Abstract
Aims
In field studies assessing cognitive function in illicit ecstasy users, there are several frequent confounding factors that might plausibly bias the findings toward an overestimate of ecstasy-induced neurocognitive toxicity. We designed an investigation seeking to minimize these possible sources of bias.
Design
We compared illicit ecstasy users and non-users while 1) excluding individuals with significant lifetime exposure to other illicit drugs or alcohol; 2) requiring that all participants be members of the “rave” subculture; and 3) testing all participants with breath, urine, and hair samples at the time of evaluation to exclude possible surreptitious substance use. We compared groups with adjustment for age, gender, race/ethnicity, family-of-origin variables, and childhood history of conduct disorder and attention deficit hyperactivity disorder. We provide significance levels without correction for multiple comparisons.
Setting
Field study.
Participants
Fifty-two illicit ecstasy users and 59 non-users, age 18-45.
Measurements
Battery of 15 neuropsychological tests tapping a range of cognitive functions.
Findings
We found little evidence of decreased cognitive performance in ecstasy users, save for poorer strategic-self-regulation, possibly reflecting increased impulsivity. However this finding might have reflected a premorbid attribute of ecstasy users, rather than a residual neurotoxic effect of the drug.
Conclusions
In a study designed to minimize limitations found in many prior investigations, we failed to demonstrate marked residual cognitive effects in ecstasy users. This finding contrasts with many previous findings—including our own—and emphasizes the need for continued caution in interpreting field studies of cognitive function in illicit ecstasy users.
Keywords: ecstasy, MDMA, substance abuse, cognitive function, neurotoxicity
INTRODUCTION
“Ecstasy,” as used below, refers to illicit ±3,4-methylenedioxymethamphetamine (MDMA), with the recognition that actual “street” preparations may be adulterated or even contain no MDMA at all (1). Ecstasy has been used by some 12 million individuals in the United States alone and millions more worldwide (2-5). An extensive animal literature suggests that ecstasy can be neurotoxic, especially to the 5-HT system, with consequent possible effects on cognitive performance (6-9)—, but it is unclear whether these findings can be fully extrapolated to humans (10). To address this question, numerous naturalistic studies have assessed cognitive function in illicit ecstasy users. These studies, reviewed in several recent papers , generally suggest that illicit ecstasy users display negative residual effects on various cognitive measures, with the most consistent and robust finding being lowered verbal memory (3, 11-13). Such findings are of concern not only with regard to illicit ecstasy use, but for recent studies proposing therapeutic applications for MDMA, such as in treatment of posttraumatic stress disorder (14).
However, as we (15) and others (3, 10, 16, 17) have discussed, such naturalistic studies are vulnerable to methodological limitations, many of which might plausibly bias findings towards an overestimate of differences between ecstasy users and non-users. First, comparison non-users in many studies were not members of the “rave” subculture. Thus, unlike ecstasy users, they lacked repeated exposure to sleep and fluid deprivation from all-night dancing—factors that themselves can produce long-lasting cognitive effects (18). Second, few studies screened participants for MDMA, other illicit drugs, and alcohol on the day of testing—leaving open the possibility of surreptitious recent drug use. Third, ecstasy users in virtually all studies reported extensive lifetime use of other drugs, including cannabis, amphetamine, other hallucinogens, and cocaine—which might themselves contribute neurotoxicity. Studies have typically addressed this issue by statistically adjusting for other drug use or by matching groups for non-ecstasy drug use—but such methods are likely imperfect.
Fourth, cognitive difficulties in ecstasy users might be attributable to premorbid attributes rather than ecstasy exposure. For example, users might be less intelligent or more impulsive than non-users even before using ecstasy—possibilities that can be explored, but never eliminated, in cross-sectional studies.
To address these problems, we performed a 2004 pilot study (15) assessing cognitive function in 23 ecstasy users and 16 non-users, all reporting minimal exposure to other illicit drugs or alcohol and all reporting a history of all-night dancing. We tested all participants for alcohol and illicit drugs, including MDMA, at the time of testing, and excluded positive cases. We then compared cognitive test results in non-users vs. “moderate” users (reporting 22-50 lifetime episodes of use) vs. “heavy” users (60-450 episodes) while adjusting for numerous potentially confounding attributes, including age; gender; family-of-origin attributes; estimated verbal IQ; and Beck Depression Inventory (BDI) scores. Moderate users exhibited virtually no significant differences vs. non-users, but heavy users differed significantly from non-users on several measures, involving mental processing speed, strategic self-regulation, and executive functioning. These findings seemed unlikely to represent an artifact of the methodological limitations enumerated above, since each had been addressed in the study design. We than sought to replicate the findings in a similar larger investigation, reported here.
MATERIALS AND METHODS
Participants
Case finders in Salt Lake City, Utah advertised for study participants at raves and other sites frequented by the local all-night dance subculture. Potential participants were screened by telephone for lifetime use of ecstasy and other drugs, together with the other inclusion and exclusion criteria described below. The telephone-screening instrument intentionally included irrelevant questions (e.g., questions about tobacco and caffeine consumption) to reduce the chances that individuals might guess the study criteria and then misrepresent their histories simply to gain entrance into the study.
We recruited participants aged 18-45 years who reported 1) at least 17 lifetime episodes of ecstasy use or 2) no lifetime ecstasy use. These participants represented a fresh sample, not including any participants from the prior pilot study (15). Participants in both groups were required to be native speakers of English and to report experience in the rave culture, as demonstrated by having attended at least 10 all-night dance parties, defined as staying awake until at least 4:30 AM. We excluded participants reporting: 1) more than 100 lifetime episodes of using cannabis, or more than 10 episodes of using any other class of illicit drugs other than ecstasy (cocaine, stimulants, opioids, hallucinogens, sedative-hypnotics, gamma hydroxybutyrate, phencyclidine, ketamine, or hydrocarbon inhalants); 2) more than 50 lifetime episodes of alcohol intoxication, defined as consuming, at least 4 drinks (defined as 12 ounces of beer, 4 ounces of wine, or 1.5 ounces of distilled spirits) within a 4-hour period; 3) history of head injury with loss of consciousness judged clinically significant, or history of other medical illnesses that might affect cognitive function; or 4) current use of psychoactive medications, such as antidepressants or benzodiazepines. Our criteria for maximum lifetime episodes of alcohol and illicit drug use were chosen, based on practical experience from our pilot study (15), to exclude non-ecstasy drug use as much as possible without being so strict that we would excessivelyreduce the participant pool. Note that participants reporting psychiatric disorders were not excluded, since some psychiatric syndromes might plausibly be caused by ecstasy use, and exclusion of such cases might bias the sample of ecstasy users. Individuals rejected on telephone screen were not told the reasons for rejection, to minimize the possibility that others might deduce the study criteria and then misrepresent their histories to gain entry to the study.
