Abstract
Objective
To explore the interplay of maternal depressive symptoms on the infant limbic-hypothalamic-pituitary-axis (LHPA) and neurological development.
Design
Pregnant women were monitored for depressive symptoms using the Beck Depression Inventory (BDI) at 28, 32, and 37 weeks of gestation and at delivery. A mixture growth curve analysis divided the women into three risk groups: low/stable, intermediate, and high/increasing depression based on BDI scores. The infant neuroendocrine system was examined using cord blood for adrenocorticotrophic hormone (ACTH) and cortisol measurements. Two week old infants were examined using Neonatal Intensive Care Unit Neurobehavioral Scale (NNNS).
Results
Infants born to women of the high/increasing depression group had significant ACTH elevation at birth. On NNNS examination, these infants were more hypotonic and habituated to auditory and visual stimuli.
Conclusion
When compared to non-depressed women, maternal depressive symptoms, even in the absence of major depressive disorder, appeared to facilitate a different developmental pathway for the infant LHPA and early neurological development.
Keywords: Depression, Pregnancy, Infant Outcomes, Neuroendocrine System
1.0 Introduction
1.1 Women, especially those of childbearing age, are at a high risk for depression (O'Hara, Neunaber, & Zekoski, 1984), and up to 20% may experience depressive symptoms during pregnancy (Marcus, Flynn, Blow, & Barry, 2003). Likewise, studies of the prevalence of major depressive disorder (MDD) during the postpartum period suggest a 10–15% incidence in all deliveries, making it one of the most common complications of pregnancy.
1.2 Impact of depressive symptoms on the fetus and neonate
1.3 Considerable literature has been devoted to the impact of maternal depressive symptoms on the fetus and newborn. Neonates of women with mood symptoms may be more likely to be born prematurely (Jesse, Seaver, & Wallace, 2003) or of low birth weight (Hoffman & Hatch, 2000). Newborns of women meeting criteria for dysthymia, a more chronic form of depression, were found to have lower fetal weight, fetal length, femur length and abdominal circumference in studies performed prenatally. The newborns affected in this manner also had higher cortisol levels and lower dopamine/serotonin levels when compared to neonates born to non-depressed women and their hormonal/neurotransmitter profile was similar to that of their mothers prior to delivery (Field, Diego, & Hernandez-Reif, 2008).
1.4 Immediately following birth, infant neurobehavioral dysregulation has been studied using the Brazelton Neonatal Behavioral Assessment Scale (NBAS). In these studies, infants have shown more irritability and diminished activity (Abrams, Field, Scafidi, & Prodromidis, 1995; Als, Tronick, Lester, & Brazelton, 1977) and have had lower scores on the orientation cluster of the Brazelton scale (Lundy, Field, & Pickens, 1996). In a more recent study (Field, Diego, & Hernandez-Reif, 2006), infants born to women diagnosed with MDD or dysthymia during pregnancy and the immediate postpartum period, were found to perform less optimally on hand-to-mouth activity items and cuddliness. Moreover, they were found to be less attentive on the orientation items of the NBAS scale. Such studies underscore the possibility that prenatal depressive symptoms may impact both maternal and infant neuroregulation as well as infant performance on neurobehavioral measures. Collectively, the studies to date suggest that it is possible that in genetically vulnerable individuals, developmental events initiated by a specific in utero hormonal environment may act as early catalysts in a process that alters gene expression, neuroendocrine function and neuronal circuitry in the developing infant and young child. This process may set the stage for increased risk for behavioral disorders and subsequent psychopathology in children.
1.5 The limbic-hypothalamic-pituitary axis (LHPA) during pregnancy
1.6 The LHPA is the most investigated and pertinent neurobiological system that is clearly altered in individuals suffering from depression (Carroll, Curtis, & Mendels, 1976a; Carroll, Curtis, & Mendels, 1976b). The main purpose of this classic neuroendocrine system is to respond to stress and facilitate an alert state upon awaking via the rapid synthesis of cortisol from the adrenal cortex. Circadian variation and stressful stimuli increase the synthesis and release of corticotrophin releasing hormone (CRH) from the hypothalamus. The hypothalamus, in turn, acts on the anterior pituitary to release ACTH, which interacts with receptors in the adrenal cortex to release cortisol. In pregnancy, bioactive CRH with the same molecular structure as that which is synthesized in the hypothalamus is also secreted from the placenta. However, as the pregnancy progresses into the second and third trimester a binding protein (CRHbp) limits the bioavailability of CRH and the activation of a woman’s LHPA. Precocious elevation of CRH during the 2nd and early 3rd trimester of human pregnancy is associated with spontaneous preterm birth (Sandman, Wadhwa, Chicz-DeMet, Dunkel-Schetter, & Porto, 1997). CRH is also associated with the development of the nervous system in humans (Sandman, Wadhwa, Chicz-DeMet, Porto, & Garite, 1999). For the patients suffering from depression, the LHPA is in an ‘over drive” state (Heim & Nemeroff, 1999). The ‘over-activity’ is marked by: (1) increased basal levels of cortisol, especially at the nadir of the circadian rhythm (Carroll et al., 1976b; Krishnan et al., 1990), (2) evidence of increased central drive, i.e. high levels of central nervous system (CNS) CRH (Nemeroff et al., 1984), and (3) alterations in the inhibition of the LHPA axis (Young, Haskett, Murphy-Weinberg, Watson, & Akil, 1991).
1.7 Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS)
1.8 The Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS) is a standardized neurobehavioral examination developed by Lester and Tronick as a comprehensive assessment of the ‘at risk infant’ performed within the first month of life (Lester, Tronick, & Brazelton, 2004). The NNNS addresses neurological integrity, behavioral functioning, and general signs of stress in the infant. This stress scale is unique to the NNNS and is particularly well suited to assess infant neurobehavioral outcomes within clinical settings, including infants who may be at risk for withdrawal symptoms (Boukydis, Bigsby, & Lester, 2004). The NNNS was developed using the basic concepts and clinical evaluation strategies of the Neonatal Behavioral Assessment Scale (NBAS) (Brazelton, 1973). The stress scale added to the NNNS includes components of previous standardized neonatal examinations (Als, Lester, Tronick, & Brazelton, 1982; Amiel-Tison, 1968; Finnegan, 1986; Prechtl, van Eykern, & O'Brien, 1977).
2.0 Methods
2.1 Screening
Prior to initiating the study, all research procedures and surveys were reviewed and approved by the Institutional Review Board at the University of Michigan Medical School. Pregnant women (n=2466) between eight and twenty-eight gestational weeks were recruited over a period of three years from 2003–2006 from three obstetrics clinics in southeastern Michigan. The screened population represented the population in southeastern Michigan, and included both private and training clinic populations. Women were asked to complete a fifteen-minute initial screening survey that included two instruments: The Edinburgh Postpartum Depression Scale (EPDS) and a General Health Questionnaire. The EPDS is a widely used 10-item instrument used in standard clinical practice at many institutions to screen for perinatal depressive symptoms since it relies less on somatic complaints common in pregnancy (Cox, 1988). Scores of 10 or higher indicate depressive symptoms and were used as an initial clinical screening. The General Health Questionnaire (GHQ) asked about estimated date of delivery, the use or discontinuation of antidepressant medication, use of alcohol and tobacco during pregnancy, as well as demographic information (age, race/ethnicity), past medical illnesses and present prescribed and ‘over the counter’ medications that may interfere with the planned hormonal assessments.
2.2 Eligibility and Enrollment
2.3 Women (n=415) met the following eligibility criteria: over 20 years of age, fluency with the English language, no plans to move from the area within the two year study period, no adoption plan, no chronic medical conditions or use of medications that impact the LHPA (e.g. corticosteroids, sex hormones), no substance dependence, current substance abuse, eating disorder, or bipolar illness. Of the eligible women, 154 (37%) agreed to participate and consent was obtained. As with the screened population, the recruited women were representative of the population in southeast Michigan and their demographics are described in Table 1. There were no differences in either depression severity or demographics which differentiated the 154 women who agreed to participate vs. those women who declined participation. Following enrollment, The Structured Clinical Interview for DSM IV (SCID) (Mazure, Kincare, & Schaffer, 1995) was performed by SCID trained personnel who demonstrated reliability of scoring between 85 and 90%. The purpose was to identify current symptoms or past history of major depressive disorder, as well as past history of an anxiety disorder. The data from women with MDD are not reported in this sample, to emphasize that even depressive symptoms falling short of a clinical diagnosis of MDD have significant impact on infant outcomes. This is salient, as up to 18% of women have depressive symptoms, but far fewer meet criteria for MDD during pregnancy (Marcus et al., 2003), and thus this subsyndromal depression is likely the most common complication of pregnancy. Infants who were born with congenital anomalies, those born < 35 gestational weeks, or who had other substantial neonatal complications which would confound results of the neuroendocrine data (e.g., cerebral hemorrhage, sepsis, any surgical intervention) were later excluded from data analysis. Only women who were not treated with psychotropic medication during their pregnancy were retained in the analyses to rule out the confounding effect of pharmacotherapy, and 14 women with depressive symptoms on medication were excluded for this reason. Patient report regarding utilization of service, and pharmacotherapy use was verified through the electronic medical record.
Table 1.
Demographic Variables (Total N=103)
| Demographic Variable | N | % |
|---|---|---|
| Age | ||
| 20 – 30 | 50 | 0.49 |
| 31 – 40 | 51 | 0.50 |
| >40 | 2 | 0.02 |
| Education | ||
| High school or less | 9 | 0.09 |
| Vocational or technical degree | 6 | 0.06 |
| College degree | 40 | 0.39 |
| Graduate education | 41 | 0.40 |
| Students | 7 | 0.07 |
| SES (Household Income) | ||
| Very Low (< $25,000 ) | 13 | 0.13 |
| Low ($25,000–44,999) | 13 | 0.13 |
| Mod ($45,000–64,999) | 12 | 0.12 |
| High (> $65,000) | 64 | 0.62 |
| Ethnic Group | ||
| Black | 11 | 0.11 |
| White | 74 | 0.72 |
| Hispanic | 6 | 0.06 |
| Asian or Pacific Islander | 8 | 0.08 |
| American Indian or Alaskan Native | 0 | 0.00 |
| Others | 4 | 0.04 |
| Marital Status | ||
| Married | 90 | 0.87 |
| Live-in Partner | 3 | 0.03 |
| Total Couples | 93 | 0.90 |
2.4 Research Design
2.5 Longitudinal assessments of maternal depressive symptoms were conducted at 28 weeks, 32 weeks, and 37 weeks gestation and again at birth using the 21 questions within the Beck Depression Inventory (BDI), a highly reliable (alpha = 0.93 and test-retest r = 0.74) instrument for measurement of ongoing mood symptoms (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). The BDI was chosen as a longitudinal instrument because its robustness for repeated measures exceeded that of the EPDS, which had been used for clinical screening. The Beck consists of 21 questions, all of which were included in the evaluation of the pregnant women. During the study, a score at or greater that 20 was followed by SCID diagnostic interview. However, for the purpose of the present report, the analytical strategy defined a depressive “cutoff” that grouped the women into one of three groups: (1) low–stable non-depressive trajectory=low depression; (2) intermediate–stable depressive symptoms=intermediate depression, and (3) high-elevated depression symptoms trajectory=high depression. This was based on a mixture growth curve approach proposed by Nagin (2005) which was used to identify different trajectory patterns of depressive symptoms reported by the mothers based on their BDI scores obtained at 28, 32, and 37 weeks gestation. In this study, we hypothesized that women, who have depressive symptoms as measured by elevations in the Beck Depression Inventory (BDI), would have infants with unique patterns of neonatal LHPA activity and infant central nervous system (CNS) responses. Infant ACTH and cortisol levels were analyzed from blood obtained from the umbilical cord artery upon delivery. Chilled tubes containing the blood were placed on ice, immediately refrigerated, centrifuged and the plasma component was aliquoted into tubes free of subject identifiers. Plasma samples were stored in a −80 degree freezer, and two replicates were performed on each analyzed sample. Researcher assistants performing the assays and the investigators analyzing the data were not aware of the women’s depression status.
2.6 Neurobehavioral assessment of infant functioning
2.7 A neurobehavioral evaluation was performed by staff members when the infants were two weeks old using the Neonatal Intensive Care Network Neurobehavioral Scale (NNNS). Staff were trained and certified to conduct the NNNS by Boukydis (Boukydis et al., 2004), who was a member of the team of investigators that developed the NNNS. A training period of 6–12 weeks preceded individual scoring of the NNNS examination. The NNNS was administered according to the procedures specified in "The Neonatal Intensive Care Unit Network Neurobehavioral Scale Procedures" (Lester et al., 2004). The NNNS examination was developed to evaluate stress, neurologic symptoms and withdrawal signs, establishing its uniqueness from other tools such as the NBAS. Because this study focused on neonates born to women with depressive symptoms and potential adverse sequelae related to intrauterine exposure to stress hormones, this instrument was uniquely suited to these analyses. Reliability was set to the criteria used on other neurobehavioral examinations of no more than two 2-point disagreements on items with ≥9 scale points throughout the examination. For items with ≤5 scale points, agreement had to be exact with no more than five disagreements throughout the NNNS entire examination. A certified NNNS consultant was available throughout the study period for questions regarding individual examination results that were discrepant among the staff members. The examiners were blind to the mother’s depression status. The 30 minute examination was conducted in a quiet, well-heated room with dim lights to prevent these variables from altering the examination. Parents were not present within the room, but if requested could watch the examination through a two-way mirror, accessed from an adjacent observation room.
2.8 Infant status regarding feeding and sleep schedule and general health of the infant were reported by the mother for the day of the examination and a week prior to the examination. The exam included three parts: (1) the more classical neurological items that assess active and passive tone and primitive reflexes; (2) behavioral items including state, sensory and interactive responses; and (3) stress/abstinence items particularly appropriate for high-risk infants. The neonatal behaviors assessed are elicited in relation to a range of environmental stimuli, yielding scores on measures of orientation, motor behavior, the range and regulation of state, autonomic stability, reflexes, irritability and stress. The scores obtained across items were reduced by an NNNS software program to means and standard deviations calculated for the following NNNS summary scores: habituation, attention, arousal, regulation, number of handling procedures, quality of movement, number of non-optimal reflexes, number of asymmetric reflexes, hypertonicity, hypotonicity, and stress (skin, visual, autonomic), excitability, and lethargy. The summary scores for these NNNS items are described in Table 2, and reference typical scores for a developing neonate. Maternal depressive symptoms, mother’s and the newborn’s neurotransmitter/neurohormone profiles have been assessed in previous studies (e.g. Field et al., 2004); and this study adds to that literature in that it examines infant LHPA activity from cord blood at birth and neonatal neurobehavioral functioning as a function of the mother’s prenatal history of depressive symptoms.
Table 2.
Neonatal Intensive Care Unit Network Neurobehavioral Scale: Definition and Interpretations of Summary Scores (Adopted from Lester et. al. in Appendix 2 PEDIATRICS Vol. 113 No. 3 March 2004)
| Summary Item | Expected Response | Normative Score |
|---|---|---|
| Habituation | Response decrement to repeated auditory and visual stimuli; mean of items (3 items; range: 1–9) | High range |
| Attention | Response to animate and inanimate auditory and visual stimuli; mean of items (7 items; range: 1–9) | High range |
| Handling | Handling Handling strategies used during orientation to maintain alert state; mean number of strategies used (8 items, range 0–1) | 1 |
| Quality of movement | Quality of movement Measure of motor control including smoothness, maturity, lack of startles and tremors; mean of items recoded for good motor control (6 items; range: 1–9) | Middle range |
| Regulation | Capacity to organize motor activity, physiology, and state during the examination and to respond to cuddling, consoling, and negative stimuli; mean of items recoded for good regulation (15 items; range: 1–9) | Middle range |
| Nonoptimal reflexes | Any nonoptimal response to reflex elicitation; sum of items recoded for nonoptimal reflexes (15 items; range: 0–15) | Hign range |
| Asymmetric reflexes | Any asymmetric response to reflex elicitation; sum of items recoded for asymmetric responses (16 items; range: 0–16) | <16 |
| Stress/abstinence | Amount of stress and abstinence signs observed during examination; mean number of observed stress signs (50 items; range: 0–1) | Low range |
| Arousal | Level of arousal including state and motor activity during the examination; mean of items recoded for high arousal (7 items; range: 1–9) | Middle range |
| Hypertonicity | Hypertonic response in arms, legs, or trunk or in general tone; sum of items recoded for hypertonic indicators (10 items; range: 0–10) | 0 |
| Hypotonicity | Hypotonic response in arms, legs, or trunk or in general tone; sum of items recoded for hypotonic indicators (10 items; range: 0–10) | 0 |
| Excitability | Measure of high levels of motor, state, and physiologic reactivity; sum of items recoded for excitable behavior (15 items; range: 0–15) | High range |
| Lethargy | Measure of low levels of motor, state, and physiologic reactivity; sum of items recoded for lethargic behavior (15 items; range: 0–15) | Low range |
3.0 Statistical Analyses
3.1 A mixture growth curve approach proposed by Nagin (2005) was used to identify different trajectory patterns of depressive symptoms reported by the mothers based on their BDI scores obtained at 28, 32, and 37 weeks gestation. Expectation Maximization (EM) algorithm as described by Dempster Laird, & Rubin (1977) was used to derive different BDI patterns and estimates the corresponding parameters for each pattern. The group curves that were derived with this process were deemed to represent developmental patterns of depressive symptoms in the women. The trajectories consider that within group variation in depression scores is less informative than between-group variation in the pattern of scores over time. Thus, this analytic approach identifies distinct groups within the overall population that vary in their level and pattern of scores over time, whereas general growth-mixture modeling focuses on individual variability around the overall population trajectory (Bilmes, 1998). (The data from all the subjects who had at least two BDI measures were used for the analysis with the missing values being imputed using Proc MI in SAS). The Bayesian Information Criteria (BIC) was used to estimate the optimal number of groups. Specifically, BIC = −839.2 was highest when three trajectories were identified. Once three trajectories were determined the groups were compared on demographic variables. Analysis of variance was used initially to test whether the trajectory groups differed on continuous sociodemographic indicators: maternal age, maternal education, and income. For categorical variables (marital stability, and ethnicicty), logistic regression models were used to test for group differences. Mean differences across trajectory groups over time were examined using repeated measures ANOVA. Finally, general linear model (controlling for possible confounders) was used to test for group differences on the infant outcomes for: cord blood ACTH and cortisol, APGAR score at 1 minute and 5 minutes and NNNS measures at two weeks. There were no Bonferoni adjustments made in the calculations.
4.0 Results
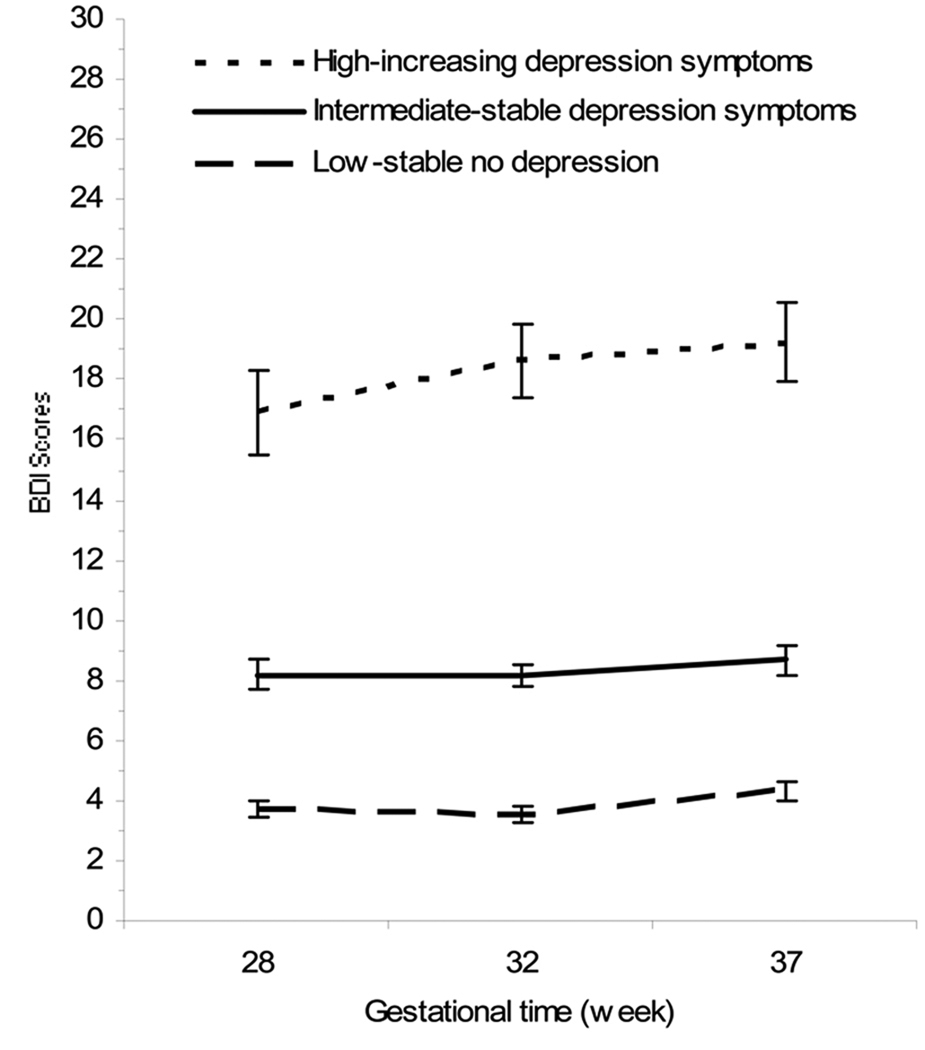
4.1 Figure 1 depicts the three distinct trajectories of maternal depressive symptoms identified in the analysis. The actual trajectories represent the mean depressive symptom scores for mothers who were assigned to each group on the basis of the classification by the expectation-maximization (EM) algorithm. There were a total of 140 women in all three groups. The first trajectory identified is one of consistently low levels of maternal depressive symptoms (low–stable non-depressive trajectory=low depression). Fifty women (36%) in the sample fall into this trajectory. The mean BDI score was 3.74 (SD +/− 2.0) at 28 weeks gestation 3.56 (SD +/− 1.84) at 32 wks and increasing to a mean of 4.32 (SD +/− 2.11) by 37 weeks of pregnancy. The next and largest group (56%, n=78) is characterized by moderate symptomatology (intermediate–stable depressive symptoms=intermediate depression), with a mean BDI score of 8.2 (SD +/− 3.37) at 28 weeks of gestation, with minimal increase across the third trimester (32 weeks, M=8.16, SD +/− 2.7 and 37 weeks, M= 8.67 SD +/− 3.19). The final trajectory group captured 8% (n=12) of the women with elevated symptoms increasing over time (high-elevated depression symptoms trajectory=high depression). These women report marked variations in levels of symptoms over time with high scores at 28 weeks gestation (M=16.92, SD +/− 4.78), followed by a significant (p=.021) increase at 32 weeks of pregnancy (M=18.63, SD +/− 3.42) that remain stable until 37 weeks (M= 19.25, SD +/− 3.73). These results are summarized in Table 3.
Figure 1.
Depicts the symptom severity trajectories of the recruited women in each of the 3 groups: high with increasing depressive symptoms, intermediate with stable depressive symptoms and low (no depression) with stable symptoms. Symptoms severity as measured by the BDI is shown on the y axis, with gestation on the x axis.
Table 3.
BDI Score by Trajectory Group
| Gestational time (week) |
Low-stable no depression |
Intermediate-stable depression symptoms |
High-increasing depression symptoms |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| 28 | 3.74 | 2 | 8.2 | 3.37 | 16.92 | 4.78 |
| 32 | 3.56 | 1.84 | 8.16 | 2.7 | 18.63 | 3.42 |
| 37 | 4.32 | 2.11 | 8.67 | 3.19 | 19.25 | 3.73 |
4.2 In order to ascertain whether other psychosocial variables might confound the results of symptom severity findings, we compared the socioeconomic and demographic data obtained from the women of the three depression groups. The overall demographic information for the population of recruited women is summarized in Table 1 across the three different groups of women, and was controlled in analyses.
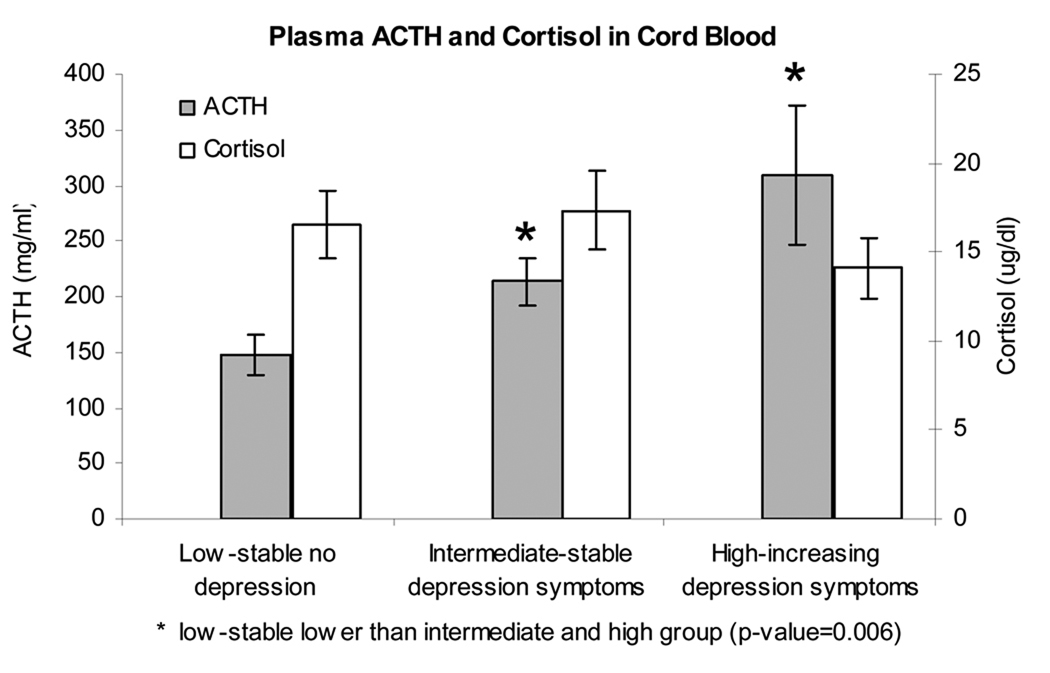
4.3 Results from analysis of variance indicated that the infants born to women within these subgroups showed differences in neuroendocrine profiles at birth (Figure 2). As can be seen, infants born to the women in the low-stable group had ACTH levels within cord blood that was significantly lower than the infants born to women in either of the other groups (p=.006). It is of note, however, that cord cortisol levels within all three groups were not statistically different from one another. There were no differences in delivery type (including Caesarian delivery), delivery complications or APGAR scores between the three depression trajectories. Thus, the neuroendocrine findings cannot be explained by differences in obstetric outcomes.
Figure 2.
Depicts a graphic representation of cord blood ACTH and cord blood cortisol obtained from the infants born to the three groups of women (high/increasing depression symptoms, intermediate/stable depression symptoms, low/stable no depression), at the time of birth.
4.4 In addition to the neuroendocrine findings, analyses revealed subtle differences in infant neurobehavioral functioning at two weeks of age across depression trajectories. Table 2 describes each of the summary NNNS scores. Additionally, a summary of all infants’ NNNS score means and standard deviation with the maximal and minimum values obtained for each item is shown in Table 4. Of the 13 summary scores, only two revealed statistically significant differences across all depression groups: hypotonia and habituation. Visual stress was significantly different between the infants of the high depression women relative to the low depression group.
Table 4.
Summary of NNNS Scores for all Samples
| Trajectories group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low-stable no depression (N=50) |
Intermedium-stable depression symptoms (N=45) |
High-increasing depression symptoms (N=9) |
|||||||
| NNNS summary item | N | Mean | SD | N | Mean | SD | N | Mean | SD |
| Habituation | 23 | 6.72 | 1.63 | 24 | 7.94 | 0.9 | 5 | 7.93 | 1.19 |
| Attention | 37 | 5.41 | 1.07 | 36 | 5.6 | 1.52 | 8 | 5.52 | 1.75 |
| Handling | 38 | 0.55 | 0.27 | 37 | 0.52 | 0.31 | 6 | 0.46 | 0.38 |
| Quality of movement | 40 | 4.73 | 0.58 | 40 | 4.72 | 0.49 | 9 | 4.57 | 0.38 |
| Regulation | 40 | 5.42 | 1.07 | 39 | 5.45 | 1.16 | 8 | 5.71 | 1.16 |
| Nonoptimal reflexes | 41 | 4.41 | 2.48 | 40 | 3.93 | 3.14 | 9 | 4.56 | 3.09 |
| Asymmetric reflexes | 41 | 0.83 | 1.43 | 40 | 0.83 | 1.15 | 9 | 0.89 | 0.93 |
| Stress/abstinence | |||||||||
| Stress abstinence | 39 | 0.14 | 0.08 | 39 | 0.12 | 0.1 | 9 | 0.12 | 0.11 |
| Autonomic stress | 40 | 0.21 | 0.18 | 40 | 0.17 | 0.18 | 9 | 0.19 | 0.21 |
| CNS stress | 40 | 0.17 | 0.14 | 40 | 0.14 | 0.14 | 9 | 0.16 | 0.16 |
| Gastrointestinal stress | 39 | 0.13 | 0.21 | 39 | 0.13 | 0.22 | 9 | 0.07 | 0.15 |
| Physiological stress | 40 | 0.08 | 0.21 | 40 | 0.06 | 0.23 | 9 | 0.11 | 0.22 |
| Skin stress | 40 | 0.09 | 0.11 | 40 | 0.11 | 0.19 | 9 | 0.2 | 0.26 |
| Visual stress | 39 | 0.1 | 0.08 | 39 | 0.07 | 0.07 | 9 | 0.03 | 0.06 |
| State abstinence | 39 | 0.14 | 0.18 | 39 | 0.15 | 0.18 | 9 | 0.08 | 0.14 |
| Arousal | 40 | 4.03 | 0.75 | 40 | 3.95 | 0.77 | 9 | 3.49 | 1 |
| Hypertonicity | 40 | 0.15 | 0.48 | 40 | 0.03 | 0.16 | 9 | 0 | 0 |
| Hypotonicity | 40 | 0.3 | 0.52 | 40 | 0.4 | 0.81 | 9 | 1.78 | 1.92 |
| Excitability | 40 | 3.25 | 2.61 | 40 | 3.03 | 2.63 | 9 | 3 | 2.96 |
| Lethargy | 41 | 3.95 | 2.01 | 40 | 4.18 | 2.27 | 9 | 4.78 | 3.73 |
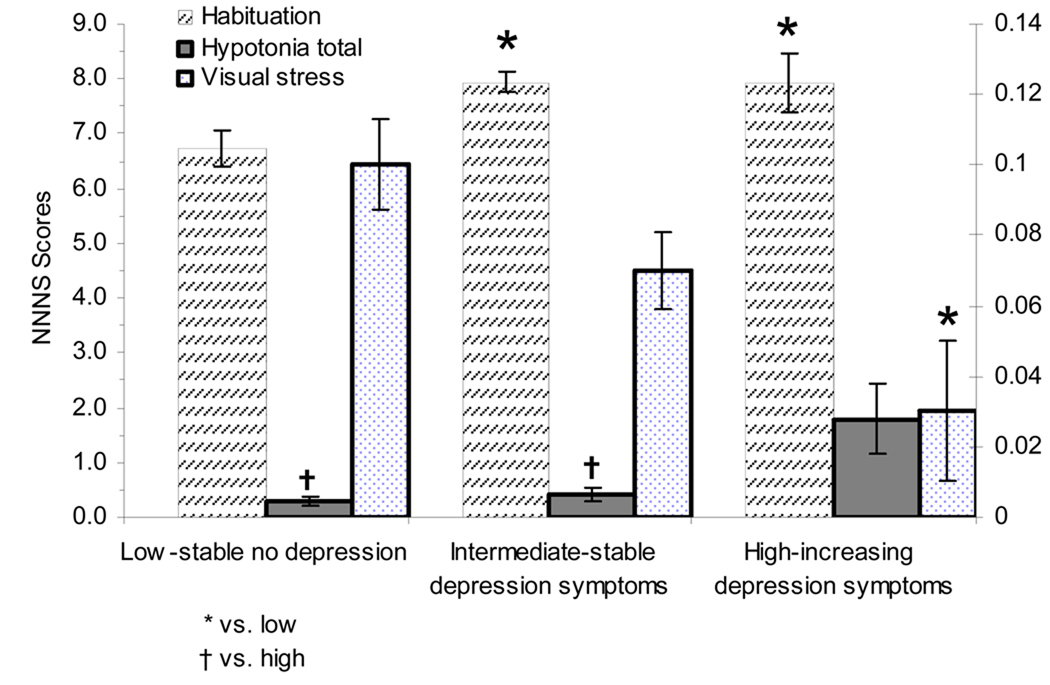
4.5 Hypotonicity detects the presence of hypotonic responses in the arms, legs, trunk, or in general tone of the infant. Infants with typical neonatal adaptation have scores at or near 0. The infants born to the mothers in the high depression group had scores which were significantly different from both the low risk infants (p <.0001) and from the intermediate risk infants (p<.0001). These results are depicted in Figure 3.
Figure 3.
These figures depicts those NNNS summary score variables that were significant, or trended toward significance, when the infants born to women from the three groups were compared at 2 weeks of age.
4.6 The Habituation scale is based on an infants response to repeated stimulation (by rattle, by bell and by light), and how the infants habituate to this stimulation over time. It is based on a 9-point scale, some infants habituate more quickly, which gives them a higher score on this scale. The intermediate and high depression groups were significantly different from the low risk group (p=.0006). These scores indicate that the infants born to women within the intermediate and high depression groups habituated much more quickly to stimuli, relative to infants in the low depression group.
4.7 Visual stress refers to a number of visual signs (nystagmus, gaze aversion, hyper alertness, setting sun, strabismus, and other abnormal visual responses). More mature infants have a low score on this scale. Both the low and the intermediate depression groups had visual stress scores which were significantly higher than the group of babies born to the high depression women (p=.01).
4.8 The variable number of infants undergoing the NNNS subtests was largely determined by the infant’s state/arousal during the exam, which accounted for the different sample size across NNNS scales. The greatest number of infants were missing data for the habituation subscale as this exam required the infants to be in light or deep sleep and many infants did not reach this state during the 30-minute exam. Other subtests were missed if the infants reached high levels of arousal and distress such that the exam needed to be truncated. All infant data presented were obtained from NNNS examinations, which could be scored.
5.0 Discussion
5.1 This preliminary investigation does suggest that maternal depressive symptoms may be associated with the development of the infant limbic-hypothalamic-pituitary axis (LHPA). Elevation in cord ACTH in infants born to women greater depressive symptoms suggests that the fetal brain of these infants may be more sensitive to stress signals and has responded with increased release of ACTH from the anterior pituitary at the time of birth. Another explanation might be that infants of women with depressed mood have a lack of sensitivity to the normally functioning feedback loop by which cortisol diminishes the production of ACTH and CRH.
5.2 Umbilical cord cortisol did not significantly differ between the three groups of infants. It is theoretically possible that the adrenal “end organ” of LHPA activation is maximally saturated and stimulated by ACTH even at low levels of CRH, and is simply unable to produce any more cortisol, or has reset its secretory capacity in the face of an elevated ACTH level.
5.3 Several studies have evaluated the mother’s and the newborn’s hormonal profiles and maternal depressive symptoms in an effort to associate these to perinatal outcome measures and/or infant neurological functioning (Field et al., 2004; Lundy et al., 1999). In these studies, newborns and mothers were evaluated for their urine hormonal profiles within 24 hours of delivery. Infants of depressed mothers showed hormonal and neurotransmitter profiles that were similar. Our evaluation of LHPA hormones at birth is in agreement with an activation of the LHPA axis in the infants of depressed women given the high levels of cord blood ACTH in neonates born to women with intermediate and high BDI scores. In contrast to previous reports in the neonate, cortisol levels were not significantly high in the babies born to depressed women. There are several possible explanations for this discrepancy. Unlike previous reports, our measures are performed at birth, a time that can be construed as that of maximum physical stress to the mother and newborn. Therefore, it is less surprising to us that the cortisol levels are very similar across groups. As previously mentioned, the lack of difference may be indicative of maximal secretion of this hormone by the adrenal gland (ceiling effect). Another important difference between this study and previous studies is the source of hormone measure and the time-frame represented. This study utilized a plasma sample derived from cord blood, whereas other studies have performed biochemical measurements in urine within 24 hrs after birth (Field et al., 2004). Therefore, hormones obtained from cord blood represent hormonal secretion by the neonate close to the time of birth, whereas the levels in previous studies reflect an integrated or mean measure of the hormones or neurotransmitters secreted over a period of hours. This is because urine collects in the urinary bladder, an organ that serves as a reservoir of body fluid filtered over time by the kidney. Thus, hormones measured from cord blood represent the hormone stress response of the infant closest to the time of delivery. In contrast, hormone levels measured from the infant’s urine represent hormones secreted over a longer time frame. Taken together, our study and previous studies suggest that what is of most importance is that the events that have transpired during gestation, namely the depression severity in the mother, may have impacted the development and adaptation of the developing LHPA in the infant.
5.4 What is more intriguing is to speculate how these changes may alter neurodevelopmental adaptation. DiPietro and colleagues (DiPietro, Hodgson, Costigan, Hilton, & Johnson, 1996) have noted that infants of mothers with elevated CRH exhibit decreased motor movements in utero. The data presented here, expands these findings beyond gestation, noting that infants born to the women with high depressive symptoms are more hypotonic. These infants born to depressed mothers also “habituate” to stimuli quickly. This finding is counterintuitive since previous studies on infants of depressed mothers have shown more irritability during a Brazelton neonatal behavior assessment (Abrams, 1995). Though differences in habituation were not reported in Abram’s study, more irritability would suggest that we should have observed a limited ability to habituate to the repeated auditory and visual stimuli used for this part of the NNNS assessment. Indeed, another study reporting on the NBAS results from two day old infants born to mothers who experienced depressive symptoms showed less optimal scores on several areas, including habituation, orientation, motor, range of state, autonomic stability and depression scales (Field et al., 2004). The infants showing these alterations were born to women who had increased urinary cortisol and decreased dopamine urinary levels during the prenatal and postnatal assessments, suggesting that this hormonal/neurotransmitter profile may be associated with the observed results. It is possible that the time of evaluation in the study reported here (two weeks vs. twenty-four hours after birth) may explain these differences.
5.5 Glucocorticoids have been associated with correction of visual reflexes attributed to the vestibular system, which may explain the absence of nystagmus in the infants of women with high depressive symptoms. Though negative and positive effects are suggested by the data, this is not completely unexpected. A number of investigators, including de Kloet and his colleagues (de Kloet, Vreugdenhil, Oitzl, & Joels, 1998), have delineated an inverted U pattern for adrenal steroid effects on the CNS such that both very low and very high concentrations are associated with adverse CNS effects.
6.0 Limitations of the Study
6.1 This is a pilot study, and as such had a relatively small number of infants in the highest risk (high depression) subgroup. There were also a variable number of infants who received the NNNS subtests (largely driven by factors such as level of arousal, which precluded some of the subtests). This is a frequent occurrence when administering neonatal assessments in a laboratory environment. These findings do raise many important questions within the field of obstetrics and suggest that maternal mood symptoms may impact the development of the LHPA and neurobehavioral systems in their neonates.
Acknowledgements
Funding support was provided by the National Institute of Mental Health grant MH065062 and the National Institute of Health General Clinical Research Center Grant M01 RR00042. Additional bridge funding was provided by the University of Michigan Depression Center and Department of Psychiatry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams SM, Field T, Scafidi F, Prodromidis M. Newborns of depressed mothers. Infant Mental Health Journal. 1995;16:233–239. [Google Scholar]
- Als H, Tronick E, Lester BM, Brazelton TB. The Brazelton Neonatal Behavioral Assessment Scale (BNBAS) Journal of Abnormal Child Psychology. 1977;5:215–231. doi: 10.1007/BF00913693. [DOI] [PubMed] [Google Scholar]
- Als H, Lester BM, Tronick E, Brazelton TB. Towards a research instrument for the assessment of preterm infants’ behavior (A.P.I. B.) In: Fitzgerald HE, Lester BM, Yogman MW, editors. Theory and Research in Behavioral Pediatrics. Plenum Press: New York; 1982. pp. 85–132. [Google Scholar]
- Amiel-Tison C. Neurological evaluation of the maturity of newborn infants. Archives of Disease in Childhood. 1968;43:89–93. doi: 10.1136/adc.43.227.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bilmes J. A gentle tutorial of the EM algorithm and its application to parameter estimation for Gaussian mixture and hidden Markov models, ICSI-TR-97-021. 1998 Retrieved from http://www.ee.columbia.edu/~sfchang/course/svia-F03/ee6882-F03-paper-list.htm.
- Boukydis CF, Bigsby R, Lester BM. Clinical use of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113:679–689. [PubMed] [Google Scholar]
- Brazelton TB. Neonatal Behavioral Assessment Scale. Philadelphia, PA: JB Lippincott; 1973. [Google Scholar]
- Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. I. Limbic system-adrenocortical dysfunction. Archives of General Psychiatry. 1976a;33:1039–1044. doi: 10.1001/archpsyc.1976.01770090029002. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Archives of General Psychiatry. 1976b;33:1051–1058. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- Cox AD. Maternal depression and impact on children's development. Archives of Disease in Childhood. 1988;63:90–95. doi: 10.1136/adc.63.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocrine Reviews. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. Journal of Royal Statistical Society. Series B (Methodological) 1977;39:1–38. [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TR. Fetal neurobehavioral development. Child Development. 1996;67:2553–2567. [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Prenatal dysthymia versus major depression effects on the neonate. Infant Behavior and Development. 2008;31:190–193. doi: 10.1016/j.infbeh.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: a review. Infant Behavior and Development. 2006;29:445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Dieter JN, Hernandez-Reif M, Schanberg S, Kuhn C, Bendell D. Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development. 2004;27:216–229. [Google Scholar]
- Finnegan LP. Neonatal abstinence syndrome: assessment and pharmacotherapy. In: Rubaltelli FF, Granati B, editors. Neonatal therapy: an update. New York, NY: Excerpta Medica; 1986. pp. 122–146. [Google Scholar]
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biological Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- Hoffman S, Hatch MC. Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychology. 2000;19:535–543. [PubMed] [Google Scholar]
- Jesse DE, Seaver W, Wallace DC. Maternal psychosocial risks predict preterm birth in a group of women from Appalachia. Midwifery. 2003;19:191–202. doi: 10.1016/s0266-6138(03)00031-7. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Ritchie JC, Saunders W, Wilson W, Nemeroff CB, Carroll BJ. Nocturnal and early morning secretion of ACTH and cortisol in humans. Biological Psychiatry. 1990;28:47–57. doi: 10.1016/0006-3223(90)90431-z. [DOI] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113:641–667. [PubMed] [Google Scholar]
- Lundy B, Field T, Pickens J. Newborns of mothers with depressive symptoms are less expressive. Infant Behavior and Development. 1996;19:419–424. [Google Scholar]
- Lundy BL, Jones NA, Field T, Nearing G, Davalos M, Pietro PA, Kuhn C. Prenatal depression effects on neonates. Infant Behavior and Development. 1999;22:119–129. [Google Scholar]
- Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. Journal of Women’s Health. 2003;12:373–380. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- Mazure CM, Kincare P, Schaffer CE. DSM-III-R Axis IV: Clinician reliability and comparability to patients' reports of stressor severity. Psychiatry. 1995;58:56–64. doi: 10.1080/00332747.1995.11024711. [DOI] [PubMed] [Google Scholar]
- Nagin D. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Neunaber DJ, Zekoski EM. Prospective study of postpartum depression: prevalence, course, and predictive factors. Journal of Abnormal Psychology. 1984;93:158–171. doi: 10.1037//0021-843x.93.2.158. [DOI] [PubMed] [Google Scholar]
- Prechtl HF, van Eykern LA, O'Brien MJ. Respiratory muscle EMG in newborns: a non-intrusive method. Early Human Development. 1977;1:265–283. doi: 10.1016/0378-3782(77)90040-8. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Dunkel-Schetter C, Porto M. Maternal stress, HPA activity, and fetal/infant outcome. In: Beckwith BE, Saria A, Chronwall BM, Sandman C, Strand FL, editors. Annals of the New York Academy of Sciences. Vol. 814. NY: New York Academy of Sciences; 1997. pp. 266–275. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology. 1999;34:163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Young EA, Haskett RF, Murphy-Weinberg V, Watson SJ, Akil H. Loss of glucocorticoid fast feedback in depression. Archives of General Psychiatry. 1991;48:693–699. doi: 10.1001/archpsyc.1991.01810320017003. [DOI] [PubMed] [Google Scholar]