Summary
During adaptive radiations, animals colonize diverse environments, which requires adaptation in multiple phenotypic traits [1]. Because hormones mediate the dynamic regulation of suites of phenotypic traits [2–4], evolutionary changes in hormonal signaling pathways might contribute to adaptation to new environments. Here, we report changes in the thyroid hormone signaling pathway in stream-resident ecotypes of threespine stickleback fish (Gasterosteus aculeatus), which have repeatedly evolved from ancestral marine ecotypes [5–8]. Stream-resident fish exhibit a lower plasma concentration of thyroid hormone and a lower metabolic rate, which is likely adaptive for permanent residency in small streams. The thyroid stimulating hormone-β2 (TSHβ2) gene exhibited significantly lower mRNA expression in pituitary glands of stream-resident sticklebacks relative to marine sticklebacks. Some of the difference in TSHβ2 transcript levels can be explained by cis-regulatory differences at the TSHβ2 gene locus. Consistent with these expression differences, a strong signature of divergent natural selection was found at the TSHβ2 genomic locus. By contrast, there were no differences between the marine and stream-resident ecotypes in mRNA levels or genomic sequence in the paralogous TSHβ1 gene. Our data indicate that evolutionary changes in hormonal signaling have played an important role in the postglacial adaptive radiation of sticklebacks.
Highlights.
Low plasma concentration of thyroid hormone has evolved in stream sticklebacks.
Lower metabolic rate of stream sticklebacks may be adaptive.
Pituitary TSHβ2 mRNA level is reduced in stream sticklebacks.
Evidence of cis-regulatory divergence and signatures of natural selection in TSHβ2.
Results and Discussion
Divergence in Thyroid Hormone Concentration
Hormones mediate the regulation of diverse phenotypic traits. Therefore, hormonal divergence between ecotypes can facilitate or constrain the adaptive evolution of a suite of correlated phenotypic traits in diverse environments and is likely to underlie several life history trade-offs [2]. Although suites of morphological, behavioral, and physiological changes are common in adaptive radiations [1], little is known about the hormonal basis of these changes. The threespine stickleback (Gasterosteus aculeatus) provides a great model system to explore the hormonal basis for adaptive radiation. After the last glacial recession about 12,000 years ago, ancestral marine sticklebacks colonized newly formed freshwater streams and lakes in many coastal regions of the Northern hemisphere, resulting in the repeated evolution of freshwater-resident ecotypes (Figures 1A and B) [5–8]. The genetic basis of morphological traits that have evolved repeatedly during freshwater colonization has been investigated [7, 9–14], but the hormonal and genetic basis for adaptive physiological traits is less well understood.
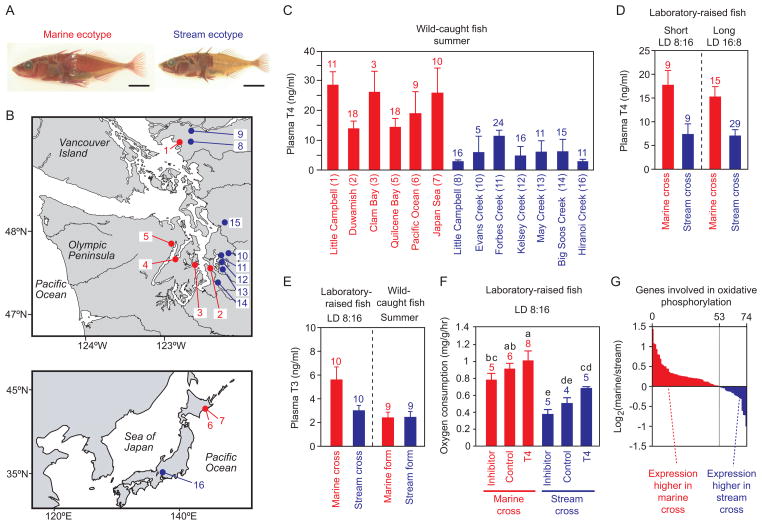
Figure 1. Low plasma thyroid hormone concentration and metabolic rate in stream-resident stickleback.
(A) Representative images of a marine ecotype (Duwamish River, Site 2 in Figure 1B) and a stream-resident ecotype of threespine stickleback (Big Soos Creek, Site 14 in Figure 1B). Skeletal structures are visualized with alizarin red staining. Scale bar = 10 mm.
(B) Map of collection sites in the Pacific Northwest of North America (upper panel) and the Japanese Archipelago (lower panel). Red dots indicate the collection sites of marine fish, while blue dots indicate the collection sites of stream-resident fish. Numbers indicate the sampling sites: (1) Little Campbell River marine; (2) Duwamish River marine; (3) Clam Bay marine; (4) Seabeck Bay marine; (5) Quilcene Bay marine; (6) Akkeshi Pacific Ocean marine; (7) Akkeshi Japan Sea marine; (8) Little Campbell River stream; (9) Salmon River stream; (10) Evans Creek stream; (11) Forbes Creek stream; (12) Kelsey Creek stream; (13) May Creek stream; (14) Big Soos Creek stream; (15) Allen Creek stream; (16) Hiranoi Creek stream.
(C) Plasma T4 concentration (mean ± SEM) of sticklebacks caught in early summer. Red bars indicate marine populations, while blue bars indicate stream-resident populations. Sample sizes for each population are shown above each bar. The number in parenthesis indicates the collection site shown in Figure 1B.
