While botulinum toxin injections are effective in reducing muscle spasms in blepharospasm and hemifacial spasm patients, they have a relatively short duration of action due to formation of new functional neuromuscular junctions. Co-injection of botulinum toxin–treated eyelids with corticotrophin-releasing factor or antibody to insulin growth factor–receptor prevented neuromuscular junction increases. The long-term goal is to increase the duration of effectiveness of botulinum toxin and reduce the lifetime exposure in patients.
Abstract
Purpose.
Botulinum toxin A is the most commonly used treatment for blepharospasm, hemifacial spasm, and other focal dystonias. Its main drawback is its relatively short duration of effect. The goal of this study was to examine the ability of corticotropin releasing factor (CRF) or antibody to insulin growth factor I–receptor (anti-IGFIR) to reduce the up-regulation of neuromuscular junctions that are associated with return of muscle function after botulinum toxin treatment.
Methods.
Eyelids of adult rabbits were locally injected with either botulinum toxin alone or botulinum toxin treatment followed by injection of either CRF or anti-IGFIR. After one, two, or four weeks, the orbicularis oculi muscles within the treated eyelids were examined for density of neuromuscular junctions histologically.
Results.
Injection of botulinum toxin into rabbit eyelids resulted in a significant increase in the density of neuromuscular junctions at one and two weeks, and an even greater increase in neuromuscular junction density by four weeks after treatment. Treatment with either CRF or anti-IGFIR completely prevented this increase in neuromuscular junction density.
Conclusions.
The return of function after botulinum toxin–induced muscle paralysis is due to terminal sprouting and formation of new neuromuscular junctions within the paralyzed muscles. Injection with CRF or anti-IGFIR after botulinum toxin treatment prevents this sprouting, which in turn should increase the duration of effectiveness of single botulinum toxin treatments. Future physiology studies will address this. Prolonging botulinum toxin's clinical efficacy should decrease the number of injections needed for patient muscle spasm relief, decreasing the risk of negative side effects and changes in drug effectiveness that often occurs over a lifetime of botulinum toxin exposure.
Botulinum toxin is the most common medical treatment for blepharospasm and hemifacial spasm. Developed in the 1970s,1 it produces a chemodenervation by binding to and paralyzing the neuromuscular junction specifically by blocking neurotransmitter release. This is an excellent treatment; however, its main limitation is the relatively short duration of its action. The average reinjection interval for blepharospasm in the published literature is between two and three months.2 In addition, many patients desire more frequent injections, partly to remain spasm-free and partly from decreasing sensitivity to the drug's effects.3 Additionally, some patients develop antibodies to botulinum toxin, requiring increased dosing to achieve paralysis or rendering them unresponsive to treatment.4
The return of muscle function after botulinum toxin injection is caused by sprouting of axonal collaterals from the presynaptic nerve endings at the neuromuscular junctions of the paralyzed muscles.5,6 Nerve sprouting after botulinum toxin treatment results in a significant increase in new acetylcholine receptors on the treated muscle compared to normal. These newly formed acetylcholine receptors are in locations distinct from those of the original, paralyzed neuromuscular junctions.7 Peripheral nerve sprouting can be measured as early as three days after botulinum injection.8 Compound action potentials demonstrate the return of 20% of normal activity in patients as soon as seven days after botulinum toxin injection.9 This rapid and early sprouting results in some muscle function returning as quickly as the sixth day.10 Quantification of neuromuscular junction number in rabbit extraocular muscle at various times after botulinum toxin injection showed doubling of neuromuscular junctions within the first month after treatment.11 This is one of the major limitations of botulinum toxin use in patients with focal dystonias; the duration of effectiveness is too short to allow permanent alteration of innervation and muscle force. Increasing the duration of effectiveness of botulinum toxin would reduce both the need for frequent repeat injections and the lifetime exposure of patients to the drug. This in turn should reduce the chance for the decreased sensitivity to the treatment. This is an important concern, because there are few other widely accepted choices for medical management of blepharospasm and hemifacial spasm, and none that rival botulinum toxin in clinical efficacy.
