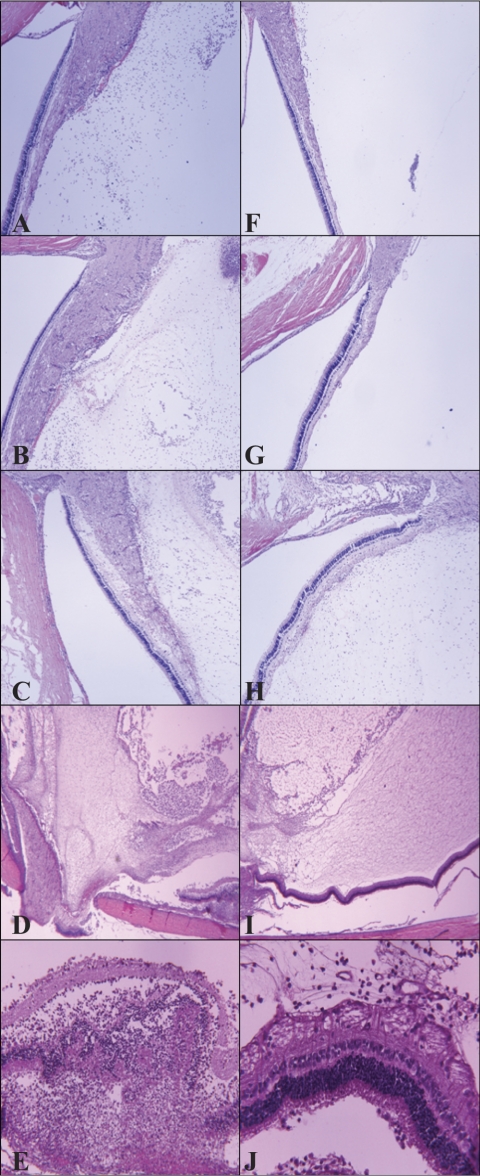
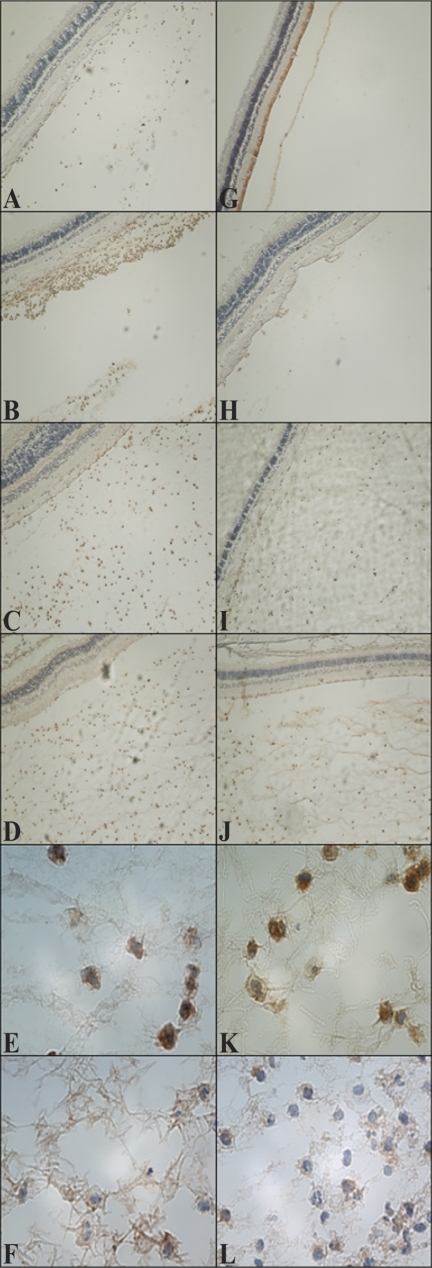
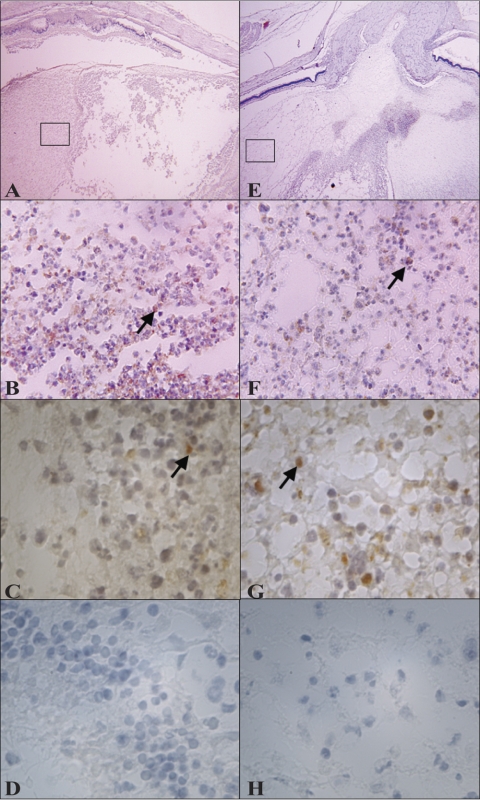
All eyes were removed in whole, sectioned, and stained with hematoxylin and eosin for visualization of general immune cell infiltration and retinal destruction (Fig. 4), an anti–mouse neutrophil stain that cross-reacts with rabbit for visualization of neutrophils (Fig. 5), or an anti–rabbit macrophage stain for visualization of macrophages (Fig. 6). To observe differences in infiltration at earlier time points during infection, eyes were harvested at 12, 24, and 36 hours PI. At all three time points, less severe infiltration was observed in eyes infected with the mutant strain (Figs. 4F–J, 5G–L) compared with eyes infected with the parent strain (Figs. 4A–E, 5A–F). Inflammation observed in the eyes infected with the parent strain was more pronounced and destructive than in the eyes infected with the mutant strain. In addition, inflammation of the vitreous, retina, and choroid was observed in the eyes infected with the parent strain; the retinas became detached from the optic disc and were destroyed (Figs. 4D, 4E). Retinas were largely intact in the eyes infected with the mutant strain (Figs. 4I, 4J), and fewer neutrophils were detected (Figs. 5G–J), which reflected the lower MPO activity determined. All these data together strengthen the argument that capsule is important in this infection model. Macrophages were present in the vitreous of rabbits infected with either strain (Fig. 6). Depending on the area of each histology section chosen for observation, the general amount of macrophages appeared to be similar, greater, or fewer in eyes infected with either strain; therefore, it was concluded that there were no overt differences in macrophage recruitment.
