Retinal arteriolar and venular calibers have a high phenotypic correlation caused by a shared genetic correlation. Identifying vessel pleiotropic genes will elucidate the nature of vessel association with specific cardiovascular disease risk factors.
Abstract
Purpose.
Retinal arteriolar and venular calibers are highly heritable and associated with cardiovascular disease. This study was designed to investigate the relative influence of genetic and environmental factors on the high phenotypic correlation (r = 0.59) between these two traits and to assess the shared and specific influence of established and novel cardiovascular disease risk factors on them.
Methods.
A total of 1463 Caucasian female twins (706 monozygotic and 757 dizygotic), between 24 and 79 years of age, underwent retinal photography from which retinal arteriolar (mean, 153.75 ± 22.1 μm, SD) and venular (mean, 232.1 ± 36.6 μm) calibers were measured with semiautomated software. A bivariate heritability model was used to assess the genetic and environmental influences underlying both specific trait variance and the covariance between the vessel traits. The investigation was an assessment of phenotypic associations between retinal arteriolar and venular calibers and cardiovascular disease risk factors.
Results.
Additive genetic factors accounted for approximately three fourths of the covariance between retinal arteriolar and venular calibers within the cohort. This finding was replicated in a sample of 1981 twins from the Australian Twins Eye Study. The partial correlation showed that known risk factors accounted for only 5% of the covariance between arteriolar and venular calibers. Novel associations were found between venular caliber and β-cell function (P = 0.011) and insulin sensitivity (P = 0.002).
Conclusions.
These results suggest that future gene-mapping studies may identify pleiotropic genetic variants influencing both retinal arteriolar and venular calibers. Genetic variants associated with retinal caliber and (risk factors for) cardiovascular disease should provide new etiologic insights into this complex disease.
Cardiovascular disease (CVD) accounts for almost one third of deaths worldwide.1 Metabolic syndrome consists of the presence of several CVD risk factors, such as central obesity, hyperglycemia, dyslipidemia, and hypertension and can itself be considered a further risk factor for CVD.2 Retinal vessel caliber has been associated with CVD risk factors and outcomes and may serve as a prognostic factor.3,4
As reviewed by Sun et al.,5 arteriolar caliber is more consistently associated with blood pressure, and venular caliber is more consistently associated with markers of inflammation and stroke. However, both arteriolar and venular calibers have been reported to be associated with several other CVD risk factors including markers of obesity, hypertension, and diabetes. Despite the evidence that these are two distinct phenotypes, arteriolar and venular calibers have a high phenotypic correlation,6 but the relative contribution of genetic and environmental factors to this correlation is unknown. Xing et al.7 observed both overlapping and distinct signals for retinal arteriolar and venular calibers in a linkage scan of 1762 individuals. By contrast, other groups have reported only distinct loci associated with arteriolar or venular caliber, but not both measures.8–10
Identification of pleiotropic and specific-effect genes that influence retinal arteriolar and venular calibers should also provide insights into those influencing CVD risk factors. Because of the apparent complexity of associations between arteriolar and venular caliber, we first must investigate the relationship between these vessel traits. The purposes of this study were to investigate (1) the relative importance of genetic and environmental factors influencing this relationship, by using a classic twin study for a sample from the Twins UK Adult Twin Registry and for a replication sample of twins participating in the Australian Twins Eye Study, and (2) the relationship between epidemiologic risk factors and each of the vessel measures and influence, if any, on the covariance between the two traits.
Methods
Subjects
The subjects were recruited from the Twins UK Adult Twin Registry, based at St. Thomas' Hospital, London.11 The study was reviewed by the St. Thomas' Local Research Ethics Committee, and the investigation conformed to the principles outlined in the Declaration of Helsinki. The twins were unaware of the proposal of an eye test and any associated hypotheses at recruitment, but gave informed consent before the visit to have eye examinations, including those described below.
A total of 1463 Caucasian female twins (706 monozygotic [MZ] and 757 dizygotic [DZ]; comprising 694 twin pairs and 75 single twins), between 24 and 79 (mean, 60) years of age, participated. Of these, 1012 twins (466 MZ and 546 DZ) were examined between 1998 and 1999 as part of the Twins UK project. The remaining 451 twins (240 MZ and 21 DZ) were examined between 2008 and 2009 as part of the Healthy Aging Twin Study (HATS). Zygosity was initially determined by using a standardized questionnaire.12 Zygosity has since been confirmed with genomewide data, where single-nucleotide polymorphism data from the dual array (317k HumanHap; Illumina, San Diego, CA) were used for all samples to estimate genomewide mean identity by descent. As a result, zygosity was reclassified for two sets of the twins included in the present study.