(D) Plasma T4 concentration (mean ± SEM) of laboratory-raised sticklebacks sampled under a short photoperiod (left) or a long photoperiod (right). A grand mean (± SEM) of two independent families for each cross is shown in the graph. Data for each family are available in Figure S1A. Sample sizes for each group are shown above each bar.
(E) Plasma T3 concentration (mean ± SEM) of laboratory-raised sticklebacks sampled under a short photoperiod (left) or wild fish (Little Campbell River marine and stream-resident ecotypes) caught in early summer (right). Sample sizes for each group are shown above each bar.
(F) Effect of thyroid hormone inhibitor (thiourea) and thyroid hormone (thyroxine, T4) treatment on oxygen consumption rate. Oxygen consumption rate was divided by body weight (g) and shown as rate per hour. Mean ± SEM is shown. Sample sizes for each group are shown above each bar. Letters above the bars indicate the results of Waller-Duncan’s posthoc tests after ANOVA.
(G) Expression levels of 74 genes involved in oxidative phosphorylation were compared between marine and stream-resident ecotypes by microarray. Red bars indicate the genes expressed more in marine fish than in stream-resident fish, while the blue bars indicate the genes expressed more in stream-resident fish than in marine fish. Fold change of transcript level (marine fish signal divided by the stream-resident fish signal) is shown as the logarithm to base 2, so 1 indicates that the signal intensity of the transcript is twice as high in marine fish than in stream-resident fish, while -1 indicates the signal intensity of the transcript is twice as high in stream-resident fish than in marine fish.
In the present study, we investigated divergence in thyroid hormone signaling pathways between marine and stream-resident sticklebacks. Marine sticklebacks have a migratory lifecycle in which adults migrate to freshwater or estuaries to spawn in the spring and summer, and juveniles migrate back to the sea in the fall, whereas stream-resident sticklebacks stay in freshwater for their entire life [5, 15]. Thyroid hormone plays a key role in regulating many of the physiological and behavioral changes associated with migration in fish and birds [16–19]. For example, thyroid hormone regulates metabolic rate [20, 21], swimming behavior [22, 23], salinity preference [24], salinity tolerance [25], silvering [26], olfactory cellular proliferation [27], and sexual maturation [23] in fishes. Therefore, we examined whether thyroid hormone physiology has diverged between marine and stream-resident sticklebacks with contrasting migratory behaviors.
We first collected marine and stream-resident sticklebacks from multiple populations in the Pacific Northwest and Japan during the summer (Figures 1A and B) and measured plasma thyroid hormone (thyroxine; T4) concentration by radioimmunoassay (Figure 1C). Marine populations had significantly higher plasma T4 concentrations than stream-resident populations (Figure 1C; nested ANOVA, F1, 11= 50.4, P < 0.001). Next, we made pure crosses from both a marine population and a stream-resident population that have a parapatric distribution in the Little Campbell River (Site 1 and Site 8 in Figure 1B; [15]); these parapatric populations were chosen because these two collection sites are at the same latitude (i.e. similar seasonal photoperiodic regime). We then measured the plasma T4 concentration of laboratory-raised fish under both short (light-dark cycle LD = 8:16) and long photoperiods (LD = 16:8). The laboratory-raised marine fish had significantly higher T4 concentration than the laboratory-raised stream-resident fish under both short and long photoperiods (Figure 1D; nested ANOVA; effect of ecotype, F1, 4 = 26.04, P = 0.007; effect of photoperiod, F1, 4 = 0.30, P = 0.614; interaction between ecotype and photoperiod, F1, 4 = 0.12, P = 0.751). Thus, the marine and stream-resident sticklebacks have genetically-based differences in T4 concentration, and the low T4 concentration in stream-resident fish does not simply result from environmental factors in fresh water, such as lower environmental levels of iodine, a precursor of thyroid hormone [28].
Animals have another form of thyroid hormone in plasma, triiodothyronine (T3). T3 is considered the more biologically active form of thyroid hormone since thyroid hormone receptors generally show a greater affinity for T3 over T4 [29]. Although T4 is the dominant thyroid hormone secreted from thyroid follicles, plasma T4 is converted to T3 in the periphery by iodothyronine deiodinase (DIO) activity [30]. We measured plasma T3 and found that the plasma T3 concentration was generally lower than the plasma T4 concentration in sticklebacks (Figure 1E). Furthermore, we found that laboratory-raised marine sticklebacks have a higher plasma T3 concentration than the laboratory-raised stream fish under the short photoperiod (ANOVA, F1,18 = 6.82, P = 0.0177). Interestingly, plasma T3 concentration did not differ between ecotypes caught at the Little Campbell River in summer (ANOVA, F1,16 = 0.022, P = 0.883), which may indicate that photoperiod influences thyroid hormone physiology. In order to further investigate divergence in thyroid hormone physiology within target tissues, we conducted a genome-wide transcriptome analysis of the gill, one of the main target tissues of thyroid hormone in teleosts. Although there was no divergence in thyroid hormone receptor mRNA levels between ecotypes, DIO3, which converts the active T3 to the inactive T4, was expressed at higher levels in stream-resident fish (Figure S1D). In addition, DIO2, which converts T4 to T3, was expressed at higher levels in marine fish than in stream-resident fish. Interestingly, an enzyme involved in iodide salvage, iodotyrosine deiodinase (IYD) was expressed at higher levels in marine fish than in stream-resident fish (Figure S1D); loss-of-function of IYD can cause hypothyroidism in humans [31]. These results suggest that stream-resident sticklebacks have diverged from marine sticklebacks not only in plasma concentration of thyroid hormone, but also in other downstream signaling pathways.
