Abstract
Rationale
Endothelial cells (ECs) have distinct mechanotransduction mechanisms responding to laminar versus disturbed flow patterns. Endothelial dysfunction, affected by imposed flow, is one of the earliest events leading to atherogenesis. The involvement of γ/δ T lymphocytes in endothelial dysfunction under flow is largely unknown.
Objective
To investigate whether shear stress regulates membrane translocation of ATP synthase β chain (ATPSβ) in ECs, leading to the increased γ/δ T-lymphocyte adhesion and the related functions.
Method and Results
We applied different flow patterns to cultured ECs. Laminar flow decreased the level of membrane-bound ATPSβ (ecto-ATPSβ) and depleted membrane cholesterol, whereas oscillatory flow increased the level of ecto-ATPSβ and membrane cholesterol. Incubating ECs with cholesterol or depleting cellular cholesterol with β-cyclodextrin mimicked the effect of oscillatory or laminar flow, respectively. Knockdown caveolin-1 by siRNA prevented ATPSβ translocation in response to laminar flow. Importantly, oscillatory flow or cholesterol treatment elevated the number of γ/δ T cells binding to ECs, which was blocked by anti-ATPSβ antibody. Furthermore, the incubation of γ/δ T cells with ECs increased TNFα and IFNγ secretion from T cells and VCAM-1 expression in ECs. In vivo, γ/δ T-cell adhesion and ATPSβ membrane translocation was elevated in the aortic inner curvature and disturbed flow areas in partially ligated carotid arteries of ApoE−/− mice fed a high-fat diet.
Conclusion
This study provides evidence that disturbed flow and hypercholesterolemia synergistically promote γ/δ T-lymphocyte activation by the membrane translocation of ATPSβ in ECs and in vivo in mice, which is a novel mechanism of endothelial activation.
Keywords: Endothelial dysfunction, T lymphocyte, Blood flow, Mechanotransduction, ATP synthase
Introduction
Fluid shear stress plays a pivotal role in vascular physiology and pathophysiology.1,2 Laminar flow imposed on the straight parts of the arterial tree enhances vascular tone, inhibits cell proliferation and thrombosis, and augments anti-inflammatory effects. In contrast, disturbed flow patterns, such as oscillatory flow, at bifurcations and curvatures predispose the endothelium to become atheroprone.3 Thus, local flow patterns in combination with other risk factors such as hyperlipidemia and vascular inflammation, result in the focal nature of atherosclerosis. This thesis is supported by the observation that atheroprone flow enhances lesion development in atherosclerosis-susceptible regions (e.g., aortic root and inner curvature of the aortic arch) of apolipoprotein E knockout (ApoE−/−) mice. However, atheroprotective flow spares atherogenesis in the straight part of vessels (e.g., thoracic aorta of ApoE−/− mice).
