Abstract
Despite recent attention in the literature, anterior cruciate ligament (ACL) injury mechanisms are controversial and incidence rates remain high. One explanation is limited data on in vivo ACL strain during high-risk, dynamic movements. The objective of this study was to quantify ACL strain during jump landing. Marker-based motion analysis techniques were integrated with fluoroscopic and magnetic resonance (MR) imaging techniques to measure dynamic ACL strain non-invasively. First, eight subjects’ knees were imaged using MR. From these images, the cortical bone and ACL attachment sites of the tibia and femur were outlined to create 3D models. Subjects underwent motion analysis while jump landing using reflective markers placed directly on the skin around the knee. Next, biplanar fluoroscopic images were taken with the markers in place so that the relative positions of each marker to the underlying bone could be quantified. Numerical optimization allowed jumping kinematics to be superimposed on the knee model, thus reproducing the dynamic in vivo joint motion. ACL length, knee flexion, and ground reaction force were measured. During jump landing, average ACL strain peaked 55 ± 14 ms (mean and 95% confidence interval) prior to ground impact, when knee flexion angles were lowest. The peak ACL strain, measured relative to its length during MR imaging, was 12 ± 7%. The observed trends were consistent with previously described neuromuscular patterns. Unrestricted by field of view or low sampling rate, this novel approach provides a means to measure kinematic patterns that elevate ACL strains and that provide new insights into ACL injury mechanisms.
Keywords: ACL, anterior cruciate ligament; In vivo; Strain; Dynamic; Motion; MR; Magnetic resonance; Fluoroscopy; Imaging; Injury mechanisms; Kinematics; Jumping; Landing; Valgus
1. Introduction
Over 200,000 anterior cruciate ligament (ACL) injuries occur in the United States every year, half of which are experienced by young athletes between 15 and 25 years of age (Miyasaka et al., 1991; AAOS, 2008). The consequences of ACL deficiency incurred from ACL injury include pain, instability, damage to the menisci, and early-onset osteoarthritis (OA) (Fairclough et al., 1990; Roos et al., 1995; Fithian et al., 2002; Hill et al., 2005). Furthermore, ACL deficiency greatly compromises the ability to participate in sports that require jumping and cutting (van Lent et al., 1994; Fink et al., 2001; Katayama et al., 2004; Maquirriain and Megey, 2006; Kapreli et al., 2009). For these reasons, between 100,000 and 175,000 patients elect to undergo ACL reconstruction annually (Koh, 2005; Griffin et al., 2006). Although surgical intervention provides good short-term outcomes, long-term results are less consistent (Asano et al., 2002; Wolf and Lemak, 2002; Lohmander et al., 2004; von Porat et al., 2004; Grossman et al., 2005). Some studies have suggested that current reconstructive techniques do not decrease the probability of developing OA when compared to non-operative treatment (Fink et al., 2001; Lohmander et al., 2004; von Porat et al., 2004; Lohmander et al., 2007; Butler et al., 2009).
Because ACL injury affects such a young population (Roos et al., 1995; Beynnon et al., 2005) and surgery has mixed results in preventing early-onset OA (Fithian et al., 2002; Lohmander et al., 2007), there has been great interest in developing ACL injury prevention programs (Hewett et al., 1999; Heidt et al., 2000; Myklebust et al., 2003; Gilchrist et al., 2008). Many studies have focused on non-contact ACL injury mechanisms, which account for 70% of all ACL injuries (McNair et al., 1990; Weinhold et al., 2007; Gianotti et al., 2008) and potentially could be prevented with an appropriate training program. However, the levels of success achieved by current prevention programs have shown varied efficacy (Hewett et al., 1999; Soderman et al., 2000; Myklebust et al., 2003; Mandelbaum et al., 2005; Pfeiffer et al., 2006; Barber-Westin et al., 2009) and despite their implementation, high rates of non-contact ACL injuries persist (Agel et al., 2005). These findings suggest that there is an incomplete understanding of the underlying injury mechanisms. Specifically, there are limited in vivo data on ACL strain, a critical parameter for predicting ACL failure.
Numerous studies have investigated ACL injury mechanisms using videographic and motion analyses (Chappell et al., 2002; Chappell et al., 2007; Krosshaug et al., 2007a, 2007b; Boden et al., 2009; Hewett et al., 2009; Nagano et al., 2009). These studies provide important kinematic data, but do not directly measure ACL strain. Many previous studies have examined ACL loading in cadavers (Draganich and Vahey, 1990; Markolf et al., 1990; Woo et al., 1991; Li et al., 1999; Kanamori et al., 2000; DeMorat et al., 2004). Although these data give valuable information on ACL function, their application to in vivo environments are restricted by an inability to recreate complex multi-planar loading conditions experienced during dynamic jumping and cutting activities. Using implantable strain gauges, some in vivo measurements of ACL strain have also been reported (Beynnon and Fleming, 1998; Fleming et al., 1999; Cerulli et al., 2003; Fleming and Beynnon, 2004). Beynnon and Fleming et al. (Beynnon et al., 1997; Fleming et al., 1998; Fleming et al., 1999) ran a series of in vivo strain studies to understand how an ACL graft would be loaded during common post-surgical rehabilitation exercises and daily tasks. These data were the basis for many pertinent clinical rehabilitation guidelines. However, there are limited data on ACL strains during sport-specific dynamic movements. Dynamic ACL strain data are needed to accurately predict what motions predispose the ACL to injury. The objective of this study was to measure in vivo ACL strain non-invasively during a dynamic jumping activity using a novel method developed by our laboratory.
2. Methods
Institutional Review Board approval was obtained for this protocol before enrollment of participants. Eight healthy male subjects (mean age: 26 years, range: 19–38 years) were recruited to participate in this study. All volunteers were physically active, participating in recreational sports or exercise a minimum of three days a week for at least one hour each day. The potential subjects were disqualified if they had a history of knee injury.
All subjects were imaged with a 3T magnet (Trio Tim, Siemens Medical Solutions USA, Malvern, PA). Coronal, sagittal, and axial images were taken with the patient supine and the knee in a relaxed position. MR images were acquired using a double-echo steady state sequence (DESS, flip angle: 25°, TR: 17 ms, TE: 6 ms) and an eight channel receive-only knee coil with a field of view of 15 × 15 cm2, a matrix of 512 × 512 pixels, and a slice thickness of 1 mm. From these images, the outer margins of the cortical bone and ACL attachment site were outlined using solid-modeling software (Rhinoceros 4.0, Robert McNeel and Associates, Seattle, WA). These tracings were compiled to create subject-specific 3D models of each tested knee. The location of the ACL was confirmed using orthogonal image sets. This methodology accurately measures the location of the ACL footprint center to within 0.3 mm, as described previously (Abebe et al., 2009).