Figure 4.
Representative histology sections (hematoxylin and eosin staining) of eyes infected intravitreously with the parent strain (A–E) or the mutant strain (F–J) at 12 (A, F), 24 (B, G), 36 (C, H), and 48 (D, E, I, J) hours PI. At 48 hours PI, the retina of eyes infected with the parent strain (E) showed a far more severe pathology than the retina of eyes infected with the mutant strain (J). Original magnification, 40× (A–D, F–I) and 400× (E, J).
Figure 5.
Representative histology sections (neutrophil staining) of eyes infected intravitreously with the parent strain (A–F) or the mutant strain (G–L) at 12 (A, G), 24 (B, H), 36 (C, I), and 48 (D–F, J–L) hours PI. Neutrophils were observed in vitreous infected with the parent strain at all time points (A–D). Infiltration of neutrophils occurred between 24 and 36 hours PI in vitreous infected with the mutant strain (H, I, respectively). (E, K) Higher magnifications of (D) and (J), respectively, to show morphology. Neutrophils are stained brown. (F, L) Isotype controls. Original magnifications, 20× (A–D, G–J); 1000× (E, F, K, L).
Figure 6.
Representative histology pictures (macrophage staining) of eyes infected intravitreously with the parent strain (A–D) or the mutant strain (E–H) at 48 hours PI. Arrows indicate macrophages (stained brown). (C, G) Magnifications of (B) and (F), respectively, to show morphology. (D, H) Isotype controls. Original magnification, 20× (A, E); 400× (B, F); 1000× (C, D, G, H). Areas from (A) and (E) magnified in (B) and (F) are boxed.
