Abstract
In addition to its role in fibrinolysis, plasminogen (Plg) influences inflammatory cell migration, and thereby plays a prominent role in cardiovascular pathology. The contribution of Plg to inflammatory cell recruitment depends upon its tethering to the surface of responding cells. Plg receptors (Plg-Rs) are heterogeneous and can be classified as tailless, lacking cytoplasmic tails, or tailed, having cytoplasmic tails. In vivo observations implicate several tailless Plg-Rs in inflammatory responses. Tailed Plg-Rs on leukocytes include several integrins, which have also been implicated in Plg-dependent responses. Surface expression of both tailless and tailed Plg-Rs can be modulated in number and/or function. A common mechanism involving intracellular calcium mobilization and calcium channels regulates expression of both classes of Plg-Rs. Data are emerging to indicate that targeting Plg and Plg-Rs may limit inflammation and cardiovascular pathology.
Introduction
Inflammation underlies many of the pathological processes leading to cardiovascular diseases. Efficient recruitment of leukocytes from one tissue to another, including from blood, is heavily dependent on proteolysis. Among the various proteases, evidence for involvement of plasmin in inflammation is particularly strong. Numerous studies conducted in Plg-deficient mice have underscored the critical role of Plg in leukocyte recruitment and inflammatory responses (reviewed in (Plow et al. 1999,Plow and Hoover-Plow 2004)). Thus, in addition to its well-established role in fibrinolysis/thrombolysis, Plg is an important mediator of inflammation and hence in cardiovascular diseases.
In this article, we briefly consider how Plg influences inflammatory cell migration. This then leads into a discussion of Plg-R, which we categorize as ones without cytoplasmic tails (tailless) and ones with cytoplasmic tails (tailed). We then postulate that a common mechanism, changes in intracellular calcium, regulates both classes of Plg-Rs.
Contributions of Plasminogen to Inflammatory Cell Recruitment
Plg, the zymogen form of the active serine protease, plasmin, circulates at high concentrations in blood (1 μM). Plasmin may contribute to leukocyte migration at several levels. First, plasmin orchestrates degradation of the extracellular matrix (ECM), which serves as a barrier to cell passage. ECM substrates of plasmin include fibronectins, thrombospondins, and laminins as well as fibrin (reviewed in Plow et al. 1995). Additionally, plasmin can activate the matrix metalloproteinases (MMPs)(reviewed in Lijnen, (2000) which degrade collagens. Further influences of Plg on inflammatory cell recruitment stems from its processing of various chemokines/cytokines. Notable in this regard is its processing of transforming growth factor-β, interleukin-1, basic fibroblast growth factor, tumor necrosis factor-α and monocyte chemotactic protein-1 (reviewed in Ploplis and Castellino, 2000). Plasmin(ogen) also can trigger intracellular signaling events. Plg-mediated signaling occurs in macrophages (Syrovets and Simmet 2004) and neutrophils (Pluskota et al. 2008), and these changes can also influence cell adhesion and migration.
Plasminogen Receptors
Efficient utilization of plasmin(ogen) by cells depends on its tethering to the responding cell. Surface localization enhances Plg activation, increases the catalytic activity of plasmin, and protects the enzyme from inactivation by protease inhibitors, a necessity in an inhibitor-rich environment (reviewed in Plow et al., 1995). The binding of Plg to cell surfaces is mediated by a diverse array of cell surface molecules, collectively Plg-Rs. Common to most Plg-Rs is their ability to interact with the lysine binding sites within the kringle domains of plasmin(ogen). Most of the identified Plg-Rs have a carboxy-terminal lysine, but others harbor internal amino acids that mimic C-terminal lysines. This common recognition mechanism results in similar affinity of Plg for most Plg-Rs (KD ~1 μM) (Miles and Plow 1987) and renders these interactions vulnerable to inhibition by basic carboxypeptidases and C-terminal lysine analogs, such as ε-aminocaproic acid.