Baseline Evaluation
Individuals qualifying on telephone screen were scheduled for an in-person baseline evaluation in Salt Lake City by a study psychiatrist. Upon arriving for this evaluation, participants were first requested to sign informed consent for the study, which was approved by the McLean Hospital Institutional Review Board. We then administered instruments similar to those used in our pilot study (15), including demographic questions; a semi-structured interview assessing lifetime episodes of use of alcohol and other drug use, plus a detailed history of episodes, doses, and settings of lifetime ecstasy use; lifetime history of psychiatric disorders as determined by the Structured Clinical Interview for DSM-IV (SCID) (19); the 21-item Hamilton Rating Scale for depression (20); the Hamilton Rating Scale for Anxiety (21); and the Symptom Checklist-90 (22). The baseline evaluation also covered other attributes potentially associated with premorbid cognitive function, including 1) history of childhood conduct disorder, assessed using questions covering the 15 DSM-IV criterion items for conduct disorder (23); 2) childhood attention deficit hyperactivity disorder (ADHD), assessed via the Wender Utah Rating Scale (24) and a modified ADHD rating scale (25); and 3) family history of substance abuse or other psychiatric disorders, assessed as in our previous studies of cannabis users (26). These measures were not grounds for excluding participants, but were used as adjustment variables in the regression analyses (see below). The baseline evaluation also included a brief neurological examination to exclude marked neurological abnormalities.
Neuropsychological Testing Visit
Participants meeting all criteria at baseline were scheduled to return at a later date (usually within 4 weeks) for neuropsychological testing. All participants were required to abstain from ecstasy, other illicit drugs, or all-night parties for at least 10 days prior to testing. The 10-day minimum criterion was based on practical experience from our pilot study (15).
Upon arriving for testing, all participants were administered a breathalyzer test for alcohol (Alco-Sensor IV, Intoximeters, Inc., St. Louis, MO) and provided a urine sample for an immediate dipstick test for tetrahydrocannabinol, opioids, cocaine metabolites, barbiturates, amphetamines, benzodiazepines, and phencyclidine (Triage Drugs of Abuse Panel, Biosite, San Diego, CA). Participants failing these tests were excluded.
A second aliquot of urine was preserved to be sent to an outside laboratory (Quest Diagnostics, Teterboro, NJ) to test for MDMA. In addition, we obtained a hair sample from the participant’s head (or lacking adequate head hair, from the axilla) to be analyzed by Psychemedics Corporation (Culver City, CA) for drug residues, including MDMA, from the past 90 days. For 10 mg of hair, the sensitivity thresholds were: cocaine, 5 ng; opiates, 2 ng; phencyclidine, 3 ng; amphetamines 5 ng; and marijuana, 0.01 ng. Specificity of hair analyses was very high, with false-positive readings expected in less than 0.1% of cases (Schaffer, M, personal communication, September, 2010). If a participant’s urine returned positive for MDMA, or hair returned positive for any drug that the participant had denied, that participant’s results were discarded from analysis.
We then administered a battery of 15 neuropsychological tests: the subtests of 1) Vocabulary, 2) Digit Span, 3) Digit Symbol, and 4) Block Design from the Wechsler Adult Intelligence Scale, Revised (WAIS-R) (27); 5) the Rey-Osterreith Complex Figure Test (28); 6) the Wisconsin Card Sorting Test (29); 7) Trail Making Tests A and B from the Reitan Battery (30); 8) Raven’s Progressive Matrices (31); 9) the Benton Controlled Verbal Fluency Task (often called the “FAS” test) (32); 10) the Stroop Test (33); 11) the California Verbal Learning Test, Second Edition (34); 12) Logical Memory, Verbal Paired Associates, and Spatial Span from the Wechsler Memory Scale, Third Edition (35); 13) the Revised Strategy Applications Test (RSAT) (36); 14) a computerized version of the Iowa Gambling Task (37); and 15) the Grooved Pegboard (Purdue Pegboard) Test (38). Participants also completed the Beck Depression Inventory (BDI) (39) to assess current depressive symptoms.
Participants received $100 for completing the baseline evaluation, another $100 for completing the neuropsychological tests, and an additional $150 via mail upon confirmation of appropriate urinary MDMA and hair testing results.
Statistical Analyses
Using the same definitions as our pilot study (15), we divided the ecstasy users into “moderate” users reporting 17-50 lifetime episodes of using ecstasy and “heavy” users reporting more than 50 lifetime episodes. We then performed 2 analyses, the first comparing all ecstasy users with non-users, and the second comparing the subgroups of moderate users and heavy users with non-users. All comparisons used linear regression adjusting for age; gender; race/ethnicity; 4 family-of-origin variables (mother’s plus father’s level of education, parents’ income when the participant was growing up, family history of psychiatric disorder, and family history of substance use disorders, modeled as previously (15)); history of childhood conduct disorder (modeled as presence versus absence of the diagnosis by DSM-IV criteria); and childhood ADHD (modeled as a continuous variable representing the score on the modified ADHD rating scale, as in our previous studies (26)).We would note in passing that although we adjusted for this entire range of variables, not all differed significantly between groups (see Table 1 below).
Table 1.
Demographic Features of Non-Users vs. Users of Ecstasy
| Non-Users (N = 59) |
Users (N = 52) |
||||
|---|---|---|---|---|---|
| Demographic Feature | Number | % | Number | % | P Value a |
| Sex, male | 38 | 64.4 | 30 | 57.7 | 0.56 |
| Ethnicity, white | 54 | 91.5 | 40 | 76.9 | 0.04 |
| Father’s education, high school or less | 16 | 27.1 | 28 | 53.9 | 0.006 |
| Mother’s education, high school or less | 16 | 27.1 | 32 | 61.5 | < 0.001 |
| Parents’ household income ≤ $30,000 | 16 | 27.1 | 16 | 30.8 | 0.68 |
| Family history of subtance abuseb | 22 | 37.9 | 26 | 50.0 | 0.25 |
| Family history of psychiatric disorderb | 18 | 31.0 | 20 | 38.5 | 0.43 |
| Current major depressive disorder | 4 | 6.8 | 3 | 5.8 | 1.0 |
| Current anxiety disorderc | 9 | 15.3 | 5 | 9.6 | 0.41 |
|
|
|||||
| Median |
Interquartile
range |
Median |
Interquartile
Range |
||
|
|
|||||
| Age | 24 | 21, 27 | 22 | 19.3, 24.8 | 0.06 |
| Lifetime episodes of ecstasy use | 0 | 0 | 43.5 | 27, 87.3 | <0.001 |
| Days since last ecstasy use when tested | . | . | 121 | 60, 209 | . |
| Lifetime all-night “raves” | 45 | 25, 90 | 98 | 45, 200 | 0.005 |
| Lifetime alcohol intoxications | 10 | 0, 24.3 | 10 | 2.3, 27.5 | 0.31 |
| Lifetime marijuana intoxications | 1 | 0, 6 | 10 | 4, 30 | <0.001 |
| Cigarattes smoked per day | 0 | 0, 0 | 0 | 0, 0.3 | 0.04 |
| Hamilton Depression Scale scored | 2 | 0, 6 | 4 | 0.25, 8 | 0.15 |
| Hamilton Anxiety Scale scored | 2 | 0, 5 | 3 | 0, 6 | 0.24 |
| Beck Depression Inventory scored | 2.5 | 1, 7 | 1 | 0, 5 | 0.10 |
| Wender Utah Rating Scale score | 12 | 8, 24 | 13 | 5, 25 | 0.72 |
| ADHD Rating Scale score (see text) | 7 | 3, 13 | 9 | 2, 15 | 0.50 |
Two-tailed significance of difference between groups by Fisher’s exact test for proportions and the Wilcoxon rank sum test for continuous variables.