Replication of the bivariate heritability analysis was performed in data from 1981 (785 MZ and 1170 DZ) male and female (817 and 1110, respectively) twins, of whom 948 were twin pairs and 85 were single twins, between 5 and 90 (mean, 21) years of age. This cohort was recruited and phenotyped as part of the Australian Twin Eye Study. Sex-limitation modeling had been performed on this cohort10 and showed that there were no differences between the sexes in arteriolar caliber but that additive genetic factors accounted for a slightly higher proportion of the variance of CRVE in the males (63.9%) than in the females (60.4%). The exclusion of male twins from our data (n = 1100) did not qualitatively affect the outcome of the bivariate model results.
Clinical Examination
Eyes were dilated with 10% phenylephrine hydrochloride and 1% tropicamide solution (n = 1333), except when the subject refused (n = 129). For the 1012 subjects in the Twins UK study, fundus photography was performed (camera; Kowa, Nagoya, Japan; film, Ektachrome 64; Eastman Kodak, Rochester, NY). Thirty-degree, simultaneous, stereoscopic fundus images of 165 of the HATS subjects were taken with a nonmydriatic fundus camera (model 3-DX; Nidek, Gamagori, Japan; film, Polaroid, Minnetonka, MN). Digital fundus images of the remaining 285 HATS subjects were taken with a nonmydriatic fundus camera (model AFC210; Nidek). Nondigital images were digitized before analysis. Twins were excluded if the images taken could not be analyzed because of incorrect illumination or poor focusing (42 MZ and 37 DZ twins).
Fifteen CVD risk factors were measured by trained personnel by standard methods: age, smoking status (categorized as former, current, or never), mean arterial blood pressure (MABP; calculated from a single measure of systolic and diastolic blood pressures), white blood cell (WBC) count, platelets, triglyceride, HDL (high density lipoprotein) cholesterol, LDL (low density lipoprotein) cholesterol, total serum cholesterol, and Hcy (homocysteine), BMI (body mass index), fasting serum glucose and insulin levels, HOMA2 (homeostasis model assessment) β-cell function (HOMA2-%B), and insulin sensitivity (HOMA2-%S) compared with a middle-aged healthy population.13,14 Blood pressure was measured with an automatic device (mb02 Marshall sphygmomanometer; Omron Healthcare, Inc., Bannockburn, IL), where subjects were sitting upright; two measurements were taken, the first of which was used for this study. Participants fasted for a minimum of 6 hours before blood tests. HOMA2-%B and HOMA2-%S were calculated from glucose and insulin values; HOMA2-%B and HOMA2-%S should be considered and interpreted in conjunction with one another.13,14
To avoid ascertainment bias, we increased the systolic blood pressure (SBP) readings from individuals taking antihypertensive medication (n = 215) by 10 mm Hg, as suggested by Tobin et al.15; diastolic blood pressure (DBP) remained unaltered. Mean arterial blood pressure (MABP) was calculated using adjusted SBP.
Vessel Measurement
Twins UK images were analyzed by two graders from the Blue Mountains Eye Study who have reported high intra and intergrader correlations.16 HATS images were analyzed by our Twins UK grader, with intragrader correlations of 0.89 and 0.99 for CRAE (central retinal artery equivalent) and CRVE (central retinal vein equivalent), respectively. All images were graded using Ivan 1.1 (University of Wisconsin),17 which uses formulas described by Knudtson et al.18 and revisions described by Parr and Spears19 and Hubbard et al.20 The measurements were summarized as central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE).
Analytical Approach
Before analysis, vessel measurements were adjusted for camera, pupil dilation, age, zygosity, and spherical equivalent refraction. The resultant CRAE and CRVE residuals were normally distributed, with skewness and kurtosis values of approximately 0 and 3, respectively, across all datasets.