Many Plg-Rs do not contain a signal sequence, transmembrane domain and/or a cytoplasmic tail. Falling into this category of tailless Plg-Rs on leukocytes are α-enolase; annexin 2, amphoterin, TIP49a, histone H2B and Plg-RKT (see Table 1). Tailed Plg-Rs contain transmembrane and cytoplasmic tails and include several integrin adhesion receptors. Of these, αVβ3, α5β1 and αMβ2 are present on leukocytes (Table 1). It should be noted that the terminology of peripheral versus integral membrane proteins could be applied to Plg-Rs with tailless corresponding to peripheral and tailed to integral membrane proteins. This designation would change only the classification of Plg-RKT, which is postulated to contain a single intracellular loop with its C-terminal and N-terminal positioned on the exterior of the cell. A hypothetical subcategory of tailless Plg-Rs also may arise from the association of Plg binding proteins released from other cells as a consequence of lysis or secretion and then bind to the surface of leukocytes. Indeed, many other Plg binding proteins, such as cytokeratin 8 (Hembrough et al 1995), might function as Plg-Rs through such a mechanism. Both tailless and tailed Plg-Rs promote Plg activation and pericellular proteolysis, but may display these functions in different biological settings. Regulation of both classes of Plg-Rs by inflammatory stimuli is particularly important in controlling leukocyte responses. Our subsequent discussion focuses on the modulation of Plg-Rs on monocytoid cells and neutrophils (PMN) as two cell types pivotal in inflammatory responses associated with cardiovascular diseases.
Table 1.
Leukocyte Plasminogen Receptors
| Tailless Plg-Rs | C-terminal lysine |
|---|---|
| a-enolase | present |
| Annexin 2 | absent (cleaved to be functional Plg-R) |
| p11 | present |
| Amphoterin | absent |
| TIP49a | present |
| Histone 2B | present |
| Plg-RTK | present |
| Tailed Plg-Rs | |
|---|---|
| αIIbβ3 | absent |
| αvβ3 | absent |
| α4β1 | absent |
| α5β1 | absent |
| αMβ2 | absent |
| gp330 | absent |
Tailless Plasminogen Receptors
α-Enolase, first identified as a Plg-R on monocytoid cells (Miles et al. 1991), bears a C-terminal lysine, and is now known to function as a Plg-R on many other eukaryotic (Nakajima et al. 1994,Seweryn et al. 2009,Lopez-Alemany et al. 2003), including transformed cells (Lopez-Alemany et al. 1994) as well as prokaryotic cells (Pancholi and Fischetti 1998). α-Enolase has been directly implicated in macrophage recruitment in LPS-induced lung inflammation in mice and its expression correlates with accumulation of inflammatory cells in the lungs of pneumonia patients (Wygrecka et al. 2009). Annexin 2 (36 kDa), first identified as a Plg-R on endothelial cells, also binds Plg activators (reviewed in Kim and Hajjar, 2002). Annexin 2 is also expressed on monocytoid cells, and its expression is enhanced on acute promyelocytic leukemia cells (Falcone et al. 2001). Annexin 2 does not contain a C-terminal lysine, but may be cleaved to become a Plg-R. However, other studies suggest that annexin 2 only binds Plg when associated with its 11-kDa binding partner, p11, which has lysine as its natural C-terminus (MacLeod et al. 2003).
Histone H2B is present on the surface of leukocytes (Holers and Kotzin 1985), where it functions as a tailless Plg-R (Herren et al. 2006,Das et al. 2007). H2B harbors a C-terminal lysine, as do several other histones (H1, H2A and H2B), which are also likely to function as Plg-Rs if they reach the cell-surface. Several of these histones are detected extracellularly (Brix et al. 1998), some associated with extracellular DNA nets (Brinkmann et al. 2004), and have been implicated in inflammatory responses (Xu et al. 2009). The most recently discovered member among tailless Plg-Rs is Plg-RKT. In contrast to the other tailless Plg-R, Plg-RKT does bear a transmembrane domain, actually two, which are connected by a short cytoplasmic loop. Both its N-terminus and its C-terminus, a lysine, are extracellular (Andronicos et al. 2009).
Blocking studies with antibodies to α-enolase, annexin 2, p11 and H2B indicate that these tailless Plg-Rs account for the preponderance of the Plg binding capacity of monocytoid cells of either murine or human origin (Das et al. 2007) although the effects of antibodies to other Plg-Rs have not been directly compared. Anti-H2B alone blocked ~50% of the Plg binding and Plg activation (Das et al. 2007). Intravenous administration of the anti-H2B Fab fragments inhibited 70% of the Plg-dependent macrophage recruitment in thioglycollate-induced peritoneal inflammation. In this model, Fab fragments of anti-enolase produced modest inhibition in contrast to the marked involvement of α-enolase in lung inflammation induced by LPS (Wygrecka et al. 2009). Also, Plg-RKT cell surface expression was reported to increase upon M-CSF stimulation (Andronicos et al. 2009). Thus, different Plg-Rs may be utilized for inflammatory cell recruitment in a tissue-specific and/or stimulus specific manner.