At least one first-degree relative reported to display symptoms judged likely to meet DSM-IV criteria for a substance abuse disorder or another Axis I disorder, respectively; note that N = 58 for non-users because one non-user was raised in foster environment.
Panic disorder, agoraphobia, social phobia, simple phobia, obsessive compulsive disorder, or posttraumatic stress disorder
Note that the two Hamilton scales were administered at screen; the Beck Depression Inventory was administered at the time of neuropsychological testing.
Finally, in our comparisons of the heavy users versus non-users, we calculated the maximum effect sizes that we could exclude at the 0.05 level of significance, using a test for non-equivalence, based on the 90% confidence intervals of our measured effect sizes (40, 41). This test generates a measure of effect size such that there is less than 5% probability that the true difference between groups exceeds this magnitude.
We fitted all models using Stata 9.2 software, with alpha set at 0.05, 2-tailed. Although our multiple comparisons increased the likelihood of type I errors, there was no good way to correct for this, since methods such as Bonferroni correction are too conservative and inflate type II error rates (42). Hence, following the advice of some prior authorities (43, 44), we present results without correction, but caution readers to consider this issue when interpreting the findings.
RESULTS
Of about 1500 potential participants screened by telephone, only about 250 qualified for the baseline evaluation, of whom only 116 met all criteria and completed neuropsychological testing. Of these, 5 were rejected for drugs subsequently found in hair or urine analyses, leaving 111 evaluable participants, comprising 52 ecstasy users and 59 non-users. Given the difficulty in recruiting fully qualifying participants, we slightly relaxed our criteria for 6 individuals near the end of the project: 2 reported lifetime cocaine use 15 and 20 times, respectively; 3 reported use of other hallucinogens 11, 14, and 22 times; and one reported cannabis 250 times.
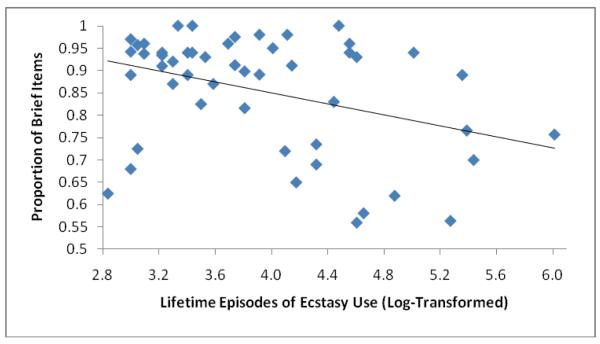
The 52 ecstasy users and 59 non-users appeared similar on many measures, but users were more frequently non-white, reported lower levels of parental education, and showed lower vocabulary scores (Table 1). Among ecstasy users, the shortest time from last ecstasy use to cognitive testing was 25 days, with only 3 (6%) participants below 40 days. The subgroups of 22 heavy and 30 moderate ecstasy users showed no significant differences (p > 0.05) on any variable in Table 1 save for lifetime episodes of ecstasy use (by definition), lifetime raves (median [interquartile range]: 150 [70, 238] versus 56 [35, 107]; P = 0.004), and Hamilton Depression Scale scores (6.5 [2.5, 10.25] versus 2 [0, 6]; P = 0.02). Comparing the overall group of users with non-users on the entire range of neuropsychological tests, we found few differences reaching statistical significance (Table 2). Comparing “moderate” and “heavy” user subgroups with non-users, we again failed to find significant differences on most outcome variables (Table 3). Performance on the Raven’s Progressive Matrices, WAIS-R Digit-Symbol Subtest, and WCST total categories was significantly reduced only among moderate users, but not heavy users. Heavy users were significantly slower than non-users when using the non-dominant hand on the grooved pegboard, but we found no comparable differences with the dominant hand. Perhaps most notably, the proportion of “brief” items on the RSAT, which represents the primary outcome variable on this test (36), was strikingly and significantly lower in heavy users--and this difference remained virtually unchanged when we adjusted further for verbal IQ and current BDI scores. Also, within the overall group of ecstasy users, the proportion of brief items was significantly associated with lifetime episodes of use (coefficient [95% confidence interval]: −1.4 [−2.3, −0.4]; P = 0.004 using log-transformed values for lifetime ecstasy episodes, and with adjustment for age, gender, and race/ethnicity). Inspection of a scatter plot (Figure 1) indicated that this association was not driven by outliers. We also repeated all of the comparisons in Tables 2 and 3, first using a simplified model adjusting only for age, gender, and race/ethnicity; and second with exclusion of the 6 individuals who slightly exceeded our criteria for other drug use. Both exercises yielded differences and significance levels very similar to those of the primary analysis.
Table 2.