Role of Thyroid Hormone in Metabolic Regulation
A previous study demonstrated that a marine stickleback population has a higher oxygen consumption rate (i.e. metabolic rate) than a sympatric stream-resident population [32]. Here, we found a similar difference between laboratory-raised marine and stream sticklebacks (Figure 1F) (ANOVA; effect of ecotype, F2, 29 = 38.9, P < 0.001), suggesting that the divergence in oxygen consumption rate has a genetic basis. We also conducted a liver metabolome analysis and found that laboratory-raised marine sticklebacks have higher levels of most intermediates in glycolysis and the TCA cycle than laboratory-raised stream-resident sticklebacks (Figure S1E). In our genome-wide transcriptome analysis, we also found that more genes involved in oxidative phosphorylation were expressed at a higher level in laboratory-raised marine fish than in laboratory-raised stream fish (Figure 1G). These collective data suggest that the marine ecotype has a higher metabolic rate than the stream-resident ecotype.
Because thyroid hormone can regulate metabolic rate in fishes [20, 21], we tested whether the differences in thyroid hormone concentration are related to differences in metabolic rate between the ecotypes. We treated fish with T4 and a T4 synthesis inhibitor (thiourea; TU). We found that treatment with T4 increased oxygen consumption rate, while treatment with TU decreased oxygen consumption rate in both ecotypes (ANOVA; effect of treatment, F2, 29 = 5.89, P = 0.007) (Figure 1F). Although the oxygen consumption rate of the stream-resident ecotype did not increase to the level of that of the marine ecotype even when exposed to T4, this is not surprising, given the fact that the stream-resident ecotypes might have a reduced ability to produce T3 in peripheral target tissues (see above). However, there are likely factors that contribute to divergence in metabolic rate between ecotypes in addition to thyroid hormone physiology.
Because thyroid hormone treatment is known to increase swimming activity in teleosts [22], some of the effects of thyroid hormone on oxygen consumption rate might be due to effects on swimming activity rather than direct effects on the basal metabolic rate. To answer this question, we observed the swimming behavior of hormone-treated fish. Fish were released into a circular tank immediately after the measurement of oxygen consumption rate, and their behavior was monitored for 20 min. T4 and TU increased and decreased, respectively, the frequency of turning behavior in both ecotypes of sticklebacks (repeated measures ANOVA; effect of treatment on turning activity, F2,27 = 7.73, P = 0.0022; effect of ecotype, F1,27 = 1.43, P = 0.242) (Figure S1C). Therefore, we added turning activity (turns/min) as a covariate and re-analyzed the effects of hormonal treatment on the oxygen consumption rate. The effect of thyroid hormone and ecotype on the oxygen consumption rate remained significant, even after including turning activity as a covariate (ANCOVA with turning activity as a covariate; effect of treatment, F2, 24 = 6.42, P = 0.006; effect of ecotype, F1, 26 = 34.6, P < 10−5; effect of activity, F1, 24 = 0.38, P = 0.543). These results suggest that thyroid hormone may be involved in the regulation of both swimming activity and basal metabolic rate.
Divergence in metabolic rate might have evolved as an adaptation to the differential abundance of nutrients and oxygen in marine and freshwater environments. Temperate marine environments generally have more abundant energy sources than freshwater environments [33]. This is the case in the Pacific Northwest of North America; primary production in a marine environment of that coastal region (eg., 1.89~3.46 gC/m2·d in Elliot Bay, Washington) [34] is higher than in small lowland streams (eg., 0.02~0.55 gC/m2·d in Kelsey Creek, Washington) [35]. In addition, dissolved oxygen is consistently high in marine environments (> 5 mg/L in the Puget Sound, Washington) [36], while it can drop below 3.0 mg/L (Evans Creek and Big Soos Creek, Washington) [37, 38] or even below 0.2 mg/L (Little Campbell Stream, British Columbia, Canada) [39] in small lowland streams. Thus, greater nutrient and oxygen availability in the marine environments may allow marine stickleback to have increased metabolic rate and swimming activity, which are likely adaptive for migration. By contrast, stream-resident stickleback with similarly high levels of thyroid hormone might be predisposed to increased risks of energy deficiency or cellular hypoxia given the comparatively limited energy sources and oxygen in their habitats [40, 41]. In fishes, both hypoxic stress and low food availability are known to induce low thyroid hormone levels [42, 43], which suggests that the genetically-based low thyroid hormone concentration we observe in stream-resident sticklebacks may be adaptive for permanent residency in oxygen- and nutrient-limited environments.
Divergence in TSHβ Expression between Marine and Resident Ecotypes
The synthesis and secretion of thyroid hormone from thyroid follicles is stimulated by a pituitary glycoprotein hormone, thyroid stimulating hormone (TSH), in many animals [44–47], although this has not yet been confirmed in sticklebacks. The functional TSH hormone is a dimer of a TSH-specific β-subunit (TSHβ) and an α-subunit (GPHα) that is shared by other pituitary glycoprotein hormones. The threespine stickleback has two TSHβ paralogs, one on linkage group (LG) 17 (TSHβι) and another on LG12 (TSHβ2) (Figure S2A). Because many paralogous genes are found on stickleback LG17 and LG12 (Figure S2B) [48], TSHβ1 and TSHβ2 are likely the products of the teleost-specific genome duplication [49]. Further supporting this conclusion, two clades of teleost TSHβ genes are found in a phylogenetic tree created using the TSHβ protein from multiple vertebrate species (Figure S2B).