Discussion
The polysaccharide capsule of S. pneumoniae has been demonstrated to be required for virulence in all the nonocular pneumococcal diseases studied to date.7–12 However, capsule has been shown to have no effect on the pathogenesis of pneumococcal keratitis. Reed et al. reported that S. pneumoniae D39 and its nonencapsulated counterpart, R6, were equally virulent in the rabbit cornea. Both strains produced statistically similar clinical scores, which suggested that factors other than capsule were important for pneumococcal corneal disease.16 One such virulence factor is pneumolysin, the major cholesterol-dependent cytolysin of S. pneumoniae, which was previously reported to be important for S. pneumoniae keratitis.31,32
Immune response to polysaccharide was observed by injecting purified polysaccharide into the vitreous of naive rabbits. Soriano et al.4 reported that capsule types 9, 19, and 23 were 3 of the 5 most common capsule types in pneumococcal endophthalmitis. Because purified polysaccharides of these types were readily available, these were used to show that purified capsule alone does not cause adverse effects in the eye.
Pneumolysin has been shown to be an important factor in pneumococcal endophthalmitis, particularly in the early stages of the disease.6 Pneumolysin is not, however, the sole component of S. pneumoniae responsible for endophthalmitis because a strain deficient in pneumolysin was able to produce symptoms as severe as the intact parent strain in the later stages of the disease in rats.6 No studies until now have been reported regarding whether the S. pneumoniae polysaccharide capsule is required for, or contributes to, pneumococcal endophthalmitis. The results presented herein demonstrate that vitreous infected with an encapsulated clinical ocular strain of S. pneumoniae had significantly more severe disease symptoms than vitreous infected with the capsule-deficient isogenic mutant strain. The encapsulated and nonencapsulated strains in the present study were also used to infect rabbit corneas to determine whether these particular strains behaved in the same manner as the strains used in the keratitis study by Reed et al.16 Similar to those findings,16 there was no significant difference in SLE scores between the two infection groups for keratitis.26
The data from this study show that capsule is an important virulence factor in bacterial endophthalmitis. Because the immune system recognizes pneumococcal capsular polysaccharides,33 the encapsulated bacteria might have elicited a stronger immune response in the vitreous than the nonencapsulated bacteria, which would explain why an abundance of neutrophils was observed in the histologic sections. The polysaccharide structure contains carbohydrates, and the immune system recognizes these antigens in a T-cell–independent manner.34
A characteristic consequence of bacterial endophthalmitis caused by activation of the immune response and breakdown of the blood-ocular fluid barrier is a massive infiltration of inflammatory cells into the intraocular cavity,35 as observed in the present study (Figs. 1B, 5D). The MPO data reflected the significantly higher SLE scores observed for eyes infected with the capsular parent strain at 48 hours PI. Moreover, retinal function as measured by ERG was significantly decreased in eyes infected with the parent strain compared with eyes infected with the mutant strain, a finding supported by the retinal destruction observed in the histopathologic sections.
The likely reason for the lack of killing of the encapsulated strain in the vitreous is that the proposed function of the pneumococcal capsule is escape from ingestion by phagocytes.7,10,36–39 The nonencapsulated mutant strain, in contrast, was able to grow above inoculum by 24 hours PI but then decreased to approximately 2 log10 units (approximately 4 log10 units below the encapsulated strain) by 48 hours PI. The nonencapsulated mutant strain was probably unable to escape phagocytosis as readily as the encapsulated parent strain.
The present study also examined whether the overall expression of selected cytokines was different in the vitreous of eyes infected with the capsular strain than in those infected with the mutant strain. Two previous studies determined that IL-1β and TNF-α were upregulated over time in rat models of Staphylococcus aureus and Staphylococcus epidermidis endophthalmitis.40,41 Additionally, Bootsma et al.42 observed the effects of three encapsulated strains of S. pneumoniae and their respective isogenic capsule mutants on cytokine production by human pharyngeal epithelial cells in vitro. Although IL-1β, IL-6, and TNF-αIP3 were all upregulated in response to pneumococcal binding to the cells, no significant differences were found in the expression of these cytokines when cell cultures infected with the encapsulated parent strains or the isogenic capsule mutants were compared. The results reported herein for cytokine induction during pneumococcal endophthalmitis agree with these previous findings.