Representative Neuropsychological Test Scores in Non-users vs. Users of Ecstasy
| Mean Scores (SD) |
Comparison Between Groups |
||||
|---|---|---|---|---|---|
| Measure | Non-Users (N=59) |
Users (N = 52) |
Estimated Mean Differencea |
95% Confidence Interval |
P Valueb |
| Wechsler Memory Scale - III: | |||||
| Logical memory - immediate recall | 49.2 (9.7) | 46.6 (11.6) | 0.7 | −3.6, 5.0 | 0.75 |
| Logical memory - delayed recall | 31.1 (7.2) | 29.4 (8.0) | 0.7 | −2.4, 3.8 | 0.65 |
| Verbal paired associations- immediate | 22.4 (7.8) | 21.6 (8.7) | 1.3 | −2.0, 4.6 | 0.44 |
| Verbal paired associations- delayed | 6.8 (1.8) | 6.8 (1.8) | 0.1 | −0.7, 0.8 | 0.90 |
| Spatial span - forward | 9.7 (1.8) | 9.0 (1.9) | 0.8 | 0.0, 1.6 | 0.04 |
| Spatial span - backward | 9.2 (1.6) | 8.8 (1.7) | 0.4 | −0.3, 1.2 | 0.22 |
| Spatial span - total | 18.9 (2.9) | 17.7 (3.2) | 1.3 | 0.0, 2.6 | 0.06 |
| Stroop test: | |||||
| Color reading time (sec) | 57.1 (10.0) | 56.3 (8.7) | −0.1 | −4.2, 3.9 | 0.94 |
| Color reading errors | 0.9 (1.1) | 1.0 (1.0) | −0.1 | −0.5, 0.4 | 0.79 |
| Word reading time (sec) | 45.0 (7.4) | 44.1 (7.2) | 0.5 | −2.6, 3.5 | 0.77 |
| Word reading errors | 0.9 (1.2) | 0.6 (0.8) | 0.2 | −0.3, 0.6 | 0.43 |
| Interference time (sec) | 103.5 (18.8) | 100.1 (16.5) | 2.7 | −4.9, 10.3 | 0.48 |
| Interference time errors | 2.3 (2.4) | 3.4 (3.5) | −0.7 | −1.9, 0.6 | 0.28 |
| Ravens Progressive Matrices- Total Score | 52.7 (5.0) | 49.8 (5.9) | 2.2 | 0.0, 4.3 | 0.05 |
| Trails: | |||||
| Trails A time (sec) | 26.0 (9.5) | 24.0 (5.6) | 2.3 | −1.1, 5.7 | 0.18 |
| Trails A errors | 0.1 (0.3) | 0.1 (0.3) | 0.0 | −0.1, 0.2 | 0.71 |
| Trails B time (sec) | 55.4 (15.5) | 56.0 (15.5) | −1.2 | −7.6, 5.1 | 0.70 |
| Trails B errors | 0.3 (0.6) | 0.3 (0.6) | 0.0 | −0.3, 0.2 | 0.87 |
| Rey-Osterreith Figure: | |||||
| Copy (number of elements) | 32.9 (2.8) | 33.2 (2.4) | −0.4 | −1.5, 0.7 | 0.44 |
| Immediate recall (number of elements) | 21.8 (6.4) | 21.3 (6.4) | 0.6 | −2.1, 3.3 | 0.66 |
| Delayed recall (number of elements) | 21.5 (6.0) | 20.7 (6.4) | 0.8 | −1.8, 3.4 | 0.55 |
| FAS Test: | |||||
| Total words | 42.1 (12.0) | 38.1 (9.4) | 2.9 | −1.5, 7.3 | 0.19 |
| Total perseverations | 0.6 (0.9) | 0.4 (0.6) | 0.2 | −0.1, 0.5 | 0.28 |
| Revised Strategy Applications Test: | |||||
| Proportion of Brief items in Total2 (%) | 88.5 (8.8) | 85.6 (12.9) | 2.2 | −2.3, 6.7 | 0.35 |
| Number of Action Slipsc,d | 0.4 (0.6) | 0.8 (1.1) | −0.1 | −0.4, 0.3 | 0.62 |
| California Verbal Learning Test: | |||||
| Level of recall - trial 1 | 8.1 (2.3) | 8.2 (2.8) | −0.3 | −1.3, 0.8 | 0.60 |
| Level of recall - trial 5 | 14.4 (1.5) | 14.1 (1.9) | 0.0 | −0.7, 0.6 | 0.91 |
| Level of recall - trials 1-5 total | 61.6 (8.3) | 60.6 (10.3) | −0.5 | −4.2, 3.2 | 0.79 |
| Level of recall - trial B | 8.1 (2.8) | 7.9 (2.6) | 0.2 | −0.9, 1.2 | 0.78 |
| Wechsler Adult Intelligence Scale-Revised: | |||||
| Vocabulary | 52.9 (8.4) | 45.8 (11.2) | 4.7 | 1.1, 8.4 | 0.01 |
| Block design | 39.8 (8.8) | 36.9 (7.9) | 2.3 | −1.3, 5.8 | 0.21 |
| Digit-symbol | 72.7 (14.6) | 64.5 (13.6) | 7.1 | 1.0, 13.2 | 0.02 |
| Digits forwards | 9.4 (2.1) | 9.3 (2.6) | 0.2 | −0.9, 1.2 | 0.77 |
| Digits backwards | 8.0 (2.3) | 7.2 (2.0) | 0.9 | 0.0, 1.9 | 0.05 |
| Verbal IQ | 108.4 (16.5) | 99.3 (13.3) | 6.0 | −0.2, 12.2 | 0.06 |
| Grooved pegboard: | |||||
| Dominant hand (time in seconds) | 63.9 (7.1) | 65.2 (8.6) | −1.6 | −4.8, 1.6 | 0.33 |
| Non-dominant hand (seconds) | 67.2 (7.6) | 72.4 (12.4) | −5.0 | −9.3, −0.7 | 0.02 |
| Iowa Gambling Taske | 28.4 (32.0) | 19.5 (32.0) | 8.1 | −5.7, 22.0 | 0.25 |
| Wisconsin Card Sorting Test: | |||||
| Total categories (decks 1 + 2) | 8.9 (1.5) | 8.4 (1.8) | 0.5 | −0.2, 1.2 | 0.16 |
| Total perseverations (decks 1 + 2)d | 2.8 (0.9) | 3.0 (1.2) | −0.2 | −0.6, 0.2 | 0.44 |
Represents nonusers minus users.
By linear regression with adjustment for age, gender, race/ethnicity, parents’ level of education, parents’ income, family history of substance abuse or psychiatric disorder, childhood attention deficit disorder, and childhood conduct disorder (see text).
Number of proscribed items performed (large items or items on pages with faces)
Square-root transformed values due to skewed distribution.
Score represents the number of cards chosen from safe decks, minus the number from risky decks, in 100 successive cards.
Table 3.