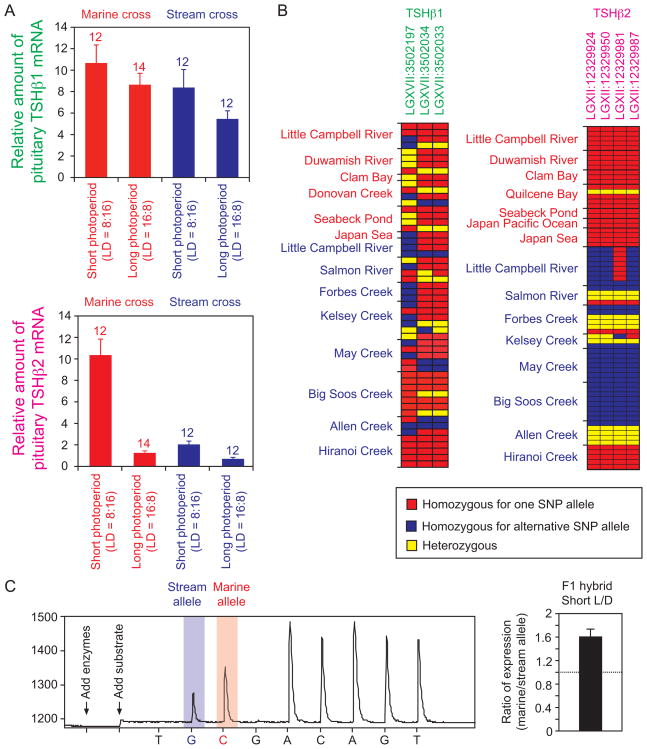
TSHβ1 and TSHβ2 mRNA levels in the stickleback pituitary gland were measured by quantitative polymerase chain reaction (qPCR) in laboratory-raised crosses from the Little Campbell marine and stream-resident populations [15]. Little variation was found in pituitary TSHβ1 mRNA levels between photoperiods or between ecotypes (Figure 2A; nested ANOVA, effect of ecotype, F1,46 = 4.03, P = 0.051; effect of photoperiod, F1,46 = 1.73, P = 0.194; interaction between ecotype and photoperiod, F1,46 = 0.49, P = 0.489). By contrast, we found significant differences in TSHβ2 mRNA levels between photoperiods as well as between ecotypes (Figure 2A). The marine ecotype has significantly higher levels of TSHβ2 mRNA than the stream-resident ecotype (nested ANOVA, effect of ecotype, F1,46 = 33.68, P < 0.001). In both ecotypes, transcript levels were high under the short photoperiod and dropped significantly under the long photoperiod (nested ANOVA, effect of photoperiod, F1,46 = 84.57, P < 0.001). However, the degree of change in TSHβ2 mRNA levels was different between the marine and stream-resident ecotypes (nested ANOVA, interaction between ecotype and photoperiod, F1,46 = 8.65, P = 0.005). In the marine ecotype, mRNA levels were 8 times higher under the short photoperiod than under the long photoperiod, while in the stream-resident ecotype, mRNA levels were only about 2 times higher under the short photoperiod than under the long photoperiod. This reduction in the magnitude of photoperiodic regulation of TSHβ2 is consistent with the fact that stream-resident fish spend their entire lives in the freshwater environment and do not perform seasonal migrations.
Figure 2. Divergent TSHβ2 transcript level and cis-regulatory sequence between marine and stream-resident sticklebacks.
(A) Photoperiodic response of total pituitary TSHβι (upper panel) and TSHβ2 (lower panel) mRNA level. A grand mean (± SEM) of two independent families is shown in the graph. Sample sizes for each group are shown above each bar. Expression level was determined by comparing the amplification threshold cycle to that of a serially diluted standard cDNA sample, followed by multiplication of the amount of RNA isolated from each pituitary gland.
(B) Three representative SNPs at the TSHβ1 locus did not show any significant segregation between marine and stream-resident ecotypes (left panel). Four representative SNPs at the TSHβ2 locus significantly differed in frequency between marine and stream-resident ecotypes (right panel). Results of genetic analyses are available in Table S1. In both panels, red squares indicate that an individual is homozygous for one SNP allele, blue squares indicate that an individual is homozygous for the alternative allele, and yellow squares indicate that an individual is heterozygous. Details of SNP information are shown in Figure S2.
(C) Pyrosequencing of pituitary cDNA in F1 hybrid fish between a Little Campbell River marine female and a Little Campbell River-resident male. The left panel indicates a representative pyrogram showing a higher peak on C than on G at a SNP site (for the SNP, see Fig. S2G). The right panel indicates the ratio (mean ± SEM) of marine to stream-resident TSHβ2 allelic expression (n = 10).