Heritability Modeling
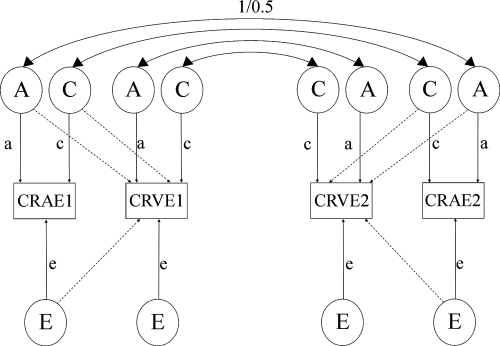
The classical twin model can be used to partition the phenotypic variance and quantify the relative influence of genetic and environmental factors on individual variation around a population mean.21 The model assumes no epistasis, gene–environment correlation or interactions and that shared environmental factors are not confounded by zygosity (the equal environments assumption). MZ twins generally share identical genes, whereas DZ twins share, on average, half their segregating genes. To infer heritability, therefore, greater MZ similarity compared to DZ twins is attributed to genetic factors. Maximum likelihood modeling was conducted, which allows detailed modeling of the variance and covariance within MZ twin pairs compared with DZ twin pairs.22 For univariate heritability estimates, the total phenotypic variance is partitioned into additive (A), dominant (D), genetic and shared familial (common; C), and unique to the individual (E) environmental components, by using a Cholesky decomposition; E, incorporates measurement error. Variance components D and C, however, cannot be modeled simultaneously for data that is limited to twin pairs reared together, because of the confounding of these components. The ratio of the genetic variance to the total phenotypic variance provides an estimate of the narrow-sense heritability (h2 = A/(A +D + C + E)), where either C or D has been fixed to 0. The Cholesky decomposition naturally extends from univariate to multivariate analyses. We implemented a bivariate Cholesky decomposition (Fig. 1), in which the covariance between CRAE and CRVE was partitioned in addition to the variance of each phenotype.23 Point estimates for each component were estimated to determine to what extent the covariation between CRAE and CRVE is due to genetic and environmental factors. A nested model design was used to assess the statistical significance of the variance components of interest. Three tests assessed fixing the following parameters of interest to 0: (1) all three additive genetic parameters (3 df); (2) additive genetic covariance parameter (1 df); and (3) all three common environmental parameters (3 df). A goodness-of-fit test was implemented to assess deterioration in model fit to the observed data, by using the Akaike's information criterion (AIC).
Figure 1.
Bivariate Cholesky decomposition ACE model. Observed phenotypes, CRAE and CRVE for twins 1 and 2 are shown in the squares, while latent factors A (additive genetic), C (common environment), and E (unique environmental) are shown in the circles. Lowercase letters represent the path coefficients. Solid arrows: proportion of variance (a2, c2, and so forth) due to one observed phenotype; dashed arrows: covariance between the two observed phenotypes. Correlations between latent genetic factors are assumed to be 1.0 in MZ twins and 0.5 in DZ twins.
The estimated genetic covariance between two traits can be presented as a proportion of the total phenotypic covariance (bivariate heritability) or alternatively, as a proportion of the total genetic variance for both traits (genetic correlation). The bivariate heritability can be interpreted as the proportion of a phenotypic correlation that can be indirectly attributed to additive polygenic causal factors in a population, whereas the genetic correlation is the proportion of total genetic variance attributable to shared additive polygenic effects between the two traits.
Epidemiologic Risk Factors
Associations between each vessel caliber measure and CVD risk factors were initially investigated by using univariate linear regression that accounts for family structure (the regression cluster option; Stata, ver. 10; Stata Corp., College Station, TX). Because of the number of variables investigated, multiple testing was considered to control the type 1 error rate. To account for the underlying correlation structure between variables, we estimated the effective number of independent tests to be 1.65 for the two vessel traits and 12.0 for the 15 correlated risk factors, when a simple linear regression method was used.24 These values were then used to perform a Bonferroni correction [α = 0.05/(1.65 × 12.0) = 0.0025].
Partial correlation analyses were used to assess to what extent the phenotypic correlation between the vessel traits could be attributed to identified associations with CVD risk factors. Univariate associated risk factors were subsequently selected for inclusion in the partial correlation analysis based on a significant association with at least one of the vessel traits, after controlling for multiple testing (P < 0.003). Partial correlation is comparable to multiple regression, but facilitates interpretation of effect size and estimates the residual correlation between two variables (CRAE and CRVE), taking into account other specified variables (CVD risk factors). A large reduction in the correlation between CRAE and CRVE in the presence of the other risk factors would implicate the role of the risk factors in the relationship between CRAE and CRVE.25
Data preparation, preliminary analyses, and statistical tests were performed in commercial software (Stata, ver. 10; Stata Corp.). Genetic modeling was performed using Mx (Department of Psychiatry, Medical College of Virginia, Richmond, VA). With the exception of smoking status, all data were analyzed as quantitative traits.
Results
Retinal vessel caliber measurements from 1384 twins (668 MZ and 716 DZ) were used in the analyses. Mean calibers (± SD) for MZ and DZ twins for CRAE were 152.3 (21.8) μm and 155.2 (22.3) μm and for CRVE, 229.0 (39.9) μm and 235.2 (39.3) μm, respectively (Table 1). No significant differences in means or variances were observed in baseline characteristics between MZs and DZs.
Table 1.