Representative Neuropsychological Test Scores in Non-users vs. Moderate Users vs. Heavy Users of Ecstasy
| Mean Scores (SD) |
Non-users vs Moderate Users |
Non-users vs Heavy Users |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Users (N=59) |
Moderate Users (N = 30) |
Heavy Users (N = 22) |
Estimated Mean Differencea |
95% Confidence Interval |
P Valueb | Estimated Mean Differencea |
95% Confidence Interval |
P Valueb | |
| Wechsler Memory Scale - III: | |||||||||
| Logical memory - immediate recall | 49.2 (9.7) | 45.9 (11.2) | 47.6 (12.4) | 2.1 | −2.8, 7.1 | 0.39 | 1.6 | −4.1, 7.2 | 0.58 |
| Logical memory - delayed recall | 31.1 (7.2) | 29.4 (7.9) | 29.8 (8.2) | 1.3 −2.2, | 4.8 | 0.46 | −0.2 | −4.3, 3.8 | 0.92 |
| Verbal paired associations - immediate | 22.4 (7.8) | 22.2 (9.5) | 20.7 (7.7) | 1.0 | −2.8, 4.8 | 0.60 | 1.8 | −2.6, 6.1 | 0.43 |
| Verbal paired associations - delayed | 6.8 (1.8) | 6.8 (2.2) | 7.0 (1.3) | 0.2 | −0.6, 1.1 | 0.62 | −0.2 | −1.2, 0.8 | 0.67 |
| Spatial span - forward | 9.7 (1.8) | 8.9 (2.2) | 9.1 (1.6) | 0.9 | 0.0, 1.8 | 0.04 | 0.7 | −0.3, 1.7 | 0.18 |
| Spatial span - backward | 9.2 (1.6) | 8.9 (1.2) | 8.5 (2.2) | 0.2 | −0.6, 1.0 | 0.61 | 0.8 | −0.1, 1.7 | 0.09 |
| Spatial span - total | 18.9 (2.9) | 17.8 (3.0) | 17.6 (3.5) | 1.1 | −0.4, 2.6 | 0.14 | 1.5 | −0.2, 3.2 | 0.09 |
| Stroop Test: | |||||||||
| Color reading time (sec) | 57.1 (10.0) | 55.8 (9.5) | 57.0 (7.5) | 0.4 | −4.2, 5.0 | 0.87 | −1.0 | −6.2, 4.3 | 0.72 |
| Color reading errors | 0.9 (1.1) | 0.9 (0.9) | 1.2 (1.1) | 0.0 | −0.5, 0.5 | 1.0 | −0.2 | −0.7, 0.4 | 0.61 |
| Word reading time (sec) | 45.0 (7.4) | 43.9 (7.5) | 44.6 (6.8) | 0.7 | −2.9, 4.2 | 0.71 | 0.2 | −3.9, 4.2 | 0.94 |
| Word reading errors | 0.9 (1.2) | 0.5 (0.7) | 0.9 (0.9) | 0.3 | −0.2, 0.8 | 0.18 | −0.1 | −0.6, 0.5 | 0.80 |
| Interference time (sec) | 103.5 (18.8) | 97.2 (15.8) | 104.1 (16.8) | 5.6 | −3.0, 14.2 | 0.20 | −1.8 | −11.6, 8.1 | 0.72 |
| Interference reading errors | 2.3 (2.4) | 3.2 (3.4) | 3.6 (3.7) | −0.5 | −1.9, 1.0 | 0.51 | −1.0 | −2.7, 0.6 | 0.22 |
| Ravens Progressive Matrices- Total Score | 52.7 (5.0) | 49.1 (5.5) | 50.8 (6.5) | 3.0 | 0.5, 5.4 | 0.02 | 1.0 | −1.8, 3.8 | 0.47 |
| Trails: | |||||||||
| Trails A time (sec) | 26.0 (9.5) | 23.4 (5.7) | 24.5 (5.5) | 2.8 | −1.1, 6.7 | 0.16 | 1.5 | −2.9, 6.0 | 0.50 |
| Trails A errors | 0.1 (0.3) | 0.1 (0.3) | 0.1 (0.4) | 0.0 | −0.1, 0.2 | 0.65 | 0.0 | −0.2, 0.2 | 0.91 |
| Trails B time (sec) | 55.4 (15.5) | 54.4 (15.8) | 58.1 (15.3) | 0.6 | −6.7, 7.8 | 0.88 | −4.0 | −12.3, 4.3 | 0.34 |
| Trails B errors | 0.3 (0.6) | 0.4 (0.7) | 0.2 (0.4) | −0.1 | −0.4, 0.2 | 0.53 | 0.1 | −0.2, 0.4 | 0.59 |
| Rey-Osterreith Figure: | |||||||||
| Copy (number of elements) | 32.9 (2.8) | 33.2 (2.4) | 33.2 (2.4) | −0.5 | −1.7, 0.8 | 0.46 | −0.4 | −1.7, 1.0 | 0.62 |
| Immediate recall (number of elements) | 21.8 (6.4) | 21.0 (5.9) | 21.7 (7.2) | 0.9 | −2.2, 4.0 | 0.46 | 0.2 | −3.4, 3.7 | 0.93 |
| Delayed recall (number of elements) | 21.5 (6.0) | 20.4 (6.3) | 21.3 (6.7) | 1.7 | −1.9, 4.2 | 0.46 | 0.2 | −3.2, 3.7 | 0.90 |
| FAS Test: | |||||||||
| Total words | 42.1 (12.0) | 37.2 (8.3) | 39.4 (10.8) | 3.3 | −1.7, 8.4 | 0.19 | 2.2 | −3.6, 8.0 | 0.45 |
| Total perseverations | 0.6 (0.9) | 0.3 (0.5) | 0.5 (0.6) | 0.2 | −0.2, 0.6 | 0.28 | 0.1 | −0.3, 0.6 | 0.51 |
| Revised Strategy Applications Test: | |||||||||
| Proportion of Brief items in Total2 (%) | 88.5 (8.7) | 90.0 (9.0) | 79.5 (15.0) | −2.1 | −7.0, 2.8 | 0.40 | 8.7 | 3.1, 14.4 | 0.003 |
| Number of Action Slipsc,d | 0.4 (0.6) | 0.8 (1.0) | 0.8 (1.2) | −0.1 | −0.5, 0.3 | 0.61 | −0.1 | −0.5, 0.4 | 0.79 |
| California Verbal Learning Test: | |||||||||
| Level of recall - trial 1 | 8.1 (2.3) | 8.0 (3.1) | 8.3 (2.3) | 0.1 | −1.2, 1.1 | 0.93 | −0.6 | −2.0, 0.7 | 0.37 |
| Level of recall - trial 5 | 14.4 (1.5) | 14.2 (1.8) | 14.0 (2.0) | 0.0 | −0.8, 0.8 | 0.98 | −0.1 | −1.0, 0.8 | 0.85 |
| Level of recall - trials 1-5 total | 61.6 (8.3) | 60.6 (10.5) | 60.5 (10.2) | 0.0 | −4.2, 4.2 | 0.99 | −1.3 | −6.1, 3.6 | 0.60 |
| Level of recall - trial B | 8.1 (2.8) | 8.2 (2.8) | 7.4 (2.3) | −0.1 | −1.3, 1.2 | 0.89 | 0.5 | −0.9, 2.0 | 0.47 |
| Wechsler Adult Intelligence Scale-Revised: | |||||||||
| Vocabulary | 52.9 (8.4) | 45.9 (11.4) | 45.5 (11.1) | 4.6 | 0.4, 8.8 | 0.03 | 5.0 | 0.2, 9.8 | 0.04 |
| Block design | 39.8 (8.8) | 37.1 (7.8) | 36.6 (8.3) | 1.9 | −2.1, 6.0 | 0.34 | 2.8 | −1.9, 7.4 | 0.24 |
| Digit-symbol | 72.7 (14.6) | 63.3 (12.2) | 66.2 (15.4) | 8.4 | 1.4, 15.3 | 0.02 | 5.2 | −2.8, 13.1 | 0.20 |
| Digits forwards | 9.4 (2.1) | 9.5 (2.7) | 9.0 (2.6) | 0.0 | −1.2, 1.2 | 0.99 | 0.4 | −1.0, 1.7 | 0.58 |
| Digits backwards | 8.0 (2.3) | 7.2 (2.2) | 7.3 (1.8) | 1.0 | −0.1, 2.0 | 0.07 | 0.9 | −0.3, 2.1 | 0.15 |
| Grooved Pegboard: | |||||||||
| Dominant hand (time in seconds) | 63.9 (7.1) | 64.3 (7.9) | 66.4 (9.5) | −0.8 | −4.5, 2.9 | 0.66 | −2.8 | −7.0, 1.5 | 0.20 |
| Non-dominant hand (seconds) | 67.2 (7.6) | 70.0 (12.1) | 75.6 (12.4) | −2.8 | −7.7, 2.0 | 0.25 | −8.4 | −13.9, −2.8 | 0.003 |
| Iowa Gambling Taske | 28.4 (32.0) | 18.0 (31.1) | 21.7 (31.8) | 10.2 | −5.9, 26.2 | 0.21 | 5.2 | −12.9, 23.3 | 0.57 |
| Wisconsin Card Sorting Test: | |||||||||
| Total categories (decks 1 + 2) | 8.9 (1.5) | 8.1 (2.0) | 8.9 (1.3) | 0.9 | 0.1, 1.7 | 0.03 | 0.0 | −0.9, 0.8 | 0.95 |
| Total perseverations (decks 1 + 2)d | 2.8 (0.89) | 3.2 (1.2) | 2.8 (1.2) | −0.3 | −0.9, 0.2 | 0.24 | 0.0 | −0.6, 0.6 | 0.98 |
|
| |||||||||
Represents nonusers minus users.