Although plasma T3 concentration exhibited a similar photoperiodic change (Figure 1E), plasma T4 did not. A previous study in killifish indicates that the relationship between TSH level and thyroid hormone concentration can change according to photoperiod because negative feedback of thyroid hormone on TSH is stronger under a long photoperiod than under a short photoperiod [50]. In sticklebacks, we saw a similar pattern; T4 treatment reduced TSHβ2 expression under the long photoperiod but not under the short photoperiod (Figure S2D). Therefore, a difference in the sensitivity of the pituitary gland to negative feedback of thyroid hormone on TSH might explain the difference in photoperiodic response between T4 and TSHβ2. Furthermore, there is evidence in mammals that TSH might act directly on peripheral tissues to stimulate DIO2 activity and the conversion of T4 to T3 [51], which is consistent with our observation that TSHβ2 mRNA level and plasma T3 concentration are higher during the short photoperiod than during the long photoperiod. Clearly, further studies on the relationship between TSHβ expression and thyroid hormone signaling are required to better understand the thyroid hormone signaling pathway in sticklebacks.
Divergent TSHβ2 Haplotypes between Marine and Resident Ecotypes
Because TSHβ2 expression was different between the marine and stream-resident ecotypes, we compared the genomic sequences of the 5′-non coding regions of TSHβ2 and TSHβ1 between the two ecotypes. This region of the TSHβ1 gene did not differ between ecotypes (Table S1); nested analysis of molecular variance (AMOVA) revealed that little of the genetic variance (−7.57 %) was explained by ecotype (P = 0.923), while most was explained by populations within ecotype (61.20%) and by individuals within populations (46.37 %). By contrast, marine and stream-resident sticklebacks diverge in the 5′-non coding region of the TSHβ2 gene. Nested AMOVA revealed that 40.83 % of the total genetic variance in the 5′-non coding region of the TSHβ2 gene was explained by ecotype (P < 0.001), while 33.03 % was explained by populations within ecotype, and 26.13 % was explained by individuals within populations (Table S1). In particular, four single nucleotide polymorphisms (SNPs) exhibited striking differences in frequency between ecotypes (Figure 2B); all marine fish except a single heterozygote have one haplotype (24/25 fish), regardless of their geographical origin. In contrast, most stream-resident fish were either homozygous or heterozygous for an alternative haplotype (39/46 fish).
The phylogenetic analysis of TSHβ2 alleles revealed that the stream-resident alleles form a single branch except that a Japanese stream-resident population (Hiranoi Creek) had an exclusively marine haplotype (Figure S2H). We confirmed that the Japanese stream-resident population also had a lower TSHβ2 mRNA expression level (Figure S2E). Interestingly, a nearby Japanese stream population was also found to have a marine haplotype at an armor plate gene, despite the fact that these fish exhibit armor reduction typical of stream-resident stickleback populations [7]. Thus, the genetic basis for freshwater adaptation in Japanese stream populations from this region may differ from the North American stream populations [7].
Divergence in the TSHβ2 haplotype is unlikely to have resulted from the phylogenetic relatedness of each ecotype. Previous phylogenetic studies of the Pacific Northwest sticklebacks indicated that stream-resident ecotypes have been independently derived from marine sticklebacks in each region [6–8, 12]. In addition, ecotype was not a significant predictor of genotype at other genes in the thyroid hormone pathway (the TSHβ1 locus, see above; Pit-1 locus, nested AMOVA, ΦCT = −0.15, P = 1.00; TSH receptor 2 locus, nested AMOVA, ΦCT = 0.029, P = 0.197) or in a microsatellite marker upstream of the TSHβ2 gene (Figure S2G; nested AMOVA, ΦCT = −0.011, P = 0.352) (Table S1).
In order to confirm that the TSHβ2 cis-regulatory region contributes to divergence in the TSHβ2 mRNA levels, we generated F1 hybrids heterozygous for marine and stream-resident TSHβ2 alleles and tested whether allele-specific expression differences can be seen in the pituitary gland of F1 hybrid fish. Differential expression of alleles in the F1 fish with an otherwise identical genetic background indicates functional cis-regulatory differences [12, 52]. Comparison of expression levels of two different alleles in the F1 fish was conducted using Pyrosequencing technology, which can compare the relative abundance of cDNA derived from the two alleles when a SNP is present in the cDNA [52]. In all of the F1 hybrids (n = 10), the marine allele was expressed at a higher level than the stream-resident allele (Figure 2C). The ratio of the marine allele to the stream-resident allele was significantly larger than one (mean ± SEM = 1.60 ± 0.13, t-test, t = 4.72, df = 9, P = 0.0010). The higher expression of the marine allele than the stream-resident allele in the same F1 hybrid fish indicates that functional cis-regulatory differences contribute to the divergence in TSHβ2 mRNA expression levels between ecotypes.
Interestingly, we found that one SNP (LGXII:12329950) of the marine haplotype is located within a consensus motif of a thyroid hormone response element (Fig. S2G), while the stream-resident allele lacks this motif due to a nucleotide substitution at this site. Furthermore, a computational analysis demonstrates that RNA secondary structure might differ between these two alleles (Fig. S2I). Although we do not know yet whether these SNPs are actually causing the cis-regulatory expression changes or altering RNA processing, further molecular studies on the functional divergence of the ecotype-specific alleles will provide novel insights into the genetic and endocrine basis for the repeated evolution of freshwater-resident sticklebacks.