Baseline Characteristics of Vessel Traits and Cardiovascular Disease Risk Factors
| MZ | n | DZ | n | |
|---|---|---|---|---|
| CRAE, μm | 152.3 (21.8) | 668 | 155.2 (22.3) | 716 |
| CRVE, μm | 229.0 (39.9) | 668 | 235.2 (39.3) | 716 |
| Age, y | 59.5 (8.1) | 668 | 59.3 (7.9) | 716 |
| Spherical equivalent, D | 0.1 (2.5) | 661 | 0.2 (2.4) | 706 |
| Never smoked, % | 57.5 | 367 | 55.7 | 383 |
| Former smoker, % | 30.3 | 193 | 30.1 | 207 |
| Current smoker, % | 12.2 | 78 | 14.2 | 98 |
| MABP, mm Hg | 95.7 (13.3) | 665 | 96.4 (12.9) | 696 |
| WBC count, ×10−9/L | 6.3 (1.9) | 453 | 6.5 (3.0) | 521 |
| Platelets, ×103/mL | 245.1 (62.5) | 453 | 245.2 (61.7) | 518 |
| Triglyceride, mmol/L | 1.3 (0.8) | 654 | 1.3 (0.8) | 678 |
| HDL, mmol/L | 1.7 (0.5) | 655 | 1.6 (0.5) | 684 |
| LDL, mmol/L | 3.5 (1.0) | 499 | 3.3 (1.0) | 494 |
| Cholesterol, mmol/L | 6.0 (1.2) | 657 | 6.0 (1.2) | 688 |
| Homocysteine, μmol/L | 11.7 (3.7) | 514 | 11.2 (4.1) | 584 |
| BMI, kg/m2 | 25.4 (4.4) | 663 | 26.1 (4.6) | 707 |
| HOMA2-% B, % of ref | 84.1 (37.6) | 482 | 90.4 (41.5) | 480 |
| HOMA2-%S, % of ref | 170.0 (107.5) | 482 | 159.2 (92.7) | 480 |
| Insulin, pmol/L | 46.8 (35.7) | 515 | 49.4 (38.0) | 509 |
| Glucose, pmol/L | 5.0 (0.6) | 527 | 4.9 (0.8) | 518 |
| Diabetic, % | 2.4 | 10 | 2.1 | 11 |
Data are expressed as the mean (±SD). HOMA2 is expressed as a percentage of a reference population of normal adults.
Heritability Modeling
Significantly higher intraclass correlations for CRAE and CRVE were observed for the 668 MZ twins (0.63 and 0.70, respectively) compared with the 716 DZ twins (0.33 and 0.33, respectively). The cross-twin, cross-trait correlations were approximately twice as large in the MZ (r = 0.44) compared with the DZ twin pairs (r = 0.23). Table 2 shows the bivariate model summary statistics for the full ACE model and nested models for three tests fitted to the U.K. cohort data (models 1–4) and the Australian replication cohort data (models 5–8). Heritability estimates for CRAE and CRVE of 66% and 72%, respectively, were observed using a bivariate heritability model for the U.K. cohort (model 1; Table 2) and were identical with those obtained from univariate heritability analyses (analysis not presented). The bivariate model suggests that approximately 77% (95% confidence interval [CI], 69%–83%) of the covariance between CRAE and CRVE is due to additive genetic factors, with the remaining 23% (95% CI, 17%–31%) attributable to unique environmental effects. Removing all three additive genetic components from the model caused a significant decline in model fit (Δχ23 = 91.3; P < 0.001; model 2), as did removing only the genetic covariance component (Δχ21 = 37.6; P < 0.001; model 3). Common environmental components for the phenotypic variance and the covariance between CRAE and CRVE in the U.K. cohort (model 1). As a result, there was no decline in model fit when these parameters were removed (model 4). Model 4 was selected as best fitting for the U.K. cohort, as it had fewer parameters than the full model without causing a significant decline in model fit. The genetic correlation for the best-fitting model for the U.K. cohort was 0.65 (95% CI, 59%–71%).
Table 2.