In contrast to the trend toward upregulation of some cytokines, IL-10 and IL-8 appeared unchanged. IL-10, an anti-inflammatory cytokine,43 was not detected at any of the time points tested. There is no commercially available positive control for this reaction. Time points of 3, 6, 9, and 12 hours PI were chosen for the detection of IL-1β, TNF-α, IL-6, and IL-8 expression, and those of 3, 6, 9, 12, 24, 36, and 48 hours PI were chosen for the detection of IL-10 and GAPDH in the present study. Time points between 3 and 12 hours PI were chosen because it was observed in a previous study that certain cytokines are detected as early as 6 hours PI and peak around 12 to 24 hours PI. The later time points were chosen for IL-10 and GAPDH to determine whether IL-10 expression was observed later in infection.43 IL-8 is a chemotactic cytokine that attracts neutrophils,44 and because neutrophils were observed in the histology specimens of eyes infected with both the parent and the mutant strains, IL-8 expression at all time points in both infections was not altogether unexpected. By 48 hours PI, more immune cells appeared to be recruited into the vitreous of eyes infected with the parent strain, as observed by neutrophil staining (Fig. 5). However, there was not a significant difference in macrophages between the two infections at this time point. There was a significant difference in SLE scores at 24 hours PI and a trend toward upregulation of IL-1β, IL-6, and TNF-α between 3 and 12 hours, but there was not a significant difference in MPO units observed at the same time point. At 48 hours PI, significantly lower MPO activity was observed in the vitreous of eyes infected with the mutant strain. By 48 hours PI, eyes infected with the parent strain had significantly higher MPO activity in recovered vitreous, more positively identified neutrophils, significantly higher SLE score, and either similar or greater amounts of macrophages as eyes infected with the mutant strain, depending on which section of the vitreous was observed by immunohistochemistry staining. One study showed several different techniques used by the pneumococcal capsule to evade phagocytosis, including blocking Fc-gamma–mediated phagocytosis, complement receptors, and nonopsonic receptors.45 The presence of macrophages in the vitreous of eyes infected with the mutant strain at 48 hours suggests that the lack of capsule on the mutant strain was failing to protect these bacteria from opsonization, allowing more efficient clearing of the bacteria.
This study showed a correlation between capsule and severity of infection. Although the eyes infected with the mutant strain had significantly lower SLE scores by 48 hours PI, the infection was severe enough to warrant euthanatization of the rabbits for humane reasons. The ability of the mutant strain to cause severe endophthalmitis, albeit delayed compared with that of the parent strain, indicates that factors other than the capsule are important in the pathogenesis of pneumococcal endophthalmitis. The mutant strain retained its ability to produce pneumolysin, which was previously reported to play a role in the early stages of endophthalmitis.5 Besides capsule and pneumolysin, the damage caused by the immune system of the host is a key factor in this disease; however, this observation does not exclude the possibility of other as yet unidentified bacterial virulence factors eliciting direct effects on the ocular tissues. The combination of multiple factors involved in pneumococcal endophthalmitis, some unknown, underscores the complexity of this disease.
Acknowledgments
The authors thank William Johnson for his consultation in using the appropriate statistics for this study, Larry McDaniel for providing the Janus cassette, Eva Bengtén and Melanie Wilson for providing their expertise with the appropriate conditions for RT-PCR of cytokines, and Sherrina Dixon for determining the capsule type of the parent strain.
Footnotes
Supported by Public Health Services Grant R01EY016195, the National Eye Institute, the National Institutes of Health, and the University of Mississippi Medical Center.