Conclusions
We found divergence between marine and stream-resident sticklebacks in thyroid hormone physiology. Because thyroid hormone has diverse functions related to migration, divergence in thyroid hormone physiology might contribute to adaptation not only in metabolic rate and swimming activity, but also in other phenotypic traits that are known to differ between marine and freshwater sticklebacks. For example, marine and stream-resident sticklebacks differ in salinity preference behavior [24], salinity tolerance [21], the timing of sexual maturation [15], and many morphological traits [5]. There is evidence for the regulation of both osmoregulatory ability [25] and reproduction by thyroid hormone in fishes [53]. Although few studies have so far investigated the hormonal regulation of morphology in sticklebacks, thyroid hormone and TSH have been shown to regulate in skeletal development and morphogenesis in many vertebrates including fish [43, 54, 55]. Further studies investigating the role of thyroid hormone in the morphological, physiological and behavioral divergence between marine and freshwater-resident sticklebacks should lead to a better understanding of how genetic changes can alter suites of phenotypic traits.
Here, we found that cis-regulatory changes are likely important for the difference in TSHβ2 mRNA expression between the marine and stream-resident ecotypes, consistent with a signature of divergent natural selection at that locus. Similar to results for loci underlying plate reduction and color change in stickleback [7, 12, 56], we identified a single heterozygote in an ancestral marine population with an allele that is predominant in freshwater environments. These data support the idea that the pre-existing allelic variation in an ancestral population can facilitate rapid adaptation to novel environments [7, 56–58]. Overall, our results suggest that divergence in hormonal signaling may play a substantial role in the evolution of multiple phenotypic traits during an adaptive radiation in animals. The threespine stickleback system provides an opportunity to further explore the molecular basis for hormonal divergence underlying multiple adaptive traits.
Supplementary Material
Acknowledgments
We thank Ikumi Nakamura, Masakado Kawata, Manabu Kume, Jon Dickey, Felicity Jones, Frank Chan, David Kingsley, Yoshio Takei, Susumu Hyodo, Sanae Ogasawara, Shaun McCann as well as all members of the Peichel and Swanson Labs for valuable advice, discussion, and technical assistance. This work was supported by the Uehara Memorial Foundation (J.K.), the Brain Science Foundation (J.K.), JST PRESTO program (J.K.), and Grant-in-Aid for Young Scientist (B) from the Ministry of Education, Science, Sports, and Culture (J.K.), the NOAA Oceans and Human Health Initiative (S.C.L.), a Burroughs Wellcome Fund Career Award in the Biomedical Sciences (C.L.P.), and National Institutes of Health grant P50 HG02568 (C.L.P.). Sampling in Washington State was conducted under Washington Department of Fish and Wildlife permits to CLP (05-049, 06-159, 07-047). Canadian and Japanese fish were collected with the courtesy of Dolph Schluter and Manabu Kume, respectively. All experiments were reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committees (protocol #1575).
Footnotes
Accession Numbers
Microarray data reported herein have been deposited at the Center for Information Biology Gene Expression (http://cibex.nig.ac.jp/index.jsp) with the accession number CBX139.
Supplemental Information includes Supplemental Experimental Procedures, two figures, and one table.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schluter D. The Ecology of Adaptive Radiation. New York: Oxford University Press; 2000. [Google Scholar]
- 2.McGlothlin JW, Ketterson ED. Hormone-mediated suites as adaptations and evolutionary constraints. Phil Trans Roy Soc B. 2008;363:1611–1620. doi: 10.1098/rstb.2007.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradshaw D. Environmental endocrinology. Gen Comp Endocrinol. 2007;152:125–141. doi: 10.1016/j.ygcen.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Wingfield JC, Williams TD, Visser ME. Introduction. Integration of ecology and endocrinology in avian reproduction: a new synthesis. Phil Trans Roy Soc B. 2008;363:1581–1588. doi: 10.1098/rstb.2007.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell MA, Foster SA. The Evolutionary Biology of the Threespine Stickleback. Oxford: Oxford University Press; 1994. [Google Scholar]
- 6.McKinnon JS, Mori S, Blackman BK, David L, Kingsley DM, Jamieson L, Chou J, Schluter D. Evidence for ecology’s role in speciation. Nature. 2004;429:294–298. doi: 10.1038/nature02556. [DOI] [PubMed] [Google Scholar]
- 7.Colosimo PF, Hosemann KE, Balabhadra S, Villarreal G, Jr, Dickson M, Grimwood J, Schmutz J, Myers RM, Schluter D, Kingsley DM. Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science. 2005;307:1928–1933. doi: 10.1126/science.1107239. [DOI] [PubMed] [Google Scholar]
- 8.Haglund TR, Buth DG, Lawson R. Allozyme variation and phylogenetic relationships of Asian, North American, and European populations of the threespine stickleback, Gasterosteus aculeatus. Copeia. 1992;1992:432–443. [Google Scholar]