Since the first use of botulinum toxin for treating blepharospasm patients,12 there has been very little research focused on improving its duration of effect or developing new therapeutic agents to selectively weaken a single or small group of skeletal muscles.13 Some animal studies examining co-treatment strategies have been performed, including studies from our laboratory. These include co-treatment with the immunotoxin ricin-mAb35,14 insulin growth factor binding proteins,15 and bupivacaine.16 The goal of our research is to test agents that have the potential to enhance the duration of paralysis, which would potentially decrease the number of “lifetime” injections of botulinum toxin needed by patients.
The hormone corticotropin releasing factor (CRF) has potent anti-inflammatory effects when applied locally in tissues for treatment of pain.17 We recently demonstrated that, when injected into an inflamed eyelid, CRF significantly reduces inflammatory cell infiltrate and nerve fibers at the site of injection.18 The reduction in sensory nerve outgrowth caused by local CRF administration in the injured eyelids suggested that this agent might also have an effect on reducing the motor nerve sprouting and neuromuscular junction formation that occurs after botulinum toxin treatment of the orbicularis oculi muscle.
Insulin growth factor (IGF) is a neurotrophic and muscle growth factor that increases in muscles after nerve injury.19 Previous studies demonstrate that co-injection of inhibitory binding proteins specific for IGF, such as IGF binding protein-4, reduces botulinum-induced sprouting in the levator ani muscle.20 Local injection of antibodies to soluble neurotrophic factors and their receptors, such as IGF and IGF-R, have been shown to reduce collateral axonal branching after nerve injury.21 Using either antibodies to IGF or antibodies to insulin growth factor receptor, proteins with known inhibitory effects on IGF effects in tissue, has the potential to reduce effectively the botulinum toxin–induced nerve sprouting and de novo increases in neuromuscular junction density locally within treated orbicularis oculi muscle.
These experiments test the ability of local injections of CRF or IGF-receptor antibody into the eyelid muscles to reduce axonal sprouting and the de novo neuromuscular junction formation induced by botulinum toxin injection. Clinically, the long-term goal is to increase the duration of effect of botulinum toxin treatment to reduce the need for repeat injections and the overall lifetime exposure to the drug in patients with chronic focal dystonias.
Materials and Methods
Adult male New Zealand White rabbits were obtained from Bakkom Rabbitry (Viroqua, WI) and housed in the AALAC-approved animal facility at the University of Minnesota. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Minnesota, as well as complied with the guidelines for the Use of Animals in Research published by the Association for Research in Vision and Ophthalmology as well as the guidelines of the National Institutes of Health.
Rabbits were anesthetized via an intramuscular injection of ketamine/xylazine (1:1) at a dose of 10 mg/kg:2 mg/kg, respectively. The cornea was anesthetized by placement of a drop of proparacaine HCl in the conjunctival cul-de-sac. Three groups of animals were prepared. Group one received a single injection of 5 units of botulinum toxin A (Botox; Allergan, Los Angeles, CA) in 1 cc of sterile isotonic saline in one randomly selected upper eyelid and examined after one, two, or four weeks post-botulinum toxin treatment. All injections were made by inserting the needle into the central region of the eyelid with the needle tip pointing toward the medial canthus, with half the volume being dispensed and the needle slowly pulled toward the needle entry point. With the needle in place, the syringe was rotated and the needle directed toward the lateral canthus. The same slow injection procedure was performed with slow withdrawal of the needle. After dispensing the full volume, the needle was left in place for 30 seconds to prevent leakage. This method minimizes leakage, as only one needle stick is performed. Previous studies demonstrated that single injections of this volume spread into all regions of the treated eyelids.22 Group two received a single injection of 5 units of botulinum toxin, followed on post-treatment days three and five with injection of corticotropin releasing factor (CRF; Peninsula Laboratories, Belmont, CA) (150 μg in 1 mL sterile saline).18,23 Eyelids were examined two weeks after the final treatment. Group 3 received botulinum toxin injections similar to groups 1 and 2, followed on post-treatment days three and five with injections of an antibody to insulin growth factor-I receptor (anti-IGFIR; R&D Systems, Minneapolis, MN) at a dose of 30 μg/mL sterile saline.23 Again, eyelids were examined two weeks after the final treatment. The contralateral upper eyelids were injected with sterile saline only in comparable volumes.