Cells of the monocytoid lineage change their Plg binding capacity in during a variety of physiological responses, including adhesion (Kim et al. 1996, Herren et al. 2001), apoptosis (O'Mullane and Baker 1999), differentiation (Brownstein et al. 2004, Das et al. 2007, 2009), and agonist stimulation (Herren et al. 2003, Peterson et al. 2003, Wygrecka et al. 2009). In cases where delineated, up-regulation in Plg binding has been accompanied by increases in expression of tailless Plg-Rs: surface expression of H2B, α-enolase, annexin 2 and p11 increased by FACS, Western blots and immunofluorescence when moncytoid cells were differentiated toward macrophages.
Integrins as Tailed plasminogen receptors on leukocytes
Integrins are among the most highly expressed tailed Plg-Rs on leukocytes. The most prominent integrins on leukocytes are the β2 integrin subfamily. Of these, αMβ2 (Mac-1, CD11b/18), αXβ2, (p150, 95, CD11c/18), and probably αDβ2 (CD11d/18) are Plg-Rs as are α5β1 and αVβ3. Although none of the β2 integrins contain a C-terminal lysine, they interact with the LBS within Plg kringle domains. Several studies demonstrated that αMβ2 forms a tight complex with uPAR, which enhances integrin-dependent leukocyte responses(Chapman and Wei 2001). In addition to uPAR, αMβ2 also interacts directly with uPA. Assembly of Plg, uPA, and uPAR on integrin αMβ2 regulates plasmin activity on PMN surface and this tetramolecular complex plays a crucial role in fibrin degradation by PMNs as well as in mediating adhesion and migration of these cells (Pluskota et al. 2003, 2004). As a tailed Plg-R, αMβ2 is capable of directly transmitting “outside-in” signals upon ligand engagement; engagement of αMβ2 by Plg triggers activation of ERK1/2 and Akt (Pluskota et al. 2008). While studies demonstrating the assembly and signaling consequences of Plg binding to αMβ2 have been observed primarily with PMN, it is likely that these mechanisms extend to other αMβ2-bearing cells and to αXβ2 and αDβ2 integrins. Although Plg is abundant in most microenvironments, including blood, these Plg-Rs are not continuously ligated. Plg does not bind unless the integrin is in activated state. Several studies have demonstrated that αVβ3, like αMβ2, recognizes plasmin, uPAR and the kringle domain of uPA, and these interactions influence the adhesive and migratory responses of cells.(Tarui et al. 2006). Of note, not only αMβ2 but also αVβ3 enhances u-PA dependent Plg activation although their role in Plg-dependent cell migration has not been assessed in vivo.
Ca2+ Mobilization and Channels Provide a Common Pathway for Regulation of Plasminogen Receptors
During monocyte-to-macrophage differentiation, the 3-4-fold up-regulation of the tailless Plg-Rs is independent of new protein synthesis and is unaffected by inhibitors of non-classical protein secretion pathways (ABC-1 transporters, Na/K ATPases, endolysozomyl recycling). However, chelation of intracellular Ca2+ suppresses differentiation-induced Plg-R expression. Of the various Ca2+ channels, only inhibitors of the L-type Ca2+ channels (LTCC) prevented Plg-R expression; two different classes of LTCC inhibitors, amlodipine and verapamil prevented up-regulation of Plg-Rs. We detected the Cav1.2 subunit, the pore-forming subunit of the LTCC, in mouse and human macrophages at the transcript and protein levels (Das et al. 2009). Cav1.2 expression increased with differentiation towards macrophages, and knockdown of Cav1.2 with siRNA also blunted Plg-R and Plg binding to the cells. Typically LTCC are voltage gated (VGC). However, expression of LTCC in non-excitatory cells has been reported, where they appear to be gated by agonists, stimulus opened channels (SOC) rather than voltage (Grafton et al. 2003,Stokes et al. 2004).
The LTCC also have been implicated in the regulation of Plg-Rs in vivo. Amlodipine or verapamil, administered by implanted minipumps, reduced thioglycollogate induced macrophage emigration into the peritoneal cavity by up to 80% at doses that did not effect blood pressure. Macrophages did exit the blood but arrested at the peritoneal membrane, a pattern that was also observed in Plg−/− mice. In addition, the peritoneal tissue in Plg−/− and drug-treated mice became engorged in collagen, an apparent consequence of the absence of Plg-dependent MMP-9 activation (Gong et al. 2008). In the drug-treated mice, the arrested macrophages showed reduced surface expression of H2B, annexin 2 and p11, and showed little surface expression of Plg (Das et al. 2009).