- 9.Shapiro MD, Marks ME, Peichel CL, Blackman BK, Nereng KS, Jonsson B, Schluter D, Kingsley DM. Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature. 2004;428:717–723. doi: 10.1038/nature02415. [DOI] [PubMed] [Google Scholar]
- 10.Peichel CL, Nereng KS, Ohgi KA, Cole BL, Colosimo PF, Buerkle CA, Schluter D, Kingsley DM. The genetic architecture of divergence between threespine stickleback species. Nature. 2001;414:901–905. doi: 10.1038/414901a. [DOI] [PubMed] [Google Scholar]
- 11.Cresko WA, Amores A, Wilson C, Murphy J, Currey M, Phillips P, Bell MA, Kimmel CB, Postlethwait JH. Parallel genetic basis for repeated evolution of armor loss in Alaskan threespine stickleback populations. Proc Natl Acad Sci USA. 2004;101:6050–6055. doi: 10.1073/pnas.0308479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller CT, Beleza S, Pollen AA, Schluter D, Kittles RA, Shriver MD, Kingsley DM. cis-Regulatory changes in Kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell. 2007;131:1179–1189. doi: 10.1016/j.cell.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert AY, Sawaya S, Vines TH, Knecht AK, Miller CT, Summers BR, Balabhadra S, Kingsley DM, Schluter D. The genetics of adaptive shape shift in stickleback: pleiotropy and effect size. Evolution. 2008;62:76–85. doi: 10.1111/j.1558-5646.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 14.Chan YF, Marks ME, Jones FC, Villarreal G, Jr, Shapiro MD, Brady SD, Southwick AM, Absher DM, Grimwood J, Schmutz J, et al. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science. 2010;327:302–305. doi: 10.1126/science.1182213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagen DW. Isolating mechanisms in threespine sticklebacks (Gasterosteus) J Fish Res Bd Canada. 1967;24:1637–1692. [Google Scholar]
- 16.Larsen DA, Moriyama S, Dickey JT, Beckman BR, Swanson P, Dickhoff WW. Regulation of the pituitary-thyroid axis during smoltification of coho salmon: Quantification of TSH, TSH mRNA, and thyroid hormones. In: Kawashima S, Kikuyama S, editors. Advances in Comparative Endocrinology. XIIIth International Congress of Comparative Endocrinology. Bologna: Monduzzi Editore; 1997. pp. 1083–1086. [Google Scholar]
- 17.Rankin MA. Endocrine effects on migration. Am Zool. 1991;31:217–230. [Google Scholar]
- 18.Comeau LA, Campana SE, Chouinard GA, Hanson JM. Timing of Atlantic cod Gadus morhua seasonal migrations in relation to serum levels of gonadal and thyroidal hormones. Mar Ecol Prog Ser. 2001;221:245–253. [Google Scholar]
- 19.McKeown BA. Fish Migration. Sydney: Croom Helm; 1984. [Google Scholar]
- 20.Oommen OV, Streejith P, Beyo RS, Divya L, Vijayasree AS, Manju M. Thyroid hormone regulates mitochondrial respiration as well as antioxidant defense in teleosts too! J Endocrinol Reprod. 2006;10:96–105. [Google Scholar]
- 21.Gutz M. Experimentelle Untersuchungen zur Salzadaptation verschiedener Rassen des Dreistachligen Stichlings (Gasterosteus aculeatus L.) Int Rev Hydrobiol. 1970;55:845–894. [Google Scholar]
- 22.Edeline E, Bardonnet A, Bolliet V, Dufour S, Elie P. Endocrine control of Anguilla anguilla glass eel dispersal: effect of thyroid hormones on locomotor activity and rheotactic behavior. Horm Behav. 2005;48:53–63. doi: 10.1016/j.yhbeh.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Bernhardt RR, von Hippel FA. Chronic perchlorate exposure impairs stickleback reproductive behaviour and swimming performance. Behaviour. 2008;145:527–559. doi: 10.1163/156853908792451511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baggerman B. An experimental study on the timing of breeding and migration in the three-spined stickleback (Gasterosteus aculeatus L.) Arch Néerlandais Zool. 1957;12:105–317. [Google Scholar]
- 25.McCormick SD. Endocrine control of osmoregulation in teleost fish. Am Zool. 2001;41:781–794. [Google Scholar]
- 26.Ikuta K, Aida K, Okumoto N, Hanyu I. Effects of thyroxine and methyltestosterone on smoltification of masu salmon (Oncorhynchus masou) Aquaculture. 1985;45:289–303. [Google Scholar]
- 27.Lema SC, Nevitt GA. Evidence that thyroid hormone induces olfactory cellular proliferation in salmon during a sensitive period for imprinting. J Exp Biol. 2004;207:3317–3327. doi: 10.1242/jeb.01143. [DOI] [PubMed] [Google Scholar]
- 28.Koutras DA, Matovinovic J, Vought R. The Ecology of Iodine. New York: Wiley Eastern Limited; 1985. [Google Scholar]
- 29.Nelson ER, Habibi HR. Thyroid receptor subtypes: structure and function in fish. Gen Comp Endocrinol. 2009;161:90–96. doi: 10.1016/j.ygcen.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Orozco A, Valverde-R C. Thyroid hormone deiodination in fish. Thyroid. 2005;15:799–813. doi: 10.1089/thy.2005.15.799. [DOI] [PubMed] [Google Scholar]
- 31.Moreno JC, Klootwijk W, van Toor H, Pinto G, D’Alessandro M, Leger A, Goudie D, Polak M, Gruters A, Visser TJ. Mutations in the iodotyrosine deiodinase gene and hypothyroidism. New Eng J Med. 2008;358:1811–1818. doi: 10.1056/NEJMoa0706819. [DOI] [PubMed] [Google Scholar]