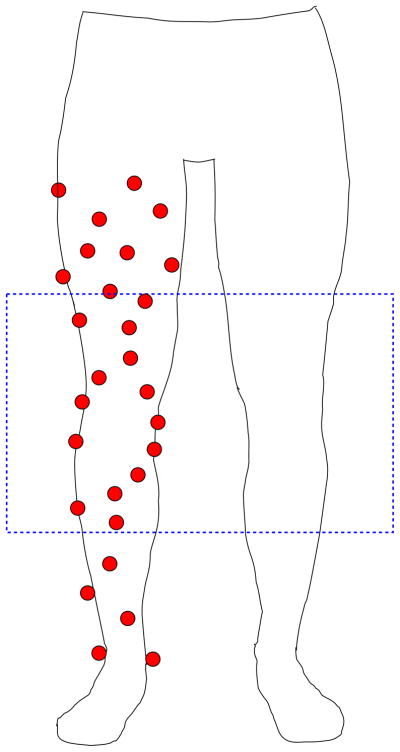
Subjects next underwent a 3D motion analysis using an eight camera motion capture system with a sampling rate of 240 Hz (Motion Analysis Corporation, Santa Rosa, CA). The calibrated capture volume for the system was 3 m long, 2 m wide, and 3 m high. Also, centered within the capture volume were four embedded force plates (AMTI, Boston, MA, USA) with a sampling rate of 2400 Hz. All subjects were provided with standardized form-fitting athletic wear and footwear. Reflective markers were placed unilaterally on various anatomic landmarks used in previous gait studies (Queen et al., 2006; Queen et al., 2008) on the subject’s leg. Additionally, non-symmetric clusters of markers were also placed on the thigh and shank until a total of 28 markers were positioned on the leg (Fig. 1). The primary goal of this complex marker set was to over-constrain each anatomical segment (thigh, shank) so that the effects of skin motion could be minimized via numerical optimization, as demonstrated by previous investigators (Andriacchi et al., 1998; Alexander and Andriacchi, 2001; Ngai et al., 2009; Ngai and Wimmer, 2009).
Fig. 1.
Twenty-eight markers were placed on the subject’s thigh and shank. Eight markers on the distal femur and six on the proximal tibia were imaged using biplanar fluroscopy, as indicated by the box. These markers were used to position the 3D model of the femur and tibia such that the knee motion of the subject during motion analysis was reproduced.
Marker data were captured initially during a static standing trial with the subject’s feet shoulder width apart for one second. Next, subjects performed five successful trials of a jump landing task. Starting from a platform 0.47 m off of the ground and half their standing height away from the force plate’s edge, subjects were asked to jump from the platform onto two force plates, then immediately jump straight up with maximal effort and land back on the same force plates again. This task is similar to previous activities studied in the literature that were designed to simulate jumping and landing activities in soccer or basketball (Onate et al., 2005; Chappell et al., 2007).
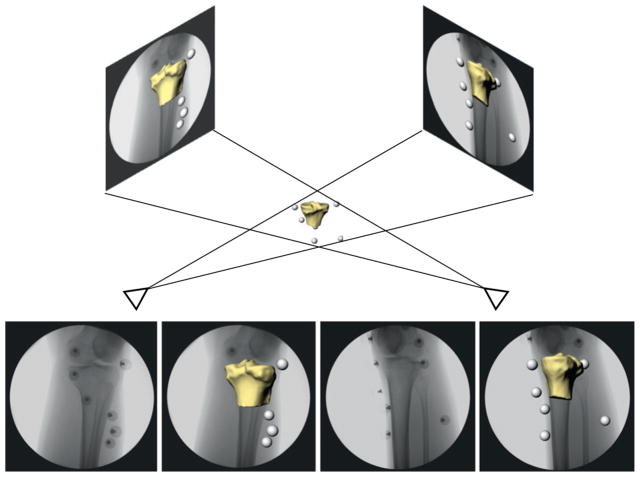
Upon completion of the 3D motion capture session, subjects were imaged with the markers still in the same positions using biplanar fluoroscopy (DeFrate et al., 2004; Caputo et al., 2009) while performing a static standing trial similar to the one performed prior to motion capture. Due to the difficulties associated with visualizing all of the markers at one time during fluoroscopy, the femur and tibia were centered individually for each imaging trial. From these image sets, the 3D orientation of the markers relative to the underlying bones was defined. First, the fluoroscopic test environment was reproduced in the solid-modeling software. Then, the 3D joint model was imported into the environment and viewed from two orthogonal directions corresponding to the location of the image sources of each fluoroscope. Next, the position and orientation of the model were manipulated manually in six degrees-of-freedom (6DoF) until their projections, as viewed from the two orthogonal directions, matched the outlines on the fluoroscopic images (Fig. 2). Previous validation has shown that this method can measure 3D kinematics to within 0.1 mm and 0.3° (DeFrate et al., 2006; Caputo et al., 2009). Positions of the markers were calculated from the area centroids of each marker on the orthogonal image set. In this fashion, the relationship of the markers to the underlying bones and ACL attachment sites was quantified.
Fig. 2.
Biplanar fluoroscopy was used to register the position of the markers relative to the underlying bone. In this case, the positions of the tibial markers were related to the position of the tibia. A similar registration was performed for the femur.
To create the dynamic in vivo model, the marker data obtained from fluoroscopy was initially registered to the static standing trial data from the motion analysis via numerical optimization one bone at a time. Assuming that the array of points measured from biplanar fluoroscopy was fixed relative to each bone, this marker set was rigidly translated and rotated in 3D space until the distance to each corresponding marker, as measured from the motion analysis, during the standing trial was minimized as such:
| (1) |
where
| (2) |
where pi is the position of the ith marker in the fluoroscopic model, qi is the position of the ith marker during the motion analysis standing trial, R is a 3D rotation matrix composed from rotations o, a, and t about the x, y, and z axes, and δ is a translation vector consisting of three translations along the x, y, and z axes (δx, δy, δz). The stated initialization step registers the underlying bone and ACL attachment to the motion analysis based global coordinate system.