Model Fit Statistics for Bivariate Heritability Analysis
| Source/Model | np | −2 LL | df | AIC | Δdf | Δχ2 | P | Variance Component | CRAE | Covariance | CRVE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UK twins | |||||||||||
| 1. ACE | 9 | 20,762.994 | 2,491 | 15,780.994 | — | — | — | A | 0.66 (0.48–0.72) | 0.77 (0.55–0.84) | 0.72 (0.58–0.77) |
| C | 0.00 (0.00–0.15) | 0.00 (−0.04–0.19) | 0.00 (0.00–0.12) | ||||||||
| E | 0.34 (0.28–0.40) | 0.23 (0.17–0.31) | 0.28 (0.23–0.34) | ||||||||
| 2. CE | 6 | 20,854.29 | 2,494 | 15,866.29 | 3 | 91.296 | <0.001 | C | 0.47 (0.40–0.52) | 0.55 (0.46–0.62) | 0.50 (0.44–0.56) |
| E | 0.53 (0.48–0.60) | 0.45 (0.38–0.54) | 0.50 (0.44–0.56) | ||||||||
| 3. ACE | 8 | 20,800.588 | 2,492 | 15,816.588 | 1 | 37.595 | <0.001 | A | 0.20 (0.07–0.31) | — | 0.33 (0.21–0.43) |
| C | 0.34 (0.24–0.44) | 0.56 (0.48–0.63) | 0.29 (0.21–0.39) | ||||||||
| E | 0.46 (0.39–0.54) | 0.44 (0.37–0.53) | 0.38 (0.32–0.45) | ||||||||
| 4. AE | 6 | 20,762.994 | 2,494 | 15,774.994 | 3 | 0 | NS | A | 0.66 (0.60–0.72) | 0.77 (0.69–0.83) | 0.72 (0.66–0.77) |
| E | 0.34 (0.28–0.40) | 0.23 (0.17–0.31) | 0.28 (0.23–0.34) | ||||||||
| Australian twins | |||||||||||
| 5. ACE | 9 | 30,088.131 | 3,759 | 22,570.131 | — | — | — | A | 0.52 (0.37–0.65) | 0.81 (0.63–0.94) | 0.73 (0.62–0.77) |
| C | 0.17 (0.05–0.29) | −0.01 (−0.11–0.15) | 0.00 (0.00–0.10) | ||||||||
| E | 0.32 (0.27–0.37) | 0.19–0.14–0.26) | 0.27 (0.23–0.32) | ||||||||
| 6. CE | 6 | 30,196.188 | 3,762 | 22,672.188 | 3 | 108.057 | <0.001 | C | 0.53 (0.48–0.57) | 0.56 (0.48–0.62) | 0.50 (0.45–0.55) |
| E | 0.47 (0.43–0.52) | 0.44 (0.38–0.52) | 0.20 (0.45–0.55) | ||||||||
| 7. ACE | 8 | 30,145.085 | 3,760 | 22,625.085 | 1 | 56.954 | <0.001 | A | 0.14 (0.01–0.26) | — | 0.40 (0.28–0.48) |
| C | 0.43 (0.33–0.53) | 0.59 (0.52–0.65) | 0.24 (0.17–0.33) | ||||||||
| E | 0.43 (0.37–0.49) | 0.41 (0.35–0.48) | 0.36 (0.32–0.42) | ||||||||
| 8. AE | 6 | 30,098.206 | 3,762 | 22,574.206 | 3 | 10.075 | 0.018 | A | 0.70 (0.65–0.74) | 0.80 (0.74–0.85) | 0.73 (0.68–0.77) |
| E | 0.30 (0.26–0.35) | 0.20 (0.15–0.26) | 0.27 (0.23–0.32) | ||||||||
| 9. ACE | 7 | 30,088.143 | 3,761 | 22,566.143 | 2 | 0.012 | 0.994 | A | 0.51 (0.39–0.63) | 0.80 (0.74–0.86) | 0.73 (0.68–0.77) |
| C | 0.17 (0.07–0.27) | — | — | ||||||||
| E | 0.32 (0.27–0.37) | 0.20 (0.14–0.26) | 0.27 (0.23–0.32) |
Values show estimated proportion of variance attributable to A, additive genetic, C, common environmental and E, unique environmental effects (95% CIs). Shaded data denote best-fitting model. Vessel traits are adjusted for age, camera, pupil dilation, zygosity, and spherical equivalent, np, number of freely estimated parameters in model; −2 LL, −2 times log likelihood.
Similar results were observed in the Australian replication cohort, with a phenotypic correlation between the vessel traits of 0.55. Removing all three additive genetic components (model 6) from the full model (model 5) caused a significant decline in model fit. However, a modest shared familial effect of 17% (95% CI, 5%–29%) was detected for CRAE in the Australian cohort. Hence, the most parsimonious model for these data was the ACE model (2 df; model 9). Consistent with the U.K. results, heritability estimates of 52% and 73% were observed in the Australian cohort for CRAE and CRVE, respectively, and approximately 81% of the covariance was observed to be attributable to additive genetic effects (model 5). The genetic correlation for the best-fitting model for the Australian cohort was 0.73 (95% CI, 64%–85%; analysis not presented). Removal of either mixed-sex twins or those less than 18 years of age did not substantially affect the results.
Epidemiologic Risk Factors
CRAE was significantly associated with age, former smoking status, and MABP, and CRVE was significantly associated with age, former and current smoking status, MABP, Hcy, BMI, insulin, HOMA2-%B, and HOMA2-%S in univariate linear regressions (P ≤ 0.011; data not shown). These variables were selected for inclusion in the partial correlation. Multiple regression showed that the combined effects of all these associated risk factors accounted for 11% and 7% of the variance of CRAE and CRVE, respectively.