Disclosure: M.E. Sanders, None; E.W. Norcross, None; Z.M. Robertson, None; Q.C. Moore III, None; J. Fratkin, None; M.E. Marquart, None
References
- 1. Mao LK, Flynn HW, Miller JD, Pflugfelder SC. Endophthalmitis caused by streptococcal species. Arch Ophthalmol. 1992;110:798–801 [DOI] [PubMed] [Google Scholar]
- 2. Miller JJ, Scott IU, Flynn HW, Smiddy WE, Corey RP, Miller D. Endophthalmitis caused by Streptococcus pneumoniae. Am J Ophthalmol. 2004;138:231–236 [DOI] [PubMed] [Google Scholar]
- 3. Nouri M, Terada H, Alfonso EC, Foster S, Durand ML, Dohlman CH. Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol. 2001;119:484–489 [DOI] [PubMed] [Google Scholar]
- 4. Soriano F, Perez-Trallero E, Pallares R, et al. Streptococcus pneumoniae endophthalmitis: a study of 36 cases with special reference to antibiotic resistance and treatment options. Clin Microbiol Infect. 2006;2:519–526 [DOI] [PubMed] [Google Scholar]
- 5. Ng EW, Samiy N, Cousins FV, et al. Implication of pneumolysin as a virulence factor in Streptococcus pneumoniae endophthalmitis. Retina. 1997;17:521–529 [PubMed] [Google Scholar]
- 6. Ng EW, Costa JR, Samiy N, et al. Contribution of pneumolysin and autolysin to the pathogenesis of experimental pneumococcal endophthalmitis. Retina. 2002;22:622–632 [DOI] [PubMed] [Google Scholar]
- 7. Johnson SE, Rubin L, Romero-Steiner S, et al. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J Infect Dis. 1999;180:133–140 [DOI] [PubMed] [Google Scholar]
- 8. Kadioglu A, Taylor S, Iannelli F, Pozzi G, Mitchell TJ, Andrew PW. Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect Immun. 2002;70:2886–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindberg J, Fangel S. Recurrent endocarditis caused by Streptococcus pneumoniae. Scand J Infect Dis. 1999;31:409–410 [DOI] [PubMed] [Google Scholar]
- 10. Llull D, Lopez R, Garcia E. Genetic bases and medical relevance of capsular polysaccharide biosynthesis in pathogenic streptococci. Curr Mol Med. 2001;1:475–491 [DOI] [PubMed] [Google Scholar]
- 11. Varon E. Severe pneumococcal infections: virulence aspects. Arch Pediatr. 2001;8(suppl 4):752s–756s [DOI] [PubMed] [Google Scholar]
- 12. Magee AD, Yother J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun. 2001;69:3755–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robbins JB, Lee CJ, Schiffman G, et al. Considerations for formulating the second- generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–1159 [DOI] [PubMed] [Google Scholar]
- 14. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460 [DOI] [PubMed] [Google Scholar]
- 15. American Academy of Pediatrics Committee on Infectious Diseases Recommendations for the prevention of Streptococcus pneumoniae infections in infants and children: use of 13-valent pneumococcal conjugate vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPSV23). Pediatrics. 2010;126:186–190 [DOI] [PubMed] [Google Scholar]
- 16. Reed JM, O'Callaghan RJ, Girgis DO, McCormick CC, Caballero AR, Marquart ME. Ocular virulence of capsule-deficient Streptococcus pneumoniae in a rabbit keratitis model. Invest Ophthalmol Vis Sci. 2005;46:604–608 [DOI] [PubMed] [Google Scholar]
- 17. Shayegani M, Parsons LM, Gibbons WE, Jr, Campbell D. Characterization of nontypable Streptococcus pneumoniae-like organisms isolated from outbreaks of conjunctivitis. J Clin Microbiol. 1982;16:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin M, Turco JH, Zegans ME, et al. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniae. N Engl J Med. 2003;348:1112–1121 [DOI] [PubMed] [Google Scholar]
- 19. Crum NF, Barrozo CP, Chapman FA, Ryan MA, Russell KL. An outbreak of conjunctivitis due to a novel unencapsulated Streptococcus pneumoniae among military trainees. Clin Infect Dis. 2004;39:1148–1154 [DOI] [PubMed] [Google Scholar]
- 20. Buck JM, Shapiro M, Glennen A, et al. A community outbreak of conjunctivitis caused by nontypeable Streptococcus pneumoniae in Minnesota. Pediatr Infect Dis J. 2006;25:906–911 [DOI] [PubMed] [Google Scholar]