Next, similar minimizations using Eqs. (1) and (2) were performed to rigidly transform the standing trial marker positions to the positions of the markers at each time step during the dynamic trials. To further reduce error, all possible combinations of ten markers on the thigh (out of a total of 17) and seven markers (out of a total of 11) on the shank were minimized. These specific subset counts were selected for their balance of solution stability and computational efficiency. From all these solutions (19,448 combinations for the thigh), the subset that best minimized the objective function was selected for each time step. In this fashion, marker sets which deviated the least from rigid body motion were chosen to optimize the motion of the underlying bony structures. Other investigators have demonstrated that over-constrained marker sets and optimization techniques, such as the point cluster technique (Andriacchi et al., 1998; Alexander and Andriacchi, 2001; Ngai et al., 2009; Ngai and Wimmer, 2009), can be used to minimize error associated with the soft tissue motion.
The 3D models were then transformed according to the optimized kinematics in order to reproduce the motion of the subject’s knee during the dynamic jumping activity. From these models, the subject’s ACL length and knee flexion were measured as a function of time. Cylinders were fit to the long shafts of the tibia and femur so that flexion could be measured about an axis fixed through the transepicondylar line of the femur. ACL length was defined as the distance between the centroids of the femoral and tibial attachments of the ligament (Li et al., 2005). Because the true resting length of the ACL is difficult to quantify, ACL strain was measured from ACL length changes relative to a reference length as follows:
| (3) |
where ε is the relative ACL strain, L is ACL length, and Lo is a reference length (defined as the length captured during MR imaging, where the fibers appeared taut). Average flexion of the subjects’ knees during MR imaging was 16.6 ± 6.2° (mean and 95% confidence interval).
Easily repeatable biomechanical milestones were chosen to define the beginning and end of the movement cycle: toe-off from the platform (0%) and peak vertical ground reaction force (100%). All data were linearly interpolated to the nearest integer percent and averaged for five jump trials from each subject and then across all individuals.
2.1. Validation study
To validate the combined motion analysis and biplanar fluoroscopy methodology, measurements of ACL length using this approach were compared to those obtained from only a biplanar fluoroscopy technique used previously (Jordan et al., 2007; Abebe et al., 2010). Since our biplanar fluoroscopy system is not equipped for high speed imaging and has a limited field of view, we could not analyze dynamic jumping activities. Therefore, a quasi-static lunge was analyzed.
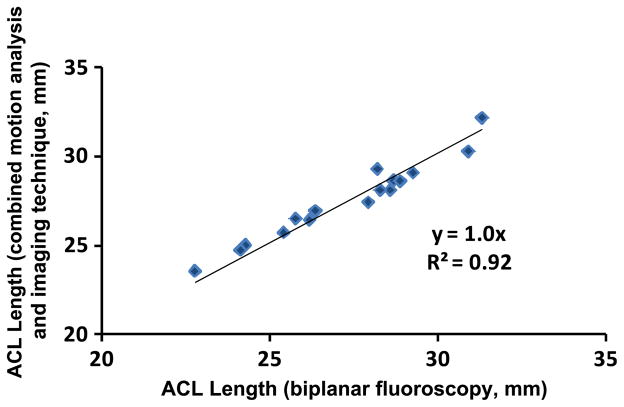
Four subjects first performed a quasi-static lunge from 0° to 45° of flexion in increments of 5° in the gait laboratory using the combined protocols outlined above. These data were used to generate a curve of length versus flexion as measured from the 3D models. Next, each subject performed a similar lunge under biplanar fluoroscopy only, and a similar graph was generated. These curves were compared at 0°, 15°, 30°, and 45° of knee flexion. From these points, a graph of ACL length as measured from the combined approach versus ACL length as measured from imaging techniques alone was produced (Fig. 3).
Fig. 3.
ACL length was measured in four subjects using the combined motion analysis and imaging method and compared to the length measured directly from biplanar fluoroscopy during a single-leg lunge. Linear regression indicated a high correlation coefficient and a slope of 1.0, indicating that the two methods resulted in similar measurements of ACL length. Root mean square error for all trials was 0.5 mm.
3. Results
3.1. Validation study
Linear regression demonstrated that the two techniques had excellent correlation, with a coefficient of determination of 0.92. Root mean square error between the measurements was 0.5 mm. These results indicate that the combined methodology accurately measures ACL deformation up to 45° of flexion during a quasi-static lunge.
3.2. Jump landing trials
The results of the jump landing trials are depicted in Fig. 4. Time of initial contact with the ground was defined as the incidence of a minimum 10 N vertical ground reaction force (GRF) (Ford et al., 2006). GRF observed in this experiment exhibited two peaks typical of a heel–toe contact sequence while landing from a jump (Kovacs et al., 1999).
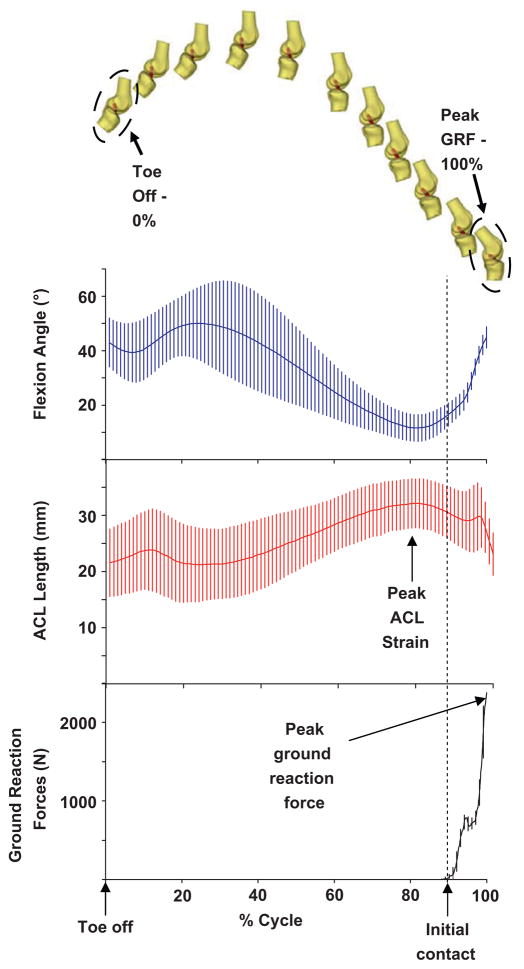
Fig. 4.