Table 3A presents partial correlation results between CRAE and CRVE, accounting for the associated risk factors, and Table 3B shows the partial correlation between CRVE and CRAE, accounting for the same risk factors. The correlation between the two vessel traits was independent of the associated risk factors (P < 0.001). The combined effects of these risk factors reduced the partial correlation between the vessel traits from r = 0.59 to r = 0.56, equivalent to only 5% of the covariance between CRAE and CRVE (Table 3). Table 3A shows evidence that the correlation between CRAE and MABP (<0.001) is independent of CRVE and the other associated risk factors. Table 3B shows evidence that the correlations between CRVE and former and current smoking status (P < 0.001), BMI (P = 0.042), and HOMA2-%S (P = 0.009) are independent of CRAE and the other associated risk factors.
Table 3.
Partial Correlation between Retinal Arteriolar and Venular Caliber Adjusted for Associated Covariates, Identified from Univariate Linear Regression
| A. Partial Correlation between CRAE and CRVE | ||
|---|---|---|
| Variable | CRAEr | P |
| CRVE | 0.56 | <0.001 |
| Age | −0.06 | 0.087 |
| Former smoker | −0.09 | 0.009 |
| Current smoker | −0.01 | 0.724 |
| MABP | −0.26 | <0.001 |
| BMI | −0.02 | 0.485 |
| Insulin | 0.02 | 0.417 |
| HOMA2-%B | 0.00 | 0.905 |
| HOMA2-%S | 0.06 | 0.042 |
| B. Partial Correlation between CRVE and CRAE | ||
|---|---|---|
| Variable | CRAEr | P |
| CRAE | 0.56 | <0.001 |
| Age | −0.05 | 0.147 |
| Former smoker | 0.11 | 0.001 |
| Current smoker | 0.14 | <0.001 |
| MABP | 0.06 | 0.069 |
| BMI | 0.07 | 0.041 |
| Insulin | −0.01 | 0.708 |
| HOMA2-%B | 0.01 | 0.868 |
| HOMA2-%S | −0.09 | 0.009 |
HOMA2, homeostasis model assessment 2 where B stands for beta cell function and S for sensitivity. HOMA2 is expressed as a percentage of a reference population of normal adults. The unadjusted phenotypic correlation between CRAE and CRVE is r = 0.59. Vessel traits are adjusted for age, camera, pupil dilation, zygosity, and spherical equivalent.
Additional partial correlations were also performed between the vessel traits, to assess each of the associated risk factors individually. Age, BMI, MABP, and insulin did not affect the partial correlation between vessel traits, and former and current smoking status reduced the partial correlation to r = 0.58, whereas HOMA2-%B and HOMA2-%S reduced the partial correlation to r = 0.57, the latter being equivalent to only 3% of the covariance between CRAE and CRVE. While HOMA2-%B and HOMA2-%S both phenotypically correlated with the venules at r = 0.1 (P = 0.002), neither correlated with the arterioles (r = −0.004, P > 0.05).
Discussion
Retinal arteriolar and venular calibers have a high phenotypic correlation yet have distinct phenotypic associations with CVD risk factors. To further investigate the complex relationship between these two traits, we performed a bivariate heritability analysis to assess the relative influences of genetic and environmental factors on the two traits. For both the U.K. and Australian twin samples, we report that approximately three fourths of the covariance between arteriolar and venular caliber was attributable to additive genetic effects, which suggests that shared genetic factors influence both arteriolar and venular calibers, even though each trait is reproducibly associated with different CVD risk factors. The genetic correlation for arteriolar and venular calibers was 65% in the U.K. cohort and 74% in the Australian cohort, indicating that most of the total genetic variance for arteriolar and venular caliber is attributable to the shared genetic covariance between them. However, the two traits are also individually heritable with specific genetic factors to each. Retinal arteriolar and venular caliber heritability was approximately 60% and 70%, respectively, for the U.K. and Australian cohorts, suggesting that the variance of each of these traits is also individually influenced by genetic factors.10,26
Overall, these results suggest that where pleiotropic effects exist, studies may be expected to have more power to detect genetic variants that influence both arteriolar and venular diameters. This finding implies a shared etiology between the vessel traits that may assist in understanding the etiology of CVD. By way of illustration, in a linkage study of 1762 individuals from 602 families, Xing et al.7 reported both distinct and overlapping signals for the two vessel traits. Among the list of candidate genes produced based on the overlapping signals, Tie1, End-1, and HGF are all genes encoding proteins that are involved in endothelium-derived nitric oxide synthase–related pathways. These genes may, therefore, be important in elucidating the role of endothelial dysfunction and inflammation in retinal vessel caliber changes.