By linear regression with adjustment for age, gender, race/ethnicity, parents’ level of education, parents’ income, family history of substance abuse or psychiatric disorder, childhood attention deficit disorder, and childhood conduct disorder (see text).
Number of proscribed items performed (large items or items on pages with faces)
Square-root transformed values due to skewed distribution.
Score represents the number of cards chosen from safe decks, minus the number from risky decks, in 100 successive cards.
Figure 1.
Scatterplot showing the association of log-transformed lifetime episodes of ecstasy use with the proportion of Brief items chosen on the Revised Strategy Applications Test. Pearson’s r = −0.37.
Finally, looking at cognitive tests where we failed to show significant differences between heavy users and non-users, we assessed the magnitude of the differences between these groups that we could exclude at the 0.05 level of probability, as explained above. We found that we could exclude even a medium effect (Cohen’s d ≥ 0.5) on many cognitive measures and could exclude a large effect (Cohen’s d ≥ 0.8) on all of the measures selected (Table 4).
Table 4.
Maximum Effect Size of Ecstasy on Various Cognitive Measures in Heavy Users versus Non-users
| Measurea | Maximum Effect Sizeb |
|---|---|
| Wechsler Memory Scale - III: | |
| Logical memory - immediate recall | 0.49 |
| Verbal paired associations- immediate recall | 0.55 |
| Spatial span - total | 0.76 |
| Stroop Test, interference time | 0.48 |
| Ravens Progressive Matrices- Total Score | 0.53 |
| Trails B time | 0.58 |
| Rey-Osterreith Test, delayed recall | 0.40 |
| Controlled Verbal Fluency Task, total words | 0.54 |
| California Verbal Learning Test, Trials 1-5 total | 0.49 |
| Wechsler Adult Intelligence Scale, Block Design Subtest | 0.64 |
| Wechsler Adult Intelligence Scale, Digit-Symbol Subtest | 0.66 |
| Wechsler Adult Intelligence Scale, Digits Backwards | 0.69 |
| Iowa Gambling Task | 0.50 |
| Wisconsin Card Sorting Test, total perseverations | 0.37 |
Chosen measures represented either the primary measure of a given test, or for tests involving multiple measures, the measures showing the largest effect sizes in the comparison of nonusers and heavy users (see Table 3).
Magnitude of effect (Cohen’s d ) that can be rejected at the 0.05 level of significance.
DISCUSSION
We assessed neurocognitive performance in 52 ecstasy users and 59 closely matched non-users in a study designed to minimize potentially confounding variables. Specifically, we chose participants reporting minimal use of drugs other than ecstasy, and we excluded all participants showing undisclosed alcohol or drug use on breath, urine, and hair analyses. We also required that participants in both groups be members of the all-night-dancing subculture. Finally, we adjusted for numerous potentially confounding variables. Using this rigorous approach, we found few consistent differences between ecstasy users and non-users on wide-ranging measures of verbal and visuospatial memory, verbal fluency, attention, processing speed, manipulative dexterity, and executive cortical functions. Ecstasy users exhibited lower vocabulary scores than non-users, but this finding likely indicates differences in premorbid ability rather than neurotoxicity of ecstasy since vocabulary is generally preserved even after neurological insults (26, 45, 46). Indeed, assuming that these differences in premorbid verbal ability are valid, the absence of significant differences on most other tests, including tests of verbal memory, becomes even more striking. Although we found a few other significant differences between the overall groups of users and non-users, these differences proved to be concentrated primarily in moderate users, rather than heavy users—suggesting that they were unlikely due to neurotoxicity of ecstasy. More likely, such differences represent chance associations—a phenomenon to be fully expected, given that we performed multiple comparisons without formal statistical correction. Exploratory analyses suggest that even the more robust difference on the grooved pegboard with the non-dominant hand in heavy users (Table 3) was likely due to chance. Overall, differences between non-users and heavy users were sufficiently modest on most cognitive measures that we could exclude a large effect of ecstasy (d ≥ 0.8) at the 0.05 level.
Our only consistently significant finding was that heavy users exhibited a lower proportion of “brief” items on the RSAT, suggesting poorer strategic self-regulation and hence perhaps greater reflection impulsivity (i.e., insufficient information-gathering before launching into the task). Notably, many prior studies have suggested associations between ecstasy use and increased impulsivity (13)—but it must be cautioned that these observed associations are complex and inconsistent (11, 13, 47, 48), perhaps in part because impulsivity is multifactorial (49, 50). Indeed, one recent study paradoxically found reflection impulsivity reduced in ecstasy users (51). Furthermore, neither our study nor other cross-sectional studies establishes that greater impulsivity is necessarily caused by ecstasy. Some prospective data suggest that impulsivity may be an effect, rather than a cause, of ecstasy use (52), whereas other studies (53, 54) favor the hypothesis that, impulsivity is a risk factor for substance abuse (for review, see (50)).
Our largely negative findings appear inconsistent with many past studies as well as some more recent investigations (55-57) that report lowered cognitive functions in ecstasy users. Indeed, our findings are inconsistent with several findings in our own pilot study (15)—possibly because heavy users in the earlier study were tested after briefer median abstinence (59.5 vs. 121 days), possibly because of differences in unmeasured confounders, or possibly because of chance alone. Conversely, our present findings appear congruent with several other recent studies suggesting that cognitive effects of ecstasy use are modest (16, 58) and perhaps mediated or confounded by trait impulsiveness (47), comorbid substance use (48), and sleep deprivation (48, 59)—although this last possibility remains uncertain (60).