At the appropriate post-injection intervals, the rabbits were anesthetized deeply with ketamine and xylazine, followed by an overdose of barbiturate anesthesia. Both eyelids were trimmed to remove the fur and dissected completely to include the muscle at both the medial and lateral canthi. They were pinned to their in situ length in embedding molds, surrounded by tragacanth gum, frozen in methylbutane chilled to a slurry on liquid nitrogen, and stored at −80°C until sectioned and processed. The muscles were sectioned completely in the longitudinal plane at 12 μm, and the sections were mounted on gelatin-subbed microslides. Every tenth section was immunostained for the presence of neuromuscular junctions using α-bungarotoxin conjugated to Alexa Fluor 488 (Molecular Probes) at a concentration of 1:100 overnight at 4°C. The slides were coverslipped with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA) and analyzed the same day they were immunostained.
The muscle sections were examined for neuromuscular junction position and number using a microscope (Leica DMR; Leica, Wetzlar, Germany). Using image analysis software (Topographer program of NovaPrime; Bioquant, Nashville, TN), the area of the entire orbicularis oculi muscle in longitudinal section was measured at 1.6×. Every neuromuscular junction was located at 20× and marked with X and Y coordinates recorded in the Topographer program. This analysis was repeated for every tenth section through the entire eyelid. The image analysis program (Bioquant Topographer) was used to reconstruct the entire muscle, including the area outlines and locations of each neuromuscular junction. This allowed for a three-dimensional reconstruction of all the neuromuscular junctions in their actual X, Y, and Z planes within the entire muscle. Four orbicularis oculi muscles were examined for each of the experimental paradigms.
Density of neuromuscular junctions was calculated as the number of neuromuscular junctions per mm2. Statistical significance was determined between the densities of neuromuscular junctions in the saline-treated control muscles, the botulinum toxin A–only treated orbicularis oculi, and the co-treated eyelid muscles. Statistics were performed using an unpaired t-test aided by statistical software (Prism and Statmate software; Graphpad, San Diego, CA). An F-test was used to verify that the variances were not significantly different. Data were considered significantly different if P ≤ 0.05.
Results
The effect of botulinum toxin A injections on neuromuscular density in orbicularis oculi of adult rabbits was determined (Figs. 1, 2, 3). The neuromuscular junction density was twofold greater in the pretarsal region of normal orbicularis oculi muscle compared to the preseptal region. The density of neuromuscular junctions was 3.3 ± 0.14/mm2 and 1.55 ± 0.09/mm2 in the pretarsal and preseptal regions, respectively. At one and two weeks after botulinum toxin injection, the density increased significantly, particularly in the preseptal region. In the pretarsal region, neuromuscular junction density was 4.0 ± 0.21/mm2 after one or two weeks. In the preseptal region, neuromuscular junction densities were two- to threefold greater than control at one and two weeks post-treatment, with densities of 4.4 ± 0.1/mm2 and 2.9 ± 0.4/mm2, respectively. At four weeks post-botulinum toxin treatment, the up-regulation of neuromuscular junctions was even greater when compared to control levels, and was also significantly greater than the density at two weeks. Densities in the pretarsal and preseptal regions at four weeks post-treatment were 5.7 ± 0.8/mm2 and 5.23 ± 0.7/mm2, respectively.
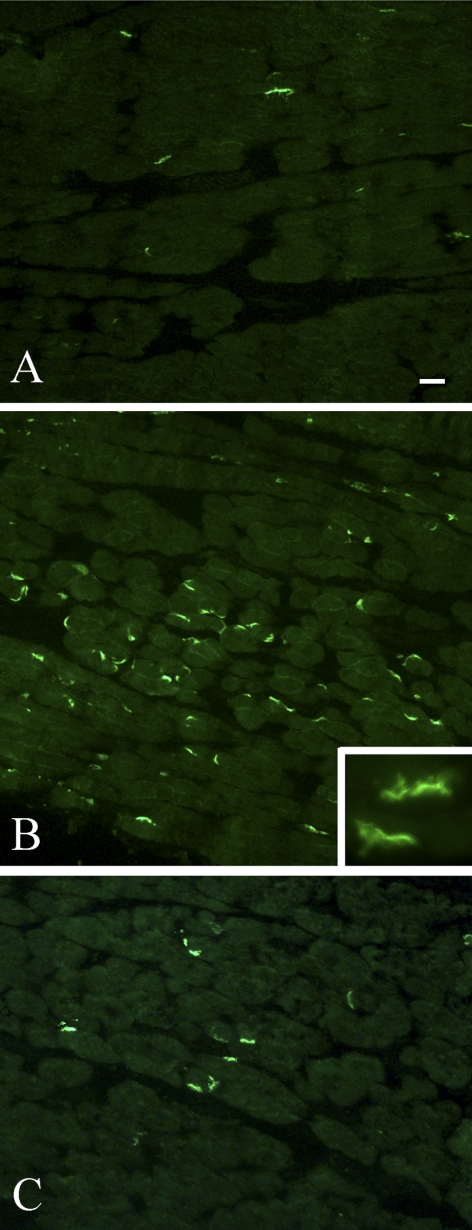
Figure 1.