On circulating leukocytes, integrins change their surface expression and functional status, and these alterations are influenced by intracellular Ca2+ and Ca2+ channels. β2 integrins are present in subcellular storage vesicles as well as in the plasma membrane. During leukocyte activation, the integrins can rapidly translocate from the storage pools to the cell surface, and “inside-out” signaling events leading to activation of their ligand binding function. A broad range of agonists, including inflammatory mediators, induce Ca2+ influx in leukocytes, and antagonists of calcium channels decrease integrin expression and function. For example, ADP/ATP induces Ca2+ influx from external stores in monocytes, which results in enhanced ligand binding to integrin αMβ2. These events are blocked by LTCC antagonists (Altieri et al., 1990). N-propargyl-nitrendipine, a specific blocker of SOC, inhibits αMβ2 cell-surface expression and αMβ2-dependent chemotaxis of PMN. SOC blockade in vivo attenuates trauma, hemorrhagic shock-induced PMN priming and lung injury without evidence of hemodynamic side effects in rats (Lee et al. 2005). Mibefradil, an antagonist of T-type Ca2+ channels also reduces surface expression of β2 integrins and L-selectin leading to impaired adhesion of human leukocytes (Nebe et al. 2002). Parallel studies have yet to be conducted to compare the relative effects of these Ca2+ blockers on the expression and function of tailed and tailless Plg-Rs, but a dual role of Ca2+ mobilization on both classes of Plg-Rs can be anticipated.
Concluding Remarks
In addition to its role as the primary fibrinolytic enzyme, plasmin facilitates cell migration through tissues, including recruitment of inflammatory cells. This function is particularly pertinent to cardiovascular diseases and depends on the binding and activation of Plg to Plg-Rs on the surface of responding cells. Multiple Plg-Rs have been identified, and here we propose a structure-based classification as tailed or tailless Plg-Rs based on the presence or absence of a cytoplasmic domain. Both classes of Plg-Rs have been implicated in the functional responses of leukocytes. Evidence is emerging to suggest that specific classes of Plg-Rs or individual Plg-R contribute to leukocyte responses. Nevertheless, a common mechanism for regulation of Plg-R expression, Ca2+ mobilization and Ca2+ channels, may be operative. These observations can be synthesized to suggest new anti-inflammatory strategies in which Plg-Rs can be generally targeted with Ca2+ channel blockers, even at doses that do not have hemodynamic effects, or can be individually targeted with antagonists of specific Plg-Rs to prevent tissue-specific or stimulus-specific inflammatory responses.
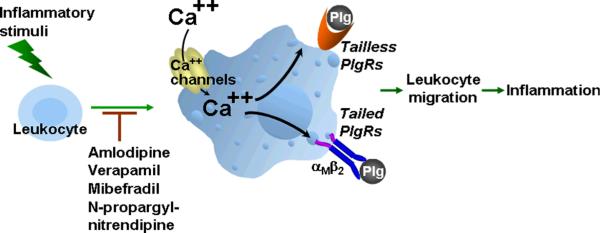
Figure 1.