Recent longitudinal studies of ecstasy users have also produced somewhat inconsistent findings. For example one study analyzed 118 individuals, all ecstasy-naïve at baseline, of whom 58 subsequently used ecstasy and 60 remained ecstasy-naïve (61). The groups showed no differences on any cognitive measures at baseline, but at follow-up, ecstasy initiators displayed significantly lower scores than still-naïve individuals on verbal memory, though not other cognitive tests. However, ecstasy initiators had consumed a median of only 1.5 lifetime tablets at follow-up—raising possible doubt about the causal role of ecstasy. By contrast, another group (62) examined memory performance in 38 ecstasy users longitudinally over 18 months. Those who stopped ecstasy following baseline examination (n=17) did not improve, and those who continued ecstasy (n=21) did not deteriorate in performance—thus questioning a causal connection between cumulative ecstasy exposure and cognitive effects.
Recent reviews of cognitive performance in ecstasy users have acknowledged these inconsistencies. One meta-analysis of 26 studies found a substantial association between ecstasy use and lowered verbal memory but noted that the lifetime number of ecstasy tablets consumed did not predict memory performance (63). Another recent meta-analysis concluded that ecstasy was associated with lowered cognitive performance but found only small to medium effect sizes (12). Other recent reviews have concluded that ecstasy-associated cognitive effects are likely modest or subtle (11, 13) and have noted that confounding effects of premorbid traits and other illict drug use cannot be excluded (11). Another recent review, enumerating many of the methodological concerns raised in our own discussion above, emphasizes the hazards of concluding that ecstasy plays a causal role in observed cognitive findings and speculates that the matter will likely remain controversial (3).
In short, our findings combine with many of the above reports to dictate continued caution in ascribing neuropsychological deficits to ecstasy exposure. On the one hand, it is possible that ecstasy indeed causes residual cognitive neurotoxicity, albeit perhaps only in individuals with high-level exposure (56), or with possible co-factors predisposing them to ecstasy-induced neurotoxicity (47). If so, we might have failed to detect a difference when a true difference exists, perhaps because we evaluated only 6 participants with very high ecstasy exposure ( ≥ 150 lifetime episodes) or perhaps because our population included few individuals with vulnerability-inducing co-factors.
On the other hand, our findings might not represent false-negative results, but might instead correctly reflect that illicit ecstasy use, by itself, does not generally produce lasting residual neurotoxicity. In support of this possibility, it should be noted that we took unusual care to minimize common methodological factors that might possibly bias results away from the null, as discussed above. Therefore it is plausible that the positive results in some prior studies were attributable to these confounding factors, and that our present negative results are valid and reflect lower levels of confounding. Whatever the case, our findings indicate that the neurotoxicity of human ecstasy use remains incompletely resolved.
Acknowledgments
The authors wish to thank Ben Stokes, Ph.D. for his important early role in making us aware of this unique population for our study and for his work as a study case finder. We also are grateful to Lynisa Lava (case finder) and Rebecca England, Ph.D. (neuropsychological testing) and to Drs. Daniel and Noémi Mattis (housing and additional logistics).
This work was supported by United States National Institute on Drug Abuse grant DA017953.
Footnotes
None of the authors reports conflicts of interest and none of the authors reports support from the tobacco, alcohol, pharmaceutical, or gaming industries in association with this study.
References
- 1.Tanner-Smith EE. Pharmacological content of tablets sold as “ecstasy”: results from an online testing service. Drug Alcohol Depend. 2006;83(3):247–54. doi: 10.1016/j.drugalcdep.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 2.United States Substance Abuse and Mental Health Services Administration . Results from the 2008 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, Maryland: 2009. (NSDUH Series H-36). HHS Publication No. SMA 09-4434. [Google Scholar]
- 3.Lyvers M. Recreational ecstasy use and the neurotoxic potential of MDMA: current status of the controversy and methodological issues. Drug Alcohol Rev. 2006;25(3):269–76. doi: 10.1080/09595230600657758. [DOI] [PubMed] [Google Scholar]
- 4.Fang YX, Wang YB, Shi J, Liu ZM, Lu L. Recent trends in drug abuse in China. Acta Pharmacol Sin. 2006;27(2):140–4. doi: 10.1111/j.1745-7254.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 5.Schifano F, Corkery J, Naidoo V, Oyefeso A, Ghodse H. Overview of amphetamine-type stimulant mortality data--UK, 1997-2007. Neuropsychobiology. 2010;61(3):122–30. doi: 10.1159/000279302. [DOI] [PubMed] [Google Scholar]
- 6.Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39(3):210–71. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- 7.Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90(2):198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11(3-4):183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- 9.Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189(4):407–24. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole JC, Sumnall HR. Altered states: the clinical effects of Ecstasy. Pharmacol Ther. 2003;98(1):35–58. doi: 10.1016/s0163-7258(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 11.Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of methylenedioxyamphetamines (MDMA; ecstasy) in humans: how strong is the evidence for persistent brain damage? Addiction. 2006;101(3):348–61. doi: 10.1111/j.1360-0443.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 12.Kalechstein AD, De La Garza R, 2nd, Mahoney JJ, 3rd, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology (Berl) 2007;189(4):531–7. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- 13.Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, Zawada A, Somerville M. The harmful health effects of recreational ecstasy: a systematic review of observational evidence. Health Technol Assess. 2009;13(6):iii–iv. ix–xii, 1–315. doi: 10.3310/hta13050. [DOI] [PubMed] [Google Scholar]
- 14.Mithoefer MC, Wagner MT, Mithoefer AT, Jerome I, Doblin R. The safety and efficacy of {+/−}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. doi: 10.1177/0269881110378371. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpern JH, Pope HG, Jr., Sherwood AR, Barry S, Hudson JI, Yurgelun-Todd D. Residual neuropsychological effects of illicit 3,4-methylenedioxymethamphetamine (MDMA) in individuals with minimal exposure to other drugs. Drug Alcohol Depend. 2004;75(2):135–47. doi: 10.1016/j.drugalcdep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Bedi G, Redman J. Ecstasy use and higher-level cognitive functions: weak effects of ecstasy after control for potential confounds. Psychol Med. 2008;38(9):1319–30. doi: 10.1017/S0033291708002730. [DOI] [PubMed] [Google Scholar]
- 17.Gouzoulis-Mayfrank E, Daumann J. The confounding problem of polydrug use in recreational ecstasy/MDMA users: a brief overview. J Psychopharmacol. 2006;20(2):188–93. doi: 10.1177/0269881106059939. [DOI] [PubMed] [Google Scholar]