The marker data during jumping were used to position each subject’s knee model, as shown for one subject (top). From each knee model, the length of the ACL (mean and 95% confidence interval) and flexion angle were measured. Data were described as a function of percentage of movement cycle (bottom) from toe-off (0%) to peak ground reaction force (100%). During the jump landing, flexion decreased initially as the subjects pushed off the platform. Flexion increased as the knee was bent to position the feet in front of the subject, and then extended to prepare for landing. Just prior to impact, flexion increased in anticipation of landing. ACL length peaked approximately 55 ± 14 ms prior to initial contact in all subjects.
During the jump landing movement cycle, flexion initially increased as the knees were brought to the front of the body (analogous to swing phase in normal walking patterns) to an average of 48.9° ± 14.7° (mean and 95% confidence interval). Next, flexion decreased for 302 ± 51 ms to a minimum of 11.2° ± 3.5° and then increased in anticipation of landing prior to the impact with the ground. Approximately 4° of flexion was recovered in this period. After ground contact, the knee began to flex rapidly as the force of impact was dissipated.
In general, the length of the ACL during the jump landing decreased with increasing flexion angle. For example, as the legs were extended in anticipation of landing, the length of the ACL increased. Peak ACL length was observed 55 ± 14 ms before initial force plate contact, when flexion angles were at their lowest values. This point corresponded to an average relative ACL strain of 12 ± 7%. After ground contact, the ACL length initially spiked to a local maximum, but quickly decreased as the knee bent. The post impact local maximum in the ACL length demonstrated 5% less relative strain than the absolute maximum prior to impact.
4. Discussion
Intrinsic loading during non-contact events are the most prevalent cause of ACL injuries. Recently, much emphasis has been placed on identifying the underlying mechanisms of these injuries with the intent of developing prevention programs to curtail their rates of incidence. Prevention programs have shown mixed results (Hewett et al., 1999; Grandstrand et al., 2006; Pfeiffer et al., 2006), and a decrease in the injury rates has yet to be realized (Agel et al., 2005). This is most likely due to a gap in the literature on the relationship of in vivo joint kinematics and ACL strain. Biplanar fluoroscopic studies have provided invaluable data on joint kinematics during various in vivo activities (Bey et al., 2008; Caputo et al., 2009; Anderst and Tashman, 2010; Brainerd et al., 2010; Torry et al., 2010; Wu et al., 2010). However, high speed biplanar fluoroscopy may not be readily available and may be potentially limited by a small field of view (Benoit et al., 2007). To address limitations in sampling rate and field of view of our biplanar fluoroscopy system, we developed a non-invasive method using a combination of MR imaging, biplanar fluoroscopy, and traditional marker-based motion analysis capable of approximating in vivo ACL strain during dynamic physical tasks.
Previous studies on ACL injury mechanisms have attempted to explain the etiology by comparing high risk populations. By examining populations who are known to suffer greater incidence rates of ACL injury, such as females, many hypothesized that because they exhibit certain unique kinematic patterns when compared to males, these discrepancies may be the principal cause of their elevated injury risk (Boden et al., 2000; Huston et al., 2001; Malinzak et al., 2001; Chappell et al., 2002; Ford et al., 2003; Hewett et al., 2005a, 2005b; Yu et al., 2005; Brown et al., 2009; Nagano et al., 2009). For some, sagittal plane knee mechanics are perceived to be the primary cause as anterior shear of the tibia loads the ACL most directly (Markolf et al., 1995), and there are significant differences in knee flexion angles between genders as observed during jump landing (Huston et al., 2001; Malinzak et al., 2001; Chappell et al., 2007). Others have theorized knee coronal plane based injury mechanisms, citing videographic evidence of an observable valgus collapse pattern within a high number of ACL injuries captured during actual sporting events (Olsen et al., 2004; Krosshaug et al., 2007a, 2007b; Hewett et al., 2009; Quatman and Hewett, 2009). More recent reports of kinematic differences at the hip, ankle, and trunk between high and low risk populations (Griffin et al., 2000; McLean et al., 2005; Boden et al., 2009; Hewett et al., 2009) suggest that an injury mechanism may not be fully explained by differences at the knee. Given the number of intrinsic variables that can affect the in vivo mechanical environment, it is difficult to predict how complex, multi-planar loading conditions experienced during jumping and cutting motions load the ligament. The system developed in this study has the potential to address these questions.
In this study, eight male subjects performed a jumping task to simulate a basketball or soccer maneuver. Average peak relative ACL strain was 12%, which falls below the 15–19% ultimate strain threshold range reported by Butler et al. (Butler et al., 1986; Butler et al., 1992). It is interesting that peak ACL strain occurred 55 ± 14 ms prior to ground impact, when the knee was maximally extended. Using implantable strain gauges, one previous study found that ACL elongation increases prior to ground contact during a jumping task, with peak elongation occuring at the maximum knee extension (Lamontagne et al., 2008). This pattern might suggest a strong quadriceps contraction in anticipation of ground impact, as suggested in a study by Chappell et al. (2007), who described a surge in quadriceps EMG activity around 50 ms before landing during a stop-jump task. Others have shown similar preparatory increases in quadriceps activity during various cutting and jump landing activities (Zazulak et al., 2005; Landry et al., 2009; Ebben et al., 2010). Following peak extension/strain, the knee began to flex before impact by approximately 4°, signifying an increase in hamstring co-contraction due to neuromuscular programming. The observed delay in hamstring activity is also supported by the literature as others found comparable increases in hamstring EMG data towards the terminus of the pre-contact flight cycles (Chappell et al., 2007; Landry et al., 2009). Landry et al. (2009) suggested that knee stability is maximized at ground impact during a cutting maneuver by the preprogrammed neuromuscular firing of hamstring muscles 50–100 ms prior to impact. Again, this corresponds to the peak ACL strain and extension angle observed in this study 55 ms prior to impact.
Because the maximum strain happens before impact, we can hypothesize that the neuromuscular timing of this milestone is crucial to when injury occurs. A hypothetical injury scenario could be attributed to disruptions in the timing of this critical sequence of events. For example, if an athlete were perturbed while in midair, the predetermined neuromuscular programming to land safely would be subject to change. In the current study, we detected peak ACL strains 55 ms prior to impact, when flexion angles were their lowest. This is consistent with the finding that non-contact ACL injuries most commonly occur with the knee in less than 30° of flexion (Griffin et al., 2000; Cochrane et al., 2007). Moreover, videographic analyses of real time sports injuries have determined that a significant number of non-contact injuries are associated with a perturbation prior to contact with the ground (Olsen et al., 2004; Krosshaug et al., 2007a, 2007b; Boden et al., 2009), a time when we observed higher ACL lengths. Regardless, further investigation is needed to confirm this theoretical mechanism.