Located at the mitochondrial inner membrane, F1,FO-ATP synthase (F1Fo) produces ATP via the proton gradient generated by the respiratory chain. As the key subunit in F1Fo for ATP production, F1 consists of a trimeric αβ hetrodimer (αβ)3 around a central stick-like γ chain. Although engaged in the F1-ATPase catalysis, ATP synthase β chain (ATPSβ) is also located on the surface of the plasma membrane.4 This mitochondrial-dissociated ATPSβ, ecto-ATPSβ, is present in many cell types, including vascular endothelial cells (ECs). Various extracellular ligands can bind to ecto-ATPSβ. In hepatocytes, surface ATPSβ serves as a receptor for ApoA-I or ApoE-enriched high-density lipoprotein.5–7 In ECs, ecto-ATPSβ binds to angiostatin, which suggests that the membrane-bound ATPS is involved in angiogenesis.8–10 Our previous study demonstrated that ECs incubated with cholesterol induced the translocation of ATPSβ from mitochondria to membrane caveolae,11 which suggests that ecto-ATPSβ may be involved in cholesterol efflux from the vessel wall. Interestingly, ecto-ATPSβ in tumor cells could be recognized by γ/δ T lymphocytes, possibly through the antigen receptor, T-cell receptor (TCR), in T cells.12
Although endothelial dysfunction is one of the earliest vascular events leading to atherogenesis,13,14 macrophages and T lymphocytes are the two major hematopoietic cell types infiltrating the vessel wall during atherogenesis. γ/δ T cells, named after their expression of TCR, represent ~5% of the T-cell population. Although the α/β subset represents the major T cells seen in atherosclerotic plaque, γ/δ T cells are also present in lesions, with a high percentage (10%–15%) of infiltrated T cells in the early stage of atherogenesis.15 Galea et al. reported that γ/δ T cells could bind to and migrate through ECs,16 and Dyugovskaya et al. found that after binding to ECs, the activated γ/δ T cells release various pro-inflammatory cytokines, including tumor necrosis factor α (TNFα).17
Because the involvement of γ/δ T cells in endothelial dysfunction is largely unknown, we investigated the regulation of ATPSβ translocation in ECs under laminar versus oscillatory flow and the consequent effect on the interaction with γ/δ T cells. Compared with static controls, laminar flow decreased the membrane translocation of ATPSβ in ECs, which reduced γ/δ T-cell adhesion. In contrast, oscillatory flow increased the level of ecto-ATPSβ and enhanced the interaction with γ/δ T cells, which initiated endothelial activation.
Methods and Materials
Reagents, EC culture and treatment
Human umbilical vein ECs (HUVECs) were isolated and cultured as previously described.18 This investigation conforms to the principles outlined in the Declaration of Helsinki for use of human tissue. All the cells used were prior to passage 5. Bovine aortic ECs (BAECs) were purchased from Cell Application, Inc. (San Diego, CA) and cultured. The flow experiments were performed as previously described.19 The applied laminar flow was steady shear stress of 12 dyne/cm2. The oscillatory flow generated by an oscillator was shear stress of 0.5±4 dyne/cm2 with a frequency of 1 Hz.20 The detail cell culture and treatment were described in online supplement methods.
Purification of lipid raft protein and Western blot analysis
Whole cell lysates, membrane protein, and mitochondrial protein were isolated from ECs by a multiple-centrifugation procedure.21 Lipid raft fractions were purified from ECs by a modified detergent-free procedure.22 Western blot analysis was performed accordingly.
RNA interference
The caveolin-1 (Cav-1) siRNA sequence was 5'-CCA GAA GGA ACA CAC AGU U-dTdT-3' corresponding to bases 223–241 of the bovine caveolin-1 mRNA.23
Isolation of γ/δ T lymphocytes, cell adhesion and determination the level of cytokines
Human peripheral monocytes were obtained from healthy volunteers and isolated on Ficoll-Hypaque density gradient centrifugation. γ/δ T cells were separated from the isolated monocytes by magnet separation,24 then labeled magnetically with a hapten-modified anti-TCR γ/δ antibody and fluorescein isothiocyanate-conjugated anti-hapten microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). In cell adhesion assay, confluent HUVECs in 96-well plates were treated with cholesterol, β-cyclodextrin (βCD), or subjected to the specified flow patterns for 2 hr. Purified human γ/δ T cells (2 × 105 cells/well) were labeled with fluorescence dye (BCECF, Invitrogen), then coincubated with HUVECs for 30 min. Human TNFα and IFNγ were measured in media by use of sandwich ELISA kits.
en face immunostaining of mouse aorta and animal experiment
HUVECs were coincubated with purified human γ/δ T cells for 24 hr, then stained with rabbit anti-VCAM-1 antibody and goat anti-rabbit Rhodamine red-conjugated secondary antibody (Jackson Immunoresearch Lab, West Grove, PA). Eight-week-old ApoE−/− and C57BL/6 male mice were obtained from the Peking University Health Science Center. Mice were fed a high-fat diet or a chow diet for 1 week as indicated. The aortic arch and thoracic aorta were fixed and excised for determination of ATPSβ, Cav-1, TCRγ, TCRα, and Mac3 levels in the intima by en face immunostaining as previously reported25. Partial ligation of the left carotid artery (LCA) was carried out as previously described26 with minor modification. The detail methods were described in online supplement methods.