- 21. Hanage WP, Kaijaleinen T, Saukkoriipi A, Rickcord JL, Spratt BG. A successful, diverse disease-associated lineage of nontypeable pneumococci that has lost the capsular biosynthesis locus. J Clin Microbiol. 2006;44:743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biagini RE, Schlottmann SA, Sammons DL, et al. Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides. Clin Diagn Lab Immunol. 2003;10:744–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol. 2006;44:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sung CK, Claverys JP, Morrison DA. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Envir Microbiol. 2001;67:5190–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trzcinski K, Thompson CM, Lipsitch M. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl Envir Microbiol. 2003;69:7364–7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Norcross EW, Tullos NA, Taylor SD, Sanders ME, Marquart ME. Assessment of Streptococcus pneumoniae capsule in conjunctivitis and keratitis in vivo: neuraminidase activity increases in nonencapsulated pneumococci following conjunctival infection. Curr Eye Res. 2010;35:787–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pozzi G, Masala L, Iannelli F, et al. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J Bacteriol. 1996;178:6087–6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Callegan MC, Booth MC, Jett BD, Gilmore MS. Pathogenesis of gram-positive bacterial endophthalmitis. Infect Immun. 1999;67:3348–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hobden JA, Hill JM, Engel LS, et al. Age and therapeutic outcome of experimental Pseudomonas aeruginosa keratitis treated with ciprofloxacin, prednisolone, and flurbiprofen. Antimicrob Agents Chemother. 1993;37:1856–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramirez K, Huerta R, Oswald E, et al. Role of EspA and intimin in expression of proinflammatory cytokines from enterocytes and lymphocytes by rabbit enteropathogenic Escherichia coli-infected rabbits. Infect Immun. 2005;73:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson MK, Hobden JA, Hagenah M, et al. The role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr Eye Res. 1990;9:1107–1114 [DOI] [PubMed] [Google Scholar]
- 32. Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6:849–858 [DOI] [PubMed] [Google Scholar]
- 34. Yoshida K, Ekstedt RD. Antibody response to Staphylococcus aureus in rabbits: sequence of immunoglobulin synthesis and its correlation with passive protection in mice. J Bacteriol. 1968;96:1540–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Metrikin DC, Wilson CA, Berkowitz BA, Lam MK, Wood GK, Peshock RM. Measurement of blood-retinal barrier breakdown in endotoxin-induced endophthalmitis. Invest Ophthalmol Vis Sci. 1995;36:1361–1370 [PubMed] [Google Scholar]
- 36. Winkelstein JA, Abramovitz AS, Tomasz A. Activation of C3 via the alternative complement pathway results in fixation of C3b to the pneumococcal cell wall. J Immunol. 1980;124:2502–2506 [PubMed] [Google Scholar]
- 37. Brown EJ, Hosea SW, Frank MM. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev Infect Dis. 1983;5(suppl 4):S797–S805 [DOI] [PubMed] [Google Scholar]
- 38. Abeyta M, Hardy GG, Yother J. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect Immun. 2003;71:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy. 2007;92:50–57 [DOI] [PubMed] [Google Scholar]
- 40. Giese MJ, Sumner HL, Berliner JA, et al. Cytokine expression in a rat model of Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci. 1998;39:2785–2790 [PubMed] [Google Scholar]
- 41. Petropoulos IK, Vantzou CV, Lamari FN, et al. Expression of TNF-alpha, IL-1beta, and IFN-gamma in Staphylococcus epidermidis slime-positive experimental endophthalmitis is closely related to clinical inflammatory scores. Graefes Arch Clin Exp Ophthalmol. 2006;244:1322–1328 [DOI] [PubMed] [Google Scholar]
- 42. Bootsma HJ, Egmont-Petersen M, Hermans PWM. Analysis of the in vitro transcriptional response of human pharyngeal epithelial cells to adherent Streptococcus pneumoniae: evidence for a distinct response to encapsulated strains. Infect Immun. 2007;75:5489–5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fiorentino DF, Zlotnick A, Viera P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451 [PubMed] [Google Scholar]
- 44. Colditz I, Zwahlen R, Dewald B, et al. In vivo inflammatory activity of neutrophil- activating factor by monomeric interleukin-8. Am J Pathol. 1989;134:755–760 [PMC free article] [PubMed] [Google Scholar]
- 45. Hyams C, Camberlein E, Cohen JM, et al. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010;78:704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]