Role of Ca2+ and Ca2+ channels in regulation of Plg-Rs. Stimulation of leukocytes, including stimuli that induce differentiation of monocytes into macrophages mobilize Ca2+ entry into cells, either through existing channels or by triggering synthesis of Ca2+ channels, particularly of the L-type Ca2+ channel variety. The pathways involved lead to enhanced expression of both tailed and tailless Plg-Rs at cell surface. Plg binding and activation on leukocytes facilitates leukocyte migration during an inflammatory response. Inhibitors of Ca2+ channels can inhibit the upregulation of Plg-Rs and suppress inflammation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altieri DC, Wiltse WL, Edgington TS. Signal transduction initiated by extracellular nucleotides regulates the high affinity ligand recognition of the adhesive receptor CD11b/CD18. J Immunol. 1990;145:662–670. [PubMed] [Google Scholar]
- Andronicos NM, Chen EI, Baik N, et al. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood. 2010;115:1319–1330. doi: 10.1182/blood-2008-11-188938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Brix K, Summa W, Lottspeich F, Herzog V. Extracellularly occurring histone H1 mediates the binding of thyroglobulin to the cell surface of mouse macrophages. J. Clin. Invest. 1998;102:283–293. doi: 10.1172/JCI1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein C, Deora AB, Jacovina AT, et al. Annexin II mediates plasminogen-dependent matrix invasion by human monocytes: enhanced expression by macrophages. Blood. 2004;103:317–324. doi: 10.1182/blood-2003-04-1304. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Wei Y. Protease crosstalk with integrins: the urokinase receptor paradigm. Thromb Haemost. 2001;86:124–129. [PubMed] [Google Scholar]
- Das R, Burke T, Plow EF. Histone H2B as a functionally important plasminogen receptor on macrophages. Blood. 2007;110:3763–3772. doi: 10.1182/blood-2007-03-079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Burke T, Van Wagoner DR, Plow EF. L-type calcium channel blockers exert an antiinflammatory effect by suppressing expression of plasminogen receptors on macrophages. Circ Res. 2009;105:167–175. doi: 10.1161/CIRCRESAHA.109.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone DJ, Borth W, Khan KMF, Hajjar KA. Plasminogen-mediated matrix invasion and degradation by macrophages is dependent on surface expression of annexin II. Blood. 2001;97:777–784. doi: 10.1182/blood.v97.3.777. [DOI] [PubMed] [Google Scholar]
- Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008;118:3012–3024. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton G, Stokes L, Toellner KM, Gordon J. A non-voltage-gated calcium channel with L-type characteristics activated by B cell receptor ligation. Biochem Pharmacol. 2003;66:2001–2009. doi: 10.1016/j.bcp.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Hembrough TA, Vasudevan J, Allietta MM, Glass WF, II, Gonias SL. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J Cell Sci. 1995;108:1071–1082. doi: 10.1242/jcs.108.3.1071. [DOI] [PubMed] [Google Scholar]
- Herren T, Burke TA, Das R, Plow EF. Identification of histone H2B as a regulated plasminogen receptor. Biochemistry. 2006;45:9463–9474. doi: 10.1021/bi060756w. [DOI] [PubMed] [Google Scholar]
- Herren T, Burke TA, Jardi M, Felez J, Plow EF. Regulation of plasminogen binding to neutrophils. Blood. 2001;97:1070–1078. doi: 10.1182/blood.v97.4.1070. [DOI] [PubMed] [Google Scholar]
- Herren T, Swaisgood CM, Plow EF. Regulation of plasminogen receptors. Front Biosci. 2003;8:d1–d8. doi: 10.2741/916. [DOI] [PubMed] [Google Scholar]
- Holers VM, Kotzin BL. Human peripheral blood monocytes display surface antigens recognized by monoclonal antinuclear antibodies. J Clin Invest. 1985;76:991–998. doi: 10.1172/JCI112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hajjar KA. Annexin II: a plasminogen-plasminogen activator co-receptor. Front Biosci. 2002;7:d341–d348. doi: 10.2741/kim. [DOI] [PubMed] [Google Scholar]
- Kim S-O, Plow EF, Miles LA. Regulation of plasminogen receptor expression on monocytoid cells by b1-integrin dependent cellular adherence to extracellular matrix proteins. J Biol Chem. 1996;271:23761–23767. doi: 10.1074/jbc.271.39.23761. [DOI] [PubMed] [Google Scholar]
- Lee C, Xu DZ, Feketeova E, et al. Store-operated calcium channel inhibition attenuates neutrophil function and postshock acute lung injury. J Trauma. 2005;59:56–63. doi: 10.1097/01.ta.0000171456.54921.fe. [DOI] [PubMed] [Google Scholar]
- Lijnen HR. Molecular interactions between the plasminogen/plasmin and matrix metalloproteinase systems. Fibrinolysis & Proteolysis. 2000;14:175–181. [Google Scholar]