Photomicrographs of neuromuscular junctions stained with fluorescently labeled α-bungarotoxin. (A) Normal control rabbit orbicularis oculi muscle. (B) Two weeks after an injection of 5 units of botulinum toxin. (C) Two weeks after an injection of 5 units of botulinum toxin A followed by two injections of corticotrophin releasing factor. Scale bar, 20 μm. Inset magnification, 40×.
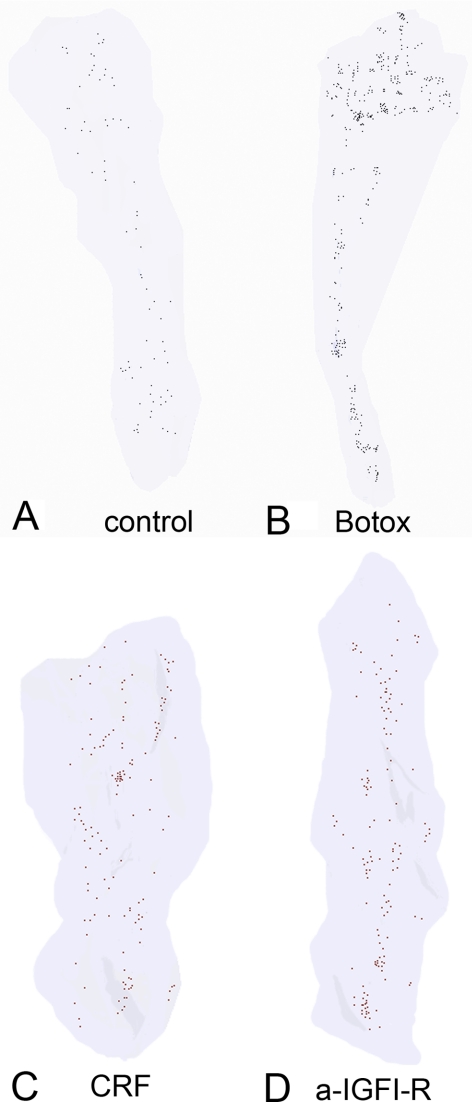
Figure 2.
Three-dimensional reconstruction of approximately 10% of the neuromuscular junctions in a representative eyelid specimen from (A) normal control orbicularis oculi muscle, (B) an eyelid treated two weeks earlier with botulinum toxin A, (C) an eyelid treated two weeks earlier with botulinum toxin A followed by CRF, and (D) an eyelid treated two weeks earlier with botulinum toxin A followed by anti-IGFIR. Each dot represents a single neuromuscular junction.
Figure 3.
Quantification of neuromuscular junction density of control orbicularis oculi muscles (white bar) as well as one (vertical hatched bar), two (black bar), and four (gray bar) weeks after an injection of 5 units botulinum toxin A (Botox). *Significantly different from control; **significantly different from control and from 1 and 2 weeks; #significantly different from pretarsal control.
Injection of CRF into the botulinum toxin–treated eyelids resulted in neuromuscular junction densities that were not significantly different from the density in the control orbicularis oculi muscles (Figs. 1, 2, 4), with the CRF-treated neuromuscular junction density at 2.39 ± 0.23/mm2 compared to 2.42 ± 0.19/mm2 in the control orbicularis oculi muscles. As the combined total muscle neuromuscular junction density in the orbicularis oculi muscle increased almost threefold after the botulinum toxin A treatment to 5.9 ± 0.43/mm2, it is quite interesting that the up-regulation of neuromuscular junctions was completely negated by the injection of CRF within days of the botulinum toxin.