One limitation of the combined method is that it was unable to be validated dynamically. This was due to limitations of our fluoroscopic system, including a small field of view and inability to measure high speed motions. Thus, a quasi-static lunge was examined in lieu of a dynamic activity. A second limitation is that the presented technique was only validated to measure ACL deformations accurately up to 45° of flexion. However, because most injuries occur at flexion angles less than 30° (Chaudhari and Andriacchi, 2006; Griffin et al., 2006; Cochrane et al., 2007; Yu and Garrett, 2007; Renstrom et al., 2008), this method would still provide useful data on ACL strains and 6DoF knee motions in the range where the ACL is thought to be susceptible to injury. Finally, strain was approximated by normalizing ACL length to the reference length measured in a relaxed position during MR imaging, where the fibers of the ACL appeared taut. It is difficult to know precisely the reference length of the ACL in vivo since it cannot support axial compression. Although there is some uncertainty with knowing the unloaded length of the ACL in our study, this measurement of relative strain is linearly related to the true strain.
Although only kinematic data on flexion angles of the knee were included with this report, it is possible for future data sets to incorporate all 6DoF motions. A complete data series that couples ACL strain with 6DoF measurements opens up the possibility for reverse engineered explanations of injury mechanisms. Instead of assuming that a set of motions loads the ACL in an unfavorable manner, an account of specific 6DoF movements that most directly deform the ACL give a much better idea of what motions should be avoided. Such data would be paramount to better focusing and improving current prevention programs.
In conclusion, we have developed a new methodology to measure in vivo ACL strain during dynamic tasks. In this pilot study, eight male subjects performed a jumping activity similar to a basketball or soccer maneuver. The study showed that the peak ACL strain occurred 55 ± 14 ms prior to impact when ACL length was 12 ± 7% longer than an MRI based reference length. In the future, this system will be used to examine kinematic parameters that elevate ACL strain. These data will provide valuable information for developing prevention programs aimed at reducing the incidence of ACL injury.
Acknowledgments
This work was supported by the National Institutes of Health (R03AR055659) and a grant from the National Football League Charities. We also gratefully acknowledge the financial support of the Department of Orthopaedic Surgery and the Department of Radiology. We would also like to thank Farshid Guilak, Ph.D. and Mansoor Haider, Ph.D. for their helpful comments regarding this work and Elizabeth C. Pennington for technical support.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare with regards to this study.
References
- AAOS. ACL Injury: Does It Require Surgery? 2008 from < http://orthoinfo.aaos.org>.
- Abebe ES, Kim JP, Utturkar GM, Spritzer CE, Moorman CT, 3rd, Taylor DC, Garrett WE, Jr, DeFrate LE. In vivo graft deformation in transtibial and tibial tunnel independent ACL reconstruction techniques. Trans Orthop Res Soc 2010 [Google Scholar]
- Abebe ES, Moorman CT, 3rd, Dziedzic TS, Spritzer CE, Cothran RL, Taylor DC, Garrett WE, Jr, DeFrate LE. Femoral tunnel placement during anterior cruciate ligament reconstruction: an in vivo imaging analysis comparing transtibial and 2-incision tibial tunnel-independent techniques. Am J Sports Med. 2009;37 (10):1904–1911. doi: 10.1177/0363546509340768. [DOI] [PubMed] [Google Scholar]
- Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33 (4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- Alexander EJ, Andriacchi TP. Correcting for deformation in skin-based marker systems. J Biomech. 2001;34 (3):355–361. doi: 10.1016/s0021-9290(00)00192-5. [DOI] [PubMed] [Google Scholar]
- Anderst WJ, Tashman S. Using relative velocity vectors to reveal axial rotation about the medial and lateral compartment of the knee. J Biomech. 2010;43 (5):994–997. doi: 10.1016/j.jbiomech.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriacchi TP, Alexander EJ, Toney MK, Dyrby C, Sum J. A point cluster method for in vivo motion analysis: applied to a study of knee kinematics. J Biomech Eng. 1998;120 (6):743–749. doi: 10.1115/1.2834888. [DOI] [PubMed] [Google Scholar]
- Asano H, Muneta T, Shinomiya K. Evaluation of clinical factors affecting knee pain after anterior cruciate ligament reconstruction. J Knee Surg. 2002;15 (1):23–28. [PubMed] [Google Scholar]
- Barber-Westin SD, Noyes FR, Smith ST, Campbell TM. Reducing the risk of noncontact anterior cruciate ligament injuries in the female athlete. Phys Sportsmed. 2009;37 (3):49–61. doi: 10.3810/psm.2009.10.1729. [DOI] [PubMed] [Google Scholar]
- Benoit DL, Ramsey DK, Lamontagne M, Xu L, Wretenberg P, Renstrom P. In vivo knee kinematics during gait reveals new rotation profiles and smaller translations. Clin Orthop Relat Res. 2007;454:81–88. doi: 10.1097/BLO.0b013e31802dc4d0. [DOI] [PubMed] [Google Scholar]
- Bey MJ, Kline SK, Tashman S, Zauel R. Accuracy of biplane X-ray imaging combined with model-based tracking for measuring in-vivo patellofemoral joint motion. J Orthop Surg Res. 2008;3:38. doi: 10.1186/1749-799X-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31 (6):519–525. doi: 10.1016/s0021-9290(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE. Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med. 2005;33 (10):1579–1602. doi: 10.1177/0363546505279913. [DOI] [PubMed] [Google Scholar]
- Beynnon BD, Johnson RJ, Fleming BC, Stankewich CJ, Renstrom PA, Nichols CE. The strain behavior of the anterior cruciate ligament during squatting and active flexion–extension. A comparison of an open and a closed kinetic chain exercise. Am J Sports Med. 1997;25 (6):823–829. doi: 10.1177/036354659702500616. [DOI] [PubMed] [Google Scholar]
- Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23 (6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- Boden BP, Torg JS, Knowles SB, Hewett TE. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;37 (2):252–259. doi: 10.1177/0363546508328107. [DOI] [PubMed] [Google Scholar]