- Lopez-Alemany R, Correc P, Camoin L, Burtin P. Purification of the plasmin receptor from human carcinoma cells and comparison to alpha-enolase. Thromb Res. 1994;75:371–381. doi: 10.1016/0049-3848(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Alemany R, Suelves M, Munoz-Canoves P. Plasmin generation dependent on alpha-enolase-type plasminogen receptor is required for myogenesis. Thromb Haemost. 2003;90:724–733. doi: 10.1160/TH03-04-0291. [DOI] [PubMed] [Google Scholar]
- MacLeod TJ, Kwon M, Filipenko NR, Waisman DM. Phospholipid-associated annexin A2-S100A10 heterotetramer and its subunits: characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin. J Biol Chem. 2003;278:25577–25584. doi: 10.1074/jbc.M301017200. [DOI] [PubMed] [Google Scholar]
- Miles LA, Dahlberg CM, Plescia J, Felez J, et al. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry. 1991;30:1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- Miles LA, Plow EF. Receptor mediated binding of the fibrinolytic components, plasminogen and urokinase, to peripheral blood cells. Thromb Haemost. 1987;58:936–942. [PubMed] [Google Scholar]
- Nakajima K, Hamanoue M, Takemoto N, et al. Plasminogen binds specifically to α-enolase on rat neuronal plasma membrane. J Neurochem. 1994;63:2048–2057. doi: 10.1046/j.1471-4159.1994.63062048.x. [DOI] [PubMed] [Google Scholar]
- Nebe B, Holzhausen C, Rychly J, Urbaszek W. Impaired mechanisms of leukocyte adhesion in vitro by the calcium channel antagonist mibefradil. Cardiovasc Drugs Ther. 2002;16:183–193. doi: 10.1023/a:1020688019792. [DOI] [PubMed] [Google Scholar]
- O'Mullane MJ, Baker MS. Elevated plasminogen receptor expression occurs as a degradative phase event in cellular apoptosis. Immunol Cell Biol. 1999;77:249–255. doi: 10.1046/j.1440-1711.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- Pancholi V, Fischetti VA. α-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J Biol Chem. 1998;273:14503–14515. doi: 10.1074/jbc.273.23.14503. [DOI] [PubMed] [Google Scholar]
- Peterson EA, Sutherland MR, Nesheim ME, Pryzdial EL. Thrombin induces endothelial cell-surface exposure of the plasminogen receptor annexin 2. J Cell Sci. 2003;116:2399–2408. doi: 10.1242/jcs.00434. [DOI] [PubMed] [Google Scholar]
- Ploplis VA, Castellino FJ. Nonfibrinolytic functions of plasminogen. Methods. 2000;21:103–110. doi: 10.1006/meth.2000.0981. [DOI] [PubMed] [Google Scholar]
- Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- Plow EF, Hoover-Plow J. The functions of plasminogen in cardiovascular disease. Trends Cardiovasc Med. 2004;14:180–186. doi: 10.1016/j.tcm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Plow EF, Ploplis VA, Carmeliet P, Collen D. Plasminogen and cell migration in vivo. Fibrinolysis & Proteolysis. 1999;13:49–53. [Google Scholar]
- Pluskota E, Soloviev DA, Bdeir K, et al. Integrin αMβ2 orchestrates and accelerates plasminogen activation and fibrinolysis by neutrophils. J Biol Chem. 2004;279:18063–18072. doi: 10.1074/jbc.M310462200. [DOI] [PubMed] [Google Scholar]
- Pluskota E, Soloviev DA, Szpak D, et al. Neutrophil apoptosis: selective regulation by different ligands of integrin alphaMbeta2. J Immunol. 2008;181:3609–3619. doi: 10.4049/jimmunol.181.5.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskota E, Solovjov DA, Plow EF. Convergence of the adhesive and fibrinolytic systems: recognition of urokinase by integrin αMβ2 as well as by the urokinase receptor regulates cell adhesion and migration. Blood. 2003;101:1582–1590. doi: 10.1182/blood-2002-06-1842. [DOI] [PubMed] [Google Scholar]
- Seweryn E, Pietkiewicz J, Bednarz-Misa IS, et al. Localization of enolase in the subfractions of a breast cancer cell line. Z Naturforsch C. 2009;64:754–758. doi: 10.1515/znc-2009-9-1023. [DOI] [PubMed] [Google Scholar]
- Stokes L, Gordon J, Grafton G. Non-voltage-gated L-type Ca2+ channels in human T cells: pharmacology and molecular characterization of the major alpha pore-forming and auxiliary beta-subunits. J Biol Chem. 2004;279:19566–19573. doi: 10.1074/jbc.M401481200. [DOI] [PubMed] [Google Scholar]
- Syrovets T, Simmet T. Novel aspects and new roles for the serine protease plasmin. Cell Mol. Life Sci. 2004;61:873–885. doi: 10.1007/s00018-003-3348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarui T, Akakura N, Majumdar M, et al. Direct interaction of the kringle domain of urokinase-type plasminogen activator (uPA) and integrin alpha v beta 3 induces signal transduction and enhances plasminogen activation. Thromb Haemost. 2006;95:524–534. doi: 10.1160/TH05-06-0457. [DOI] [PubMed] [Google Scholar]
- Wygrecka M, Marsh LM, Morty RE, et al. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood. 2009;113:5588–5598. doi: 10.1182/blood-2008-08-170837. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]