Figure 4.
Quantification of neuromuscular junction density of control orbicularis oculi muscles (white bar) compared to two weeks after an injection of 5 units of botulinum toxin A only (Botox; black bar), and two weeks after botulinum toxin followed by corticotrophin releasing factor (gray bar) or an antibody to insulin growth factor-1 receptor (hatched bar). #Significantly different from control; *significantly different from Botox treated 2 weeks.
Similarly, injection of anti-IGFIR within days of the botulinum toxin treatment resulted in no increase in neuromuscular junction density compared to control levels, with neuromuscular junction density in the anti-IGFIR-treated orbicularis oculi muscles at 2.88 ± 0.28/mm2 compared to control levels at 2.4 ± 0.19/mm2 (Figs. 1, 2, 4).
Discussion
Botulinum toxin A injection resulted in a significant increase in neuromuscular junction density, as previously demonstrated.11 Injection of the botulinum toxin–treated eyelids with either CRF or anti-IGFR negated the botulinum toxin–induced de novo formation of neuromuscular junctions on the paralyzed muscles.
Botulinum toxin A directly binds to the neuromuscular junction and prevents neurotransmitter release.24 Botulinum toxin specifically cleaves SNAP-25, a protein needed for transmitter exocytosis,25 but leaves the neuromuscular junction intact.26 This botulinum toxin–induced paralysis leads to sprouting of the terminal nerves that project to the poisoned neuromuscular junctions.27 These sprouts induce the formation of new neuromuscular junctions, and these new motor endplates are responsible for functional return at the onset of recovery.5 Eventually, over the course of one to three months, there is a return of function at the original motor endplates, and the sprouts are eliminated.28 We hypothesized that inhibition of nerve sprouting at the muscle level should extend the duration of effectiveness of botulinum toxin treatment.
The rationale for the use of CRF as an anti-sprouting treatment is based on studies that show its potent analgesic effects require the presence of peripheral nerve within injured tissue.29 Our previous work demonstrated that localized injection of CRF decreased PGP 9.5-positive nerve density in inflamed eyelid tissue.18,23 This reduction in nerve fiber density correlated with a reduction in tissue hypersensitivity to touch. In the present study, CRF prevented the formation of new motor endplates.5 CRF also can act directly by reducing synaptic transmission.30,31 This mechanism could also potentially alter muscle contractile properties. Future studies will address these complex issues. As CRF is an FDA-approved medication, local injection of CRF is a particularly attractive approach for extending the duration of botulinum toxin's paralyzing effects in the treatment of blepharospasm and related focal dystonias.
Muscle paresis and paralysis induce the expression of a number of neurotrophic molecules that play a role in peripheral nerve regeneration.32 IGFI and IGFII are particularly potent in increasing the rate of peripheral nerve regeneration, with IGFI playing a role in initial sprouting and subsequent elongation of the regenerating axons.33 It is particularly interesting to note that IGFI levels significantly increase as early as three days after a single injection of botulinum toxin and remain elevated for up to one month.15,34 Just as increased levels of IGFI can increase terminal sprouting,35 precedence exists for decreasing terminal sprouting by reduction or inactivating IGFI-related molecules. Local treatment at the site of paralyzed muscles with either IGF-binding protein-4 or -5 results in suppression of terminal sprouting.15,20 Antibodies to IGFI, when focally applied, result in reduction of terminal axon branching of injured facial nerve.21,36 In the present study, injection of antibody to IGFIR proved to be just as effective as CRF in preventing new neuromuscular junction formation caused by botulinum toxin locally within the paralyzed orbicularis oculi muscle. Future studies of longer duration as well as muscle function studies will address whether preventing new neuromuscular junctions results in functional extension of botulinum toxin–induced muscle paresis, as predicted by the present results.
In summary, local injection of either CRF or anti-IGFIR prevented the up-regulation of neuromuscular junctions that occurs after botulinum toxin A injection. There is increased evidence that long-term treatment of muscle dystonias with botulinum toxin A results in reduced duration of effect over time, as well as the development of antibodies to botulinum toxin A that reduce the drug's effectiveness.37 The ultimate goal is to develop a co-treatment strategy to increase the duration of effect of single botulinum toxin A treatments. Decreasing frequency of patient injections for treatment of blepharospasm or other focal dystonias would, in turn, decrease the life-time exposure of these patients to the toxin, with concomitant reduction in potential side-effects.38
Footnotes
Supported by the Benign Essential Blepharospasm Research Foundation, EY11375 from the National Eye Institute, the Neuro-ophthalmology Research Fund, the Minnesota Lions and Lionesses, and an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness, Inc.