- 32.Tudorache C, Blust R, de Boeck G. Swimming capacity and energetics of migrating and non-migrating morphs of three-spined stickleback Gasterosteus aculeatus L. and their ecological implications. J Fish Biol. 2007;71:1448–1456. [Google Scholar]
- 33.Gross MR, Coleman RM, McDowall RM. Aquatic productivity and the evolution of diadromous fish migration. Science. 1988;239:1291–1293. doi: 10.1126/science.239.4845.1291. [DOI] [PubMed] [Google Scholar]
- 34.Newton J, van Voorhis K. Seasonal patterns and controlling factors of primary production in Puget Sound’s central basin and possession sound. 02–03–059. Department of Ecology State of Washington; 2002. [Google Scholar]
- 35.Richey JS. Ph.D. University of Washington; 1982. Effects of urbanization on a lowland stream in western Washington. [Google Scholar]
- 36.Fresh KL. Master thesis. University of Washington; 1979. Distribution and abundance of fishes occurring in the nearshore surface waters of northern Puget Sound, Washington. [Google Scholar]
- 37.Mohamedali T, Lee S. Bear-Evans watershed temperature and dissolved oxygen total maximum daily load. 08–10–580. Department of Ecology State of Washington; 2008. [Google Scholar]
- 38.Swanson T, Mohamedali T, Roberts M, Homan C, Lee S, Jack R. Green River and Newaukum Creek temperature and dissolved oxygen total maximum daily load study: data summary report. 07–03–001. Department of Ecology State of Washington; 2007. [Google Scholar]
- 39.Bull J. Status of water quality conditions in Little Campbell, Serpentine, and Nicomeki Rivers from 1971 to 2002. British Columbia: Ministry of Water, Land and Air Pollution; 2003. [Google Scholar]
- 40.van Ginneken V, van den Thillart G. Metabolic depression in fish measured by direct calorimetry: a review. Thermochim Acta. 2009;483:1–7. [Google Scholar]
- 41.Bochdansky A, Gronkjaer P, Herra T, Leggett W. Experimental evidence for selection against fish larvae with high metabolic rate in a food limited environment. Mar Biol. 2005;147:1413–1417. [Google Scholar]
- 42.Wu RSS, Zhou BS, Randall DJ, Woo NYS, Lam PKS. Aquatic hypoxia is an endocrine disruptor and impairs fish reproduction. Environ Sci Technol. 2003;37:1137–1141. doi: 10.1021/es0258327. [DOI] [PubMed] [Google Scholar]
- 43.Lema SC, Nevitt GA. Testing an ecophysiological mechanism of morphological plasticity in pupfish and its relevance to conservation efforts for endangered Devils Hole pupfish. J Exp Biol. 2006;209:3499–3509. doi: 10.1242/jeb.02417. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. New York: Cambridge University Press; 1997. [Google Scholar]
- 45.MacKenzie DS, Jones RA, Miller TC. Thyrotorpin in teleost fish. Gen Comp Endocrinol. 2009;161:83–89. doi: 10.1016/j.ygcen.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Dayan CM, Panicker V. Novel insights into thyroid hormones from the study of common genetic variation. Nat Rev Endocrinol. 2009;5:211–218. doi: 10.1038/nrendo.2009.19. [DOI] [PubMed] [Google Scholar]
- 47.Hazlerigg D, Loudon A. New insights into ancient seasonal life times. Curr Biol. 2008;18:R795–804. doi: 10.1016/j.cub.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 48.Braasch I, Volff JN, Schartl M. The evolution of teleost pigmentation and the fish-specific genome duplication. J Fish Biol. 2008;73:1891–1918. [Google Scholar]
- 49.Jaillon O, Aury JM, Brunet F, Petit JL, Stange-Thomann N, Mauceli E, Bouneau L, Fischer C, Ozouf-Costaz C, Bernot A, et al. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate protokaryotype. Nature. 2004;431:946–957. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- 50.Brown CL, Stetson MH. Photoperiod-dependent negative feedback effects of thyroid hormones in Fundulus heteroclitus. Gen Comp Endocrinol. 1985;58:186–191. doi: 10.1016/0016-6480(85)90334-x. [DOI] [PubMed] [Google Scholar]
- 51.Wu SY, Reggio R, Florsheim WH. Characterization of thyrotropin-induced increase in iodothyronine monodeiodinating activity in mice. Endocrinology. 1985;116:901–908. doi: 10.1210/endo-116-3-901. [DOI] [PubMed] [Google Scholar]
- 52.Wittkopp PJ, Haerum BK, Clark AG. Evolutionary changes in cis and trans gene regulation. Nature. 2004;430:85–88. doi: 10.1038/nature02698. [DOI] [PubMed] [Google Scholar]
- 53.Cyr DG, Eales JG. Interrelationships between thyroidal and reproductive endocrine systems in fish. Rev Fish Biol Fisheries. 1996;6:165–200. [Google Scholar]
- 54.Bassett JHD, Williams GR. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab. 2003;14:356–364. doi: 10.1016/s1043-2760(03)00144-9. [DOI] [PubMed] [Google Scholar]
- 55.Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 56.Kitano J, Bolnick DI, Beauchamp DA, Mazur MM, Mori S, Nakano T, Peichel CL. Reverse evolution of armor plates in the threespine stickleback. Curr Biol. 2008;18:769–774. doi: 10.1016/j.cub.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 57.Barrett RDH, Schluter D. Adaptation from standing genetic variation. Trends Ecol Evol. 2008;23:38–44. doi: 10.1016/j.tree.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Hermisson J, Pennings PS. Soft sweeps: molecular population genetics of adaptation from standing genetic variation. Genetics. 2005;169:2335–2352. doi: 10.1534/genetics.104.036947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.