We have reported in this study a novel association between venular caliber with β-cell function (HOMA2-%B) and insulin sensitivity (HOMA2-%S). Genetic variants influencing only venular caliber, may therefore also be related to glucose homeostasis-related phenotypes. We may also expect to find specific genetic variants that influence only arteriolar caliber and possibly blood pressure. The literature demonstrates that arteriolar compared with venular caliber is more consistently associated with increased blood pressure.5,6 Our findings support this notion, where the effect size was approximately twice as large for arterioles compared with venules in the univariate linear regressions. However, in addition to this finding, we have reported an independent correlation between arteriolar caliber and blood pressure, but not for venular caliber and blood pressure. This confounding result may explain why the association between venular caliber and blood pressure is much less consistent in the literature. It also suggests a difference in the mechanism by which blood pressure contributes to, or is affected by, arteriolar compared to venular caliber that may be physiologically relevant.
The combined effects of measured associated risk factors accounted for little of the total covariance between these two traits (5%). To the extent that the tested risk factors did influence the relationship between these vessel traits, insulin sensitivity was the most important individual risk factor, explaining approximately 3% of the covariance between retinal arteriolar and venular caliber. Nevertheless, the effect size of insulin sensitivity on the relationship between retinal vessel calibers was not sufficiently large to facilitate a formal variance components test for the relative importance of genetic and environmental factors.
The twin subjects in this study were volunteers, but had been validated against a population-based sample of singletons. With the exception of weight, no significant differences were found between the two groups.27 In this study, both vessel calibers and baseline characteristics were similar to population-based studies.6,28–32 A common environmental effect was detected for the Australian cohort. The Australian cohort included children and was, on average, younger than the U.K. cohort. The difference suggests that familial common environmental influences may be more readily detected during childhood and adolescence, presumably due to a greater number of shared familial experiences at that age compared with adulthood. MZ and DZ means for HOMA2-%B were lower than the reference population, whereas HOMA2-%S was higher than the normal reference population from which they are calculated (Table 1).
We have demonstrated the importance of the relationship between retinal arteriolar and venular caliber in CVD research. We report that the high phenotypic correlation (r = 0.59) observed between retinal arteriolar and venular calibers is primarily genetically mediated but with little or no influence from established measures of CVD risk. The observed high genetic correlation between retinal vessel calibers may have implications for CVD research and will assist in the detection of causal functional variants. Further work is needed to understand mechanisms underlying the correlation between retinal vessel calibers with CVD. Identifying causal variants that influence retinal arteriolar and venular diameters both individually and pleiotropically will provide a useful starting point for this task.
Acknowledgments
The Twins UK authors thank Rob Marchant for his hard work in grading all the images and the volunteer twins who made available their time to research; and The Australian Twin Registry authors thank Jane MacKinnon, Shayne Brown, Lisa Kearns, Sandra Staffieri, Olivia Bigault, Colleen Wilkinson, Julie Barbour, Alex Hewitt, Jamie Craig, Jonathan Ruddle, and Paul Sanfilippo, who assisted with clinical examinations.
Footnotes
Supported by the Wellcome Trust; the European Union MyEuropia Marie Curie Research Training Network; Guide Dogs for the Blind Association; the European Community's Seventh Framework Programme (FP7/2007–2013)/grant agreement HEALTH-F2-2008-201865-GEFOS and (FP7/2007–2013); ENGAGE project grant agreement HEALTH-F4-2007-201413 and the FP-5 genomEUtwin Project (QLG2-CT-2002-01254); the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St. Thomas' NHS (National Health Service) Foundation Trust in partnership with King's College London; Biotechnology and Biological Sciences Research Council (BBSRC) Project Grant G20234; National Institutes of Health (NIH)/National Eye Institute Grant 1R01EY018246; and the NIH Center for Inherited Diseases Research (CIDR) (PI: Terri Young); Clifford Craig Medical Research Trust; the Ophthalmic Research Institute of Australia (ORIA); Glaucoma Australia; the American Health Assistance Foundation (AHAF); the Peggy and Leslie Cranbourne Foundation; the Foundation for Children; National Health and Medical Research Council (NHMRC) Project Grant 2005–2007; the Jack Brockhoff Foundation; and National Eye Institute [NEI] Project Grant 2007–2010. The Australian Twin Registry is supported by Australian NHMRC Enabling Grant 2004–2009. DAM is a recipient of the Pfizer Australia Senior Research Fellowship. TDS is an NIHR Senior Investigator and CJH is an NIHR Senior Research Fellow.