Disclosure: A.R. Harrison, None; Z. Berbos, None; R.A. Zaldivar, None; B. C. Anderson, None; M. Semmer, None; M.S. Lee, None; L.K. McLoon, None
References
- 1. Scott AB, Rosenbaum A, Collins CC. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol. 1973;12:924–927 [PubMed] [Google Scholar]
- 2. Dutton JJ, Buckley EG. Long-term results and complications of botulinum A toxin in the treatment of blepharospasm. Ophthalmology. 1988;95:1529–1534 [DOI] [PubMed] [Google Scholar]
- 3. Holds JB, Fogg SG, Anderson RL. Botulinum A toxin injection. Failures in clinical practice and a biomechanical system for the study of toxin-induced paralysis. Ophthal Plast Reconstr Surg. 1990;6:252–259 [DOI] [PubMed] [Google Scholar]
- 4. Jankovic J, Schwartz K. Response and immunoresistance to botulinum toxin injections. Neurology. 1995;45:1743–1746 [DOI] [PubMed] [Google Scholar]
- 5. de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci USA. 1999;96:3200–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holds JB, Alderson K, Fogg SG, Anderson RL. Motor nerve sprouting in human orbicularis oculi muscle after botulinum A injection. Invest Ophthalmol Vis Sci. 1990;31:964–967 [PubMed] [Google Scholar]
- 7. Thesleff S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960;151:598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wright MC, Cho WJ, Son YJ. Distinct patterns of motor nerve terminal sprouting induced by ciliary neurotrophic factor vs. botulinum toxin. J Comp Neurol. 2007;504:1–16 [DOI] [PubMed] [Google Scholar]
- 9. Eleopra R, Tugnoli V, Rossetto O, De Grandi D, Montecucco C. Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci Lett. 1998;256:135–138 [DOI] [PubMed] [Google Scholar]
- 10. Kim YI, Lomo T, Lupa MT, Thesleff S. Miniature end-plate potentials in rat skeletal muscle poisoned with botulinum toxin. J Physiol. 1984;356:587–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrison AR, Anderson BC, Thompson LV, McLoon LK. Myofiber length and three-dimensional localization of NMJs in normal and botulinum toxin treated adult extraocular muscles. Invest Ophthalmol Vis Sci. 2007;48:3594–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol. 1985;103:347–350 [DOI] [PubMed] [Google Scholar]
- 13. Harrison AR, Skladzien S, Christiansen SP, McLoon LK. Myotoxic effects of the skeletal muscle-specific immunotoxin, ricin-mAb35, on orbicularis oculi muscle after eyelid injections in rabbits. Ophthal Plast Reconstr Surg. 2004;20:312–316 [DOI] [PubMed] [Google Scholar]
- 14. Christiansen SP, Anderson BC, McLoon LK. Botulinum toxin pretreatment augments the weakening effect of injection with ricin-mAb35 in rabbit extraocular muscle. J AAPOS. 2008;12:122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caroni P, Schneider C. Signaling by insulin-like growth factors in paralyzed skeletal muscle: rapid induction of IGFI expression in muscle fibers and prevention of interstitial cell proliferation by IGF-BP5 and IGF-BP4. J Neurosci. 1994;3378–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yen MT, Wall VK. Bupivacaine-induced myotoxicity and its effect on botulinum toxin paresis. Ann Plast Surg. 2008;60:6–9 [DOI] [PubMed] [Google Scholar]
- 17. Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84:1–12 [DOI] [PubMed] [Google Scholar]
- 18. Harrison AR, McLoon LK. Reduction in touch sensitivity and hyperinnervation in vesicant-injured rabbit eyelid by direct injection of corticotropin releasing factor. Neurosci Lett. 2006;400:30–34 [DOI] [PubMed] [Google Scholar]