- 18.Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4(6):567–8. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 19.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders -- Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton M. A rating scale for anxiety. British journal of medical psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 22.Derogatis L. Symptom Checklist-90-R. Administration, Scoring, and Procedures Manual. National Computer Systems, Inc.; Minneapolis, Minnesota: 1994. [Google Scholar]
- 23.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. fourth edition (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 24.Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150(6):885–90. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- 25.DuPaul GJ. Parent and teacher ratings of ADHD symptoms: psychometric properties in a community-based sample. Journal of Clinical Child Psychology. 1991;20:245–253. [Google Scholar]
- 26.Pope HG, Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58(10):909–15. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. Psychological Corporation; Cleveland, Ohio: 1981. [Google Scholar]
- 28.Osterrieth R. Le test de copie d’une figure complexe. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- 29.Heaton R. Wisconsin Card Sorting Test Manual. Psychological Assessment Resources; Odessa, Florida: 1981. [Google Scholar]
- 30.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Second Edition Neuropsychology Press; Tucson, Arizona: 1993. [Google Scholar]
- 31.Burke H. Raven’s progressive matrices: more on norms, reliability, and validity. Journal of Clinical Psychology. 1985;41:231–235. [Google Scholar]
- 32.Lezak M. Neuropsychological assessment. third edition Oxford University Press; New York: 1995. [Google Scholar]
- 33.Stroop J. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- 34.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual. Version 1 The Psychological Corporation; San Antonio, Texas: 1987. [Google Scholar]
- 35.Wechsler D. Wechsler Memory Scale. Third Edition The Psychological Corporation; San Antonio, Texas: 1997. [Google Scholar]
- 36.Levine B, Dawson D, Boutet I, Schwartz ML, Stuss DT. Assessment of strategic self-regulation in traumatic brain injury: its relationship to injury severity and psychosocial outcome. Neuropsychology. 2000;14(4):491–500. doi: 10.1037//0894-4105.14.4.491. [DOI] [PubMed] [Google Scholar]
- 37.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1-3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 38.Tiffin J, Asher EJ. The Purdue Pegboard: norms and studies of reliability and validity. Journal of Applied Psychology. 1948;32:234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- 39.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 40.Berger RL, Hsu JC. Bioequivalence trials, intersection-union tests and equivalence confidence sets. Statistical Science. 1996;11(4):283–302. [Google Scholar]
- 41.Schuirmann DJ. A comparison of the 2 one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. Journal of Pharmacokinetics and Biopharmaceutics. 1987;15(6):657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 42.Rothman K, Greenland S. Modern Epidemiology. 2nd edition Lipppincott-Raven; Philadelphia: 1998. [Google Scholar]
- 43.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol. 1995;142(9):904–8. doi: 10.1093/oxfordjournals.aje.a117737. [DOI] [PubMed] [Google Scholar]
- 45.Pope HG., Jr. Cannabis, cognition, and residual confounding. Jama. 2002;287(9):1172–4. doi: 10.1001/jama.287.9.1172. [DOI] [PubMed] [Google Scholar]
- 46.Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69(3):303–10. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 47.Roiser JP, Rogers RD, Sahakian BJ. Neuropsychological function in ecstasy users: a study controlling for polydrug use. Psychopharmacology (Berl) 2007;189(4):505–16. doi: 10.1007/s00213-005-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pirona A, Morgan M. An investigation of the subacute effects of ecstasy on neuropsychological performance, sleep and mood in regular ecstasy users. J Psychopharmacol. 2009 doi: 10.1177/0269881109102780. [DOI] [PubMed] [Google Scholar]
- 49.Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60(5):515–22. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Clark L, Roiser JP, Robbins TW, Sahakian BJ. Disrupted ‘reflection’ impulsivity in cannabis users but not current or former ecstasy users. J Psychopharmacol. 2009;23(1):14–22. doi: 10.1177/0269881108089587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Win MM, Reneman L, Jager G, Vlieger EJ, Olabarriaga SD, Lavini C, Bisschops I, Majoie CB, Booij J, den Heeten GJ, van den Brink W. A prospective cohort study on sustained effects of low-dose ecstasy use on the brain in new ecstasy users. Neuropsychopharmacology. 2007;32(2):458–70. doi: 10.1038/sj.npp.1301225. [DOI] [PubMed] [Google Scholar]
- 53.Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468–75. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 54.Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, Glass JM, Adams K. Behavioral control and resiliency in the onset of alcohol and illicit drug use: a prospective study from preschool to adolescence. Child Dev. 2006;77(4):1016–33. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schilt T, Koeter MW, Smal JP, Gouwetor MN, van den Brink W, Schmand B. Long-term neuropsychological effects of ecstasy in middle-aged ecstasy/polydrug users. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1688-z. [DOI] [PubMed] [Google Scholar]
- 56.Schilt T, de Win MM, Jager G, Koeter MW, Ramsey NF, Schmand B, van den Brink W. Specific effects of ecstasy and other illicit drugs on cognition in poly-substance users. Psychol Med. 2008;38(9):1309–17. doi: 10.1017/S0033291707002140. [DOI] [PubMed] [Google Scholar]
- 57.Quednow BB, Jessen F, Kuhn KU, Maier W, Daum I, Wagner M. Memory deficits in abstinent MDMA (ecstasy) users: neuropsychological evidence of frontal dysfunction. J Psychopharmacol. 2006;20(3):373–84. doi: 10.1177/0269881106061200. [DOI] [PubMed] [Google Scholar]
- 58.Indlekofer F, Piechatzek M, Daamen M, Glasmacher C, Lieb R, Pfister H, Tucha O, Lange KW, Wittchen HU, Schutz CG. Reduced memory and attention performance in a population-based sample of young adults with a moderate lifetime use of cannabis, ecstasy and alcohol. J Psychopharmacol. 2009;23(5):495–509. doi: 10.1177/0269881108091076. [DOI] [PubMed] [Google Scholar]
- 59.McCann UD, Wilson MJ, Sgambati FP, Ricaurte GA. Sleep deprivation differentially impairs cognitive performance in abstinent methylenedioxymethamphetamine (“ecstasy”) users. J Neurosci. 2009;29(44):14050–6. doi: 10.1523/JNEUROSCI.4654-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montgomery C, Fisk JE, Wareing M, Murphy P. Self reported sleep quality and cognitive performance in ecstasy users. Hum Psychopharmacol. 2007;22(8):537–48. doi: 10.1002/hup.879. [DOI] [PubMed] [Google Scholar]
- 61.Schilt T, de Win MM, Koeter M, Jager G, Korf DJ, van den Brink W, Schmand B. Cognition in novice ecstasy users with minimal exposure to other drugs: a prospective cohort study. Arch Gen Psychiatry. 2007;64(6):728–36. doi: 10.1001/archpsyc.64.6.728. [DOI] [PubMed] [Google Scholar]
- 62.Gouzoulis-Mayfrank E, Fischermann T, Rezk M, Thimm B, Hensen G, Daumann J. Memory performance in polyvalent MDMA (ecstasy) users who continue or discontinue MDMA use. Drug Alcohol Depend. 2005;78(3):317–23. doi: 10.1016/j.drugalcdep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Laws KR, Kokkalis J. Ecstasy (MDMA) and memory function: a meta-analytic update. Hum Psychopharmacol. 2007;22(6):381–8. doi: 10.1002/hup.857. [DOI] [PubMed] [Google Scholar]