Statistical Analyses
Results are expressed as mean±SEM from at least 3 independent experiments. Statistical analysis involved the 2-tailed Student's t test, one-way ANOVA and Dunnett's multiple comparison test. A P<0.05 was considered statistically significant.
Results
Shear stress causes ATPSβ translocation
Steady laminar flow or high shear stress (5–20 dyn/cm2) is proposed to be anti-atherosclerotic and disturbed flow with low mean shear stress (<5 dyn/cm2) atheroprone. We investigated first whether different flow patterns could affect ATPSβ translocation between the plasma membrane and the mitochondria in cultured ECs. Under laminar flow (12 dyne/cm2), the ATPSβ level was decreased in plasma membrane but increased in mitochondria at 30 min and was maintained for 2 hr (Fig. 1A). Therefore, laminar flow might induce the translocation of ATPSβ from the plasma membrane to mitochondria. Under oscillatory flow (0.5±4 dyne/cm2), the ATPSβ level was increased in plasma membrane but decreased in mitochondria at 30 min (Fig. 1B). To examine the temporal effect of different flow patterns on ATPSβ redistribution, the flow exposure time was extended to 24 hr. Laminar flow caused a transient reduction of ATPSβ in the membrane fraction (Fig. 1C). However, oscillatory flow induced sustained membrane localization (Fig.1D). These effects were not due to the upregulation of ATPSβ because the level of total ATPSβ was not changed by laminar or oscillatory flow.
Figure 1. Flow induces ATPSβ translocation between plasma membrane and mitochondria in ECs.
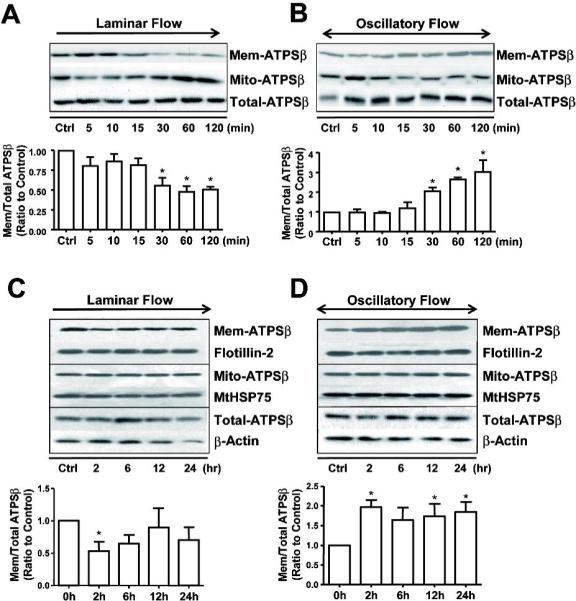
(A,B) Confluent monolayers of BAECs were subjected to laminar flow (12 dyne/cm2) or oscillatory flow (0.5±4 dyne/cm2) up to 2 hr, or kept as static controls. (C,D) The experimental conditions were the same as in A,B except the flow duration was up to 24 hr. Cells were lysed into 3 fractions (membrane, mitochondria and whole-cell lysates), and then ATPSβ was detected by western blotting. Flotillin-2 and MtHSP75 were also detected as markers for membrane or mitochondria fractions, respectively. Graph shows the ratio of membrane ATPSβ to total ATPSβ. The ratios of the static control were set to 1. Data were from 3 independent experiments. *P<0.05.
ATPSβ translocation is induced by alteration of membrane cholesterol content
Because cholesterol plays an important role in protein localization and membrane fluidity, we determined the cholesterol content in the EC plasma membrane subjected to different flow patterns. The level of membrane cholesterol decreased after exposure to laminar flow (Fig. 2A). This effect of