Disclosure: S.J. Fahy, None; C. Sun, None; G. Zhu, None; P.R. Healey, None; T.D. Spector, None; N.G. Martin, None; P. Mitchell, None; T.Y. Wong, None; D.A. Mackey, None; C.J. Hammond, None; T. Andrew, None
References
- 1. World Health Organisation Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: WHO; 2009 [Google Scholar]
- 2. Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689 [DOI] [PubMed] [Google Scholar]
- 3. McGeechan K, Liew G, Macaskill P, et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009;151:404–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGeechan K, Liew G, Macaskill P, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009;170:1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54:74–95 [DOI] [PubMed] [Google Scholar]
- 6. Liew G, Sharrett AR, Kronmal R, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48:52–57 [DOI] [PubMed] [Google Scholar]
- 7. Xing C, Klein BE, Klein R, et al. Genome-wide linkage study of retinal vessel diameters in the Beaver Dam Eye Study. Hypertension. 2006;47:797–802 [DOI] [PubMed] [Google Scholar]
- 8. Khor CC, Fan Q, Goh LK, et al. Hepatocyte growth factor and retinal arteriolar diameter in Singapore Chinese. Ophthalmology. 2010;117:939–945 [DOI] [PubMed] [Google Scholar]
- 9. Tanabe Y, Kawasaki R, Wang JJ, et al. Angiotensin-converting enzyme gene and retinal arteriolar narrowing: the Funagata Study. J Hum Hypertens. 2009;23:788–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun C, Zhu G, Wong TY, et al. Quantitative genetic analysis of the retinal vascular caliber: the Australian Twins Eye Study. Hypertension. 2009;54:788–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spector TD, Williams FM. The UK Adult Twin Registry (Twins UK). Twin Res Hum Genet. 2006;9:899–906 [DOI] [PubMed] [Google Scholar]
- 12. Martin NG, Martin PG. The inheritance of scholastric abilities in a sample of twins. I. Ascertainments of the sample and diagnosis of zygosity. Ann Hum Genet. 1975;39:213–218 [DOI] [PubMed] [Google Scholar]
- 13. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192 [DOI] [PubMed] [Google Scholar]
- 14. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 15. Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935 [DOI] [PubMed] [Google Scholar]
- 16. Sherry LM, Wang JJ, Rochtchina E, et al. Reliability of computer-assisted retinal vessel measurement in a population. Clin Exp Ophthalmol. 2002;30:179–182 [DOI] [PubMed] [Google Scholar]
- 17. Wong TY, Islam FM, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006;47:2341–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149 [DOI] [PubMed] [Google Scholar]
- 19. Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol. 1974;77:472–477 [DOI] [PubMed] [Google Scholar]
- 20. Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280 [DOI] [PubMed] [Google Scholar]
- 21. Martin N, Boomsma D, Machin G. A twin-pronged attack on complex traits. Nat Genet. 1997;17:387–392 [DOI] [PubMed] [Google Scholar]
- 22. Neale MC, Cardon LR. Model fitting functions and optimization. 1992;1st:131–148 [Google Scholar]
- 23. McCaffery JM, Snieder H, Dong Y, de Geus E. Genetics in psychosomatic medicine: research designs and statistical approaches. Psychosom Med. 2007;69:206–216 [DOI] [PubMed] [Google Scholar]
- 24. Camp NJ, Farnham JM. Correcting for multiple analyses in genomewide linkage studies. Ann Hum Genet. 2001;65:577–582 [DOI] [PubMed] [Google Scholar]
- 25. Neale MC, Cardon LR. eds. The scope of genetic analyses. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992:1–31 [Google Scholar]
- 26. Taarnhoj NC, Larsen M, Sander B, et al. Heritability of retinal vessel diameters and blood pressure: a twin study. Invest Ophthalmol Vis Sci. 2006;47:3539–3544 [DOI] [PubMed] [Google Scholar]
- 27. Andrew T, Hart DJ, Snieder H, et al. Are twins and singletons comparable?—a study of disease-related and lifestyle characteristics in adult women. Twin Res. 2001;4:464–477 [DOI] [PubMed] [Google Scholar]
- 28. Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolar abnormalities related to atherosclerosis?—The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20:1644–1650 [DOI] [PubMed] [Google Scholar]
- 29. Leung H, Wang JJ, Rochtchina E, et al. Dyslipidaemia and microvascular disease in the retina. Eye (Lond). 2005;19:861–868 [DOI] [PubMed] [Google Scholar]
- 30. Wong TY, Duncan BB, Golden SH, et al. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk in Communities study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954 [DOI] [PubMed] [Google Scholar]
- 31. Gopinath B, Wang JJ, Flood VM, et al. The associations between blood levels of homocysteine, folate, vitamin B12, and retinal vascular caliber. Am J Ophthalmol. 2009;148:902–909 [DOI] [PubMed] [Google Scholar]
- 32. Tirsi A, Bruehl H, Sweat V, et al. Retinal vessel abnormalities are associated with elevated fasting insulin levels and cerebral atrophy in nondiabetic individuals. Ophthalmology. 2009;116:1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]