- 19. Ishii DN. Relationship of insulin-like growth factor II gene expression in muscle to synaptogenesis. Proc Natl Acad Sci USA. 1989;86:2898–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caroni P, Schneider C, Kiefer MC, Zapf J. Role of muscle insulin-like growth factors in nerve sprouting: suppression of terminal sprouting in paralyzed muscle by IGF-binding protein 4. J Cell Biol. 1994;125:893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Streppel M, Azzolin N, Dohm S, et al. Focal application of neutralizing antibodies to soluble neurotrophic factors reduces collateral axonal branching after peripheral nerve lesion. Eur J Neurosci. 2002;15:1327–1342 [DOI] [PubMed] [Google Scholar]
- 22. McLoon LK, Wirtschafter J, Cameron JD. Muscle loss from doxorubicin injections into the eyelids of a patient with blepharospasm. Am J Ophthalmol. 1993;116:646–648 [DOI] [PubMed] [Google Scholar]
- 23. McLoon LK, Sandnas AM, Nockleby KJ, Wirtschafter JD. Reduction in vesicant-induced cellular inflammation and hyperalgesia by local injection of corticotropin releasing factor in rabbit eyelid. Inflamm Res. 2002;51:16–23 [DOI] [PubMed] [Google Scholar]
- 24. Molgo J, Comella JX, Angaut-Petit D, et al. Presynaptic actions of botulinal neurotoxins at vertebrate neuromuscular junctions. J Physiol (Paris). 1990;84:152–166 [PubMed] [Google Scholar]
- 25. Blasi J, Chapman ER, Link E, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163 [DOI] [PubMed] [Google Scholar]
- 26. Duchen LW, Strich SJ. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. J Exp Physiol Cogn Med Sci. 1968;53:84–89 [DOI] [PubMed] [Google Scholar]
- 27. Angaut-Petit D, Molgo J, Comella JX, Faille L, Tabti N. Terminal sprouting in mouse neuromuscular junctions poisoned with botulinum type A toxin: morphological and electrophysiological features. Neuroscience. 1990;37:799–808 [DOI] [PubMed] [Google Scholar]
- 28. Meunier FA, Schiavo G, Molgo J. Botulinum neurotoxins: from paralysis to recovery of functional neurotransmission. J Physiol (Paris). 2002;96:105–113 [DOI] [PubMed] [Google Scholar]
- 29. Veraksits A, Bileviciute-Ljungar I, Maaroos J, Vasar E, Lundeberg T. Neuronal mechanisms contribute to corticotropin-releasing factor-induced anti-oedema effect in rat hind paw. Neuropeptides. 2000;34:129–134 [DOI] [PubMed] [Google Scholar]
- 30. Schaefer M, Mousa SA, Stein C. Corticotropin-releasing factor in antinociception and inflammation. Eur J Pharmacol. 1997;323:1–10 [DOI] [PubMed] [Google Scholar]
- 31. Orozco-Cabal J, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. Regulation of synaptic transmission by CRF receptors. Rev Neurosci. 2006;17:279–307 [DOI] [PubMed] [Google Scholar]
- 32. Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. [DOI] [PubMed] [Google Scholar]
- 33. Pu SF, Zhuang HX, Ishii DN. Differential spatio-temporal expression of the insulin-like growth factor genes in regenerating sciatic nerve. Molec Brain Res. 1995;34:18–28 [DOI] [PubMed] [Google Scholar]
- 34. Shen J, Ma J, Lee C, et al. How muscles recover from paresis and atrophy after intramuscular injection of botulinum toxin A: study in juvenile rats. J Orthop Res. 2006;24:1128–1135 [DOI] [PubMed] [Google Scholar]
- 35. Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990;110:1302–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Angelov DN, Guntinas-Lichius O, Wewetzer K, Neiss WF, Streppel M. Axonal branching and recovery of coordinated muscle activity after transection of the facial nerve in adult rats. Adv Anat Embryol Cell Biol. 2005;180:1–130 [PubMed] [Google Scholar]
- 37. Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and second therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol. 32:213–218 [DOI] [PubMed] [Google Scholar]
- 38. Dutton JJ. Botulinum-A toxin in the treatment of craniocervical muscle spasms: short- and long-term, local and systemic side effects. Surv Ophthalmol. 1996;41:51–65 [DOI] [PubMed] [Google Scholar]