- Brainerd EL, Baier DB, Gatesy SM, Hedrick TL, Metzger KA, Gilbert SL, Crisco JJ. X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J Exp Zool A Ecol Genet Physiol. 2010;313 (5):262–279. doi: 10.1002/jez.589. [DOI] [PubMed] [Google Scholar]
- Brown TN, Palmieri-Smith RM, McLean SG. Differences between sexes and limbs in hip and knee kinematics and kinetics during anticipated and unanticipated jump landings: implications for ACL injury. Br J Sports Med. 2009 doi: 10.1136/bjsm.2008.055954. [DOI] [PubMed] [Google Scholar]
- Butler DL, Guan Y, Kay MD, Cummings JF, Feder SM, Levy MS. Location-dependent variations in the material properties of the anterior cruciate ligament. J Biomech. 1992;25 (5):511–518. doi: 10.1016/0021-9290(92)90091-e. [DOI] [PubMed] [Google Scholar]
- Butler DL, Kay MD, Stouffer DC. Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J Biomech. 1986;19 (6):425–432. doi: 10.1016/0021-9290(86)90019-9. [DOI] [PubMed] [Google Scholar]
- Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43 (5):366–370. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- Caputo AM, Lee JY, Spritzer CE, Easley ME, DeOrio JK, Nunley JA, 2nd, DeFrate LE. In vivo kinematics of the tibiotalar joint after lateral ankle instability. Am J Sports Med. 2009;37 (11):2241–2248. doi: 10.1177/0363546509337578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerulli G, Benoit DL, Lamontagne M, Caraffa A, Liti A. In vivo anterior cruciate ligament strain behaviour during a rapid deceleration movement: case report. Knee Surg Sports Traumatol Arthrosc. 2003;11 (5):307–311. doi: 10.1007/s00167-003-0403-6. [DOI] [PubMed] [Google Scholar]
- Chappell JD, Creighton RA, Giuliani C, Yu B, Garrett WE. Kinematics and electromyography of landing preparation in vertical stop-jump: risks for noncontact anterior cruciate ligament injury. Am J Sports Med. 2007;35 (2):235–241. doi: 10.1177/0363546506294077. [DOI] [PubMed] [Google Scholar]
- Chappell JD, Yu B, Kirkendall DT, Garrett WE. A comparison of knee kinetics between male and female recreational athletes in stop-jump tasks. Am J Sports Med. 2002;30 (2):261–267. doi: 10.1177/03635465020300021901. [DOI] [PubMed] [Google Scholar]
- Chaudhari AM, Andriacchi TP. The mechanical consequences of dynamic frontal plane limb alignment for non-contact ACL injury. J Biomech. 2006;39 (2):330–338. doi: 10.1016/j.jbiomech.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Cochrane JL, Lloyd DG, Buttfield A, Seward H, McGivern J. Characteristics of anterior cruciate ligament injuries in Australian football. J Sci Med Sport. 2007;10 (2):96–104. doi: 10.1016/j.jsams.2006.05.015. [DOI] [PubMed] [Google Scholar]
- DeFrate LE, Gill TJ, Li G. In vivo function of the posterior cruciate ligament during weight bearing knee flexion. Am J Sports Med. 2004;32 (8):1923–1928. doi: 10.1177/0363546504264896. [DOI] [PubMed] [Google Scholar]
- DeFrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34 (8):1240–1246. doi: 10.1177/0363546506287299. [DOI] [PubMed] [Google Scholar]
- DeMorat G, Weinhold P, Blackburn T, Chudik S, Garrett W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32 (2):477–483. doi: 10.1177/0363546503258928. [DOI] [PubMed] [Google Scholar]
- Draganich LF, Vahey JW. An in vitro study of anterior cruciate ligament strain induced by quadriceps and hamstrings forces. J Orthop Res. 1990;8 (1):57–63. doi: 10.1002/jor.1100080107. [DOI] [PubMed] [Google Scholar]
- Ebben WP, Fauth ML, Petushek EJ, Garceau LR, Hsu BE, Lutsch BN, Feldmann CR. Gender-based analysis of hamstring and quadriceps muscle activation during jump landings and cutting. J Strength Cond Res. 2010;24 (2):408–415. doi: 10.1519/JSC.0b013e3181c509f4. [DOI] [PubMed] [Google Scholar]
- Fairclough JA, Graham GP, Dent CM. Radiological sign of chronic anterior cruciate ligament deficiency. Injury. 1990;21 (6):401–402. doi: 10.1016/0020-1383(90)90130-m. [DOI] [PubMed] [Google Scholar]
- Fink C, Hoser C, Hackl W, Navarro RA, Benedetto KP. Long-term outcome of operative or nonoperative treatment of anterior cruciate ligament rupture—is sports activity a determining variable? Int. J Sports Med. 2001;22(4):304–309. doi: 10.1055/s-2001-13823. [DOI] [PubMed] [Google Scholar]
- Fithian DC, Paxton LW, Goltz DH. Fate of the anterior cruciate ligament-injured knee. Orthop Clin North Am. 2002;33 (4):621–636. v. doi: 10.1016/s0030-5898(02)00015-9. [DOI] [PubMed] [Google Scholar]
- Fleming BC, Beynnon BD. In vivo measurement of ligament/tendon strains and forces: a review. Ann Biomed Eng. 2004;32 (3):318–328. doi: 10.1023/b:abme.0000017542.75080.86. [DOI] [PubMed] [Google Scholar]
- Fleming BC, Beynnon BD, Renstrom PA, Johnson RJ, Nichols CE, Peura GD, Uh BS. The strain behavior of the anterior cruciate ligament during stair climbing: an in vivo study. Arthroscopy. 1999;15 (2):185–191. doi: 10.1053/ar.1999.v15.015018. [DOI] [PubMed] [Google Scholar]
- Fleming BC, Beynnon BD, Renstrom PA, Peura GD, Nichols CE, Johnson RJ. The strain behavior of the anterior cruciate ligament during bicycling. An in vivo study. Am J Sports Med. 1998;26 (1):109–118. doi: 10.1177/03635465980260010301. [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exercise. 2003;35 (10):1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Smith RL, Vianello RM, Seiwert SL, Hewett TE. A comparison of dynamic coronal plane excursion between matched male and female athletes when performing single leg landings. Clin Biomech (Bristol, Avon) 2006;21 (1):33–40. doi: 10.1016/j.clinbiomech.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2008 doi: 10.1016/j.jsams.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Gilchrist J, Mandelbaum BR, Melancon H, Ryan GW, Silvers HJ, Griffin LY, Watanabe DS, Dick RW, Dvorak J. A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. Am J Sports Med. 2008;36 (8):1476–1483. doi: 10.1177/0363546508318188. [DOI] [PubMed] [Google Scholar]
- Grandstrand SL, Pfeiffer RP, Sabick MB, DeBeliso M, Shea KG. The effects of a commercially available warm-up program on landing mechanics in female youth soccer players. J Strength Cond Res. 2006;20 (2):331–335. doi: 10.1519/R-17585.1. [DOI] [PubMed] [Google Scholar]
- Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, Garrick JG, Hewett TE, Huston L, Ireland ML, Johnson RJ, Kibler WB, Lephart S, Lewis JL, Lindenfeld TN, Mandelbaum BR, Marchak P, Teitz CC, Wojtys EM. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8 (3):141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, Dick RW, Engebretsen L, Garrett WE, Jr, Hannafin JA, Hewett TE, Huston LJ, Ireland ML, Johnson RJ, Lephart S, Mandelbaum BR, Mann BJ, Marks PH, Marshall SW, Myklebust G, Noyes FR, Powers C, Shields C, Jr, Shultz SJ, Silvers H, Slauterbeck J, Taylor DC, Teitz CC, Wojtys EM, Yu B. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34 (9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- Grossman MG, ElAttrache NS, Shields CL, Glousman RE. Revision anterior cruciate ligament reconstruction: three- to nine-year follow-up. Arthroscopy. 2005;21 (4):418–423. doi: 10.1016/j.arthro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Heidt RS, Jr, Sweeterman LM, Carlonas RL, Traub JA, Tekulve FX. Avoidance of soccer injuries with preseason conditioning. Am J Sports Med. 2000;28 (5):659–662. doi: 10.1177/03635465000280050601. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27 (6):699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR. Reducing knee and anterior cruciate ligament injuries among female athletes: a systematic review of neuromuscular training interventions. J Knee Surg. 2005a;18 (1):82–88. doi: 10.1055/s-0030-1248163. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR, Heidt RS, Jr, Colosimo AJ, McLean SG, van den Bogert AJ, Paterno MV, Succop P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005b;33 (4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact ACL injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009 doi: 10.1136/bjsm.2009.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52 (3):794–799. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- Huston LJ, Vibert B, Ashton-Miller JA, Wojtys EM. Gender differences in knee angle when landing from a drop-jump. Am J Knee Surg. 2001;14(4):215–219. discussion 219–20. [PubMed] [Google Scholar]
- Jordan SS, DeFrate LE, Nha KW, Papannagari R, Gill TJ, Li G. The in vivo kinematics of the anteromedial and posterolateral bundles of the anterior cruciate ligament during weightbearing knee flexion. Am J Sports Med. 2007;35 (4):547–554. doi: 10.1177/0363546506295941. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Woo SL, Ma CB, Zeminski J, Rudy TW, Li G, Livesay GA. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: a human cadaveric study using robotic technology. Arthroscopy. 2000;16 (6):633–639. doi: 10.1053/jars.2000.7682. [DOI] [PubMed] [Google Scholar]
- Kapreli E, Athanasopoulos S, Gliatis J, Papathanasiou M, Peeters R, Strimpakos N, Van Hecke P, Gouliamos A, Sunaert S. Anterior cruciate ligament deficiency causes brain plasticity: a functional MRI study. Am J Sports Med. 2009;37 (12):2419–2426. doi: 10.1177/0363546509343201. [DOI] [PubMed] [Google Scholar]
- Katayama M, Higuchi H, Kimura M, Kobayashi A, Hatayama K, Terauchi M, Takagishi K. Proprioception and performance after anterior cruciate ligament rupture. Int Orthop. 2004;28 (5):278–281. doi: 10.1007/s00264-004-0583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. Computer-assisted navigation and anterior cruciate ligament reconstruction: accuracy and outcomes. Orthopedics. 2005;28 (10 Suppl):s1283–s1287. doi: 10.3928/0147-7447-20051002-16. [DOI] [PubMed] [Google Scholar]
- Kovacs I, Tihanyi J, Devita P, Racz L, Barrier J, Hortobagyi T. Foot placement modifies kinematics and kinetics during drop jumping. Med Sci Sports Exercise. 1999;31 (5):708–716. doi: 10.1097/00005768-199905000-00014. [DOI] [PubMed] [Google Scholar]
- Krosshaug T, Nakamae A, Boden BP, Engebretsen L, Smith G, Slauterbeck JR, Hewett TE, Bahr R. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007a;35 (3):359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- Krosshaug T, Slauterbeck JR, Engebretsen L, Bahr R. Biomechanical analysis of anterior cruciate ligament injury mechanisms: three-dimensional motion reconstruction from video sequences. Scand J Med Sci Sports. 2007b;17 (5):508–519. doi: 10.1111/j.1600-0838.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- Lamontagne M, Benoit DL, Ramsey DK, Caraffa A, Cerulli G. In vivo biomechanical study for injury prevention. In: Hong Y, Bartlett R, editors. Routledge Handbook of Biomechanics and Human Movement Science. Routledge; London: 2008. pp. 428–445. [Google Scholar]
- Landry SC, McKean KA, Hubley-Kozey CL, Stanish WD, Deluzio KJ. Gender differences exist in neuromuscular control patterns during the pre-contact and early stance phase of an unanticipated side-cut and cross-cut maneuver in 15–18 years old adolescent soccer players. J Electromyogr Kinesiol. 2009;19 (5):e370–e379. doi: 10.1016/j.jelekin.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Li G, Defrate LE, Rubash HE, Gill TJ. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res. 2005;23 (2):340–344. doi: 10.1016/j.orthres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Li G, Rudy TW, Sakane M, Kanamori A, Ma CB, Woo SL. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32 (4):395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35 (10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50 (10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- Malinzak RA, Colby SM, Kirkendall DT, Yu B, Garrett WE. A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin Biomech (Bristol, Avon) 2001;16 (5):438–445. doi: 10.1016/s0268-0033(01)00019-5. [DOI] [PubMed] [Google Scholar]
- Mandelbaum BR, Silvers HJ, Watanabe DS, Knarr JF, Thomas SD, Griffin LY, Kirkendall DT, Garrett W., Jr Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33 (7):1003–1010. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- Maquirriain J, Megey PJ. Tennis specific limitations in players with an ACL deficient knee. Br J Sports Med. 2006;40 (5):451–453. doi: 10.1136/bjsm.2005.023390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13 (6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- Markolf KL, Gorek JF, Kabo JM, Shapiro MS. Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Joint Surg Am. 1990;72 (4):557–567. [PubMed] [Google Scholar]
- McLean SG, Walker KB, van den Bogert AJ. Effect of gender on lower extremity kinematics during rapid direction changes: an integrated analysis of three sports movements. J Sci Med Sport. 2005;8 (4):411–422. doi: 10.1016/s1440-2440(05)80056-8. [DOI] [PubMed] [Google Scholar]
- McNair PJ, Marshall RN, Matheson JA. Important features associated with acute anterior cruciate ligament injury. N Z Med J. 1990;103 (901):537–539. [PubMed] [Google Scholar]
- Miyasaka KC, Daniel DM, Stone ML. The incidence of knee ligament injuries in the general population. Am J Knee Surg. 1991;4:43–48. [Google Scholar]
- Myklebust G, Engebretsen L, Braekken IH, Skjolberg A, Olsen OE, Bahr R. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sport Med. 2003;13 (2):71–78. doi: 10.1097/00042752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Nagano Y, Ida H, Akai M, Fukubayashi T. Biomechanical characteristics of the knee joint in female athletes during tasks associated with anterior cruciate ligament injury. Knee. 2009;16 (2):153–158. doi: 10.1016/j.knee.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Ngai V, Schwenke T, Wimmer MA. In-vivo kinematics of knee prostheses patients during level walking compared with the ISO force-controlled simulator standard. Proc Inst Mech Eng H. 2009;223 (7):889–896. doi: 10.1243/09544070JEIM549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai V, Wimmer MA. Kinematic evaluation of cruciate-retaining total knee replacement patients during level walking: a comparison with the displacement-controlled ISO standard. J Biomech. 2009;42 (14):2363–2368. doi: 10.1016/j.jbiomech.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32 (4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- Onate JA, Guskiewicz KM, Marshall SW, Giuliani C, Yu B, Garrett WE. Instruction of jump-landing technique using videotape feedback: altering lower extremity motion patterns. Am J Sports Med. 2005;33 (6):831–842. doi: 10.1177/0363546504271499. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RP, Shea KG, Roberts D, Grandstrand S, Bond L. Lack of effect of a knee ligament injury prevention program on the incidence of noncontact anterior cruciate ligament injury. J Bone Joint Surg Am. 2006;88 (8):1769–1774. doi: 10.2106/JBJS.E.00616. [DOI] [PubMed] [Google Scholar]
- Quatman CE, Hewett TE. The anterior cruciate ligament injury controversy: is ”valgus collapse” a sex-specific mechanism? Br J Sports Med. 2009;43(5):328–335. doi: 10.1136/bjsm.2009.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen RM, Charnock BL, Garrett WE, Jr, Hardaker WM, Sims EL, Moorman CT., 3rd A comparison of cleat types during two football-specific tasks on FieldTurf. Br J Sports Med. 2008;42(4):278–284. doi: 10.1136/bjsm.2007.036517. discussion 284. [DOI] [PubMed] [Google Scholar]
- Queen RM, Gross MT, Liu HY. Repeatability of lower extremity kinetics and kinematics for standardized and self-selected running speeds. Gait Posture. 2006;23 (3):282–287. doi: 10.1016/j.gaitpost.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Renstrom P, Ljungqvist A, Arendt E, Beynnon B, Fukubayashi T, Garrett W, Georgoulis T, Hewett TE, Johnson R, Krosshaug T, Mandelbaum B, Micheli L, Myklebust G, Roos E, Roos H, Schamasch P, Shultz S, Werner S, Wojtys E, Engebretsen L. Non-contact ACL injuries in female athletes: an international olympic committee current concepts statement. Br J Sports Med. 2008;42 (6):394–412. doi: 10.1136/bjsm.2008.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3 (4):261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- Soderman K, Werner S, Pietila T, Engstrom B, Alfredson H. Balance board training: prevention of traumatic injuries of the lower extremities in female soccer players? A prospective randomized intervention study. Knee Surg Sports Traumatol Arthrosc. 2000;8 (6):356–363. doi: 10.1007/s001670000147. [DOI] [PubMed] [Google Scholar]
- Torry MR, Shelburne KB, Peterson DS, Giphart JE, Krong JP, Myers C, Steadman JR, Woo SL. Knee kinematic profiles during drop landings: a biplane fluoroscopy study. Med Sci Sports Exercise. 2010 doi: 10.1249/MSS.0b013e3181f1e491. [DOI] [PubMed] [Google Scholar]
- Weinhold PS, Stewart JD, Liu HY, Lin CF, Garrett WE, Yu B. The influence of gender-specific loading patterns of the stop-jump task on anterior cruciate ligament strain. Injury. 2007;38 (8):973–978. doi: 10.1016/j.injury.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Wolf RS, Lemak LJ. Revision anterior cruciate ligament reconstruction surgery. J South Orthop Assoc. 2002;11 (1):25–32. [PubMed] [Google Scholar]
- Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament–tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19 (3):217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- Wu JL, Hosseini A, Kozanek M, Gadikota HR, Gill TJt, Li G. Kinematics of the anterior cruciate ligament during gait. Am J Sports Med. 2010;38 (7):1475–1482. doi: 10.1177/0363546510364240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Garrett WE. Mechanisms of non-contact ACL injuries. Br J Sports Med. 2007;41 (Suppl 1):i47–i51. doi: 10.1136/bjsm.2007.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, McClure SB, Onate JA, Guskiewicz KM, Kirkendall DT, Garrett WE. Age and gender effects on lower extremity kinematics of youth soccer players in a stop-jump task. Am J Sports Med. 2005;33 (9):1356–1364. doi: 10.1177/0363546504273049. [DOI] [PubMed] [Google Scholar]
- Zazulak BT, Ponce PL, Straub SJ, Medvecky MJ, Avedisian L, Hewett TE. Gender comparison of hip muscle activity during single-leg landing. J Orthop Sports Phys Ther. 2005;35 (5):292–299. doi: 10.2519/jospt.2005.35.5.292. [DOI] [PubMed] [Google Scholar]
- van Lent ME, Drost MR, vd Wildenberg FA. EMG profiles of ACL-deficient patients during walking: the influence of mild fatigue. Int J Sports Med. 1994;15 (8):508–514. doi: 10.1055/s-2007-1021096. [DOI] [PubMed] [Google Scholar]
- von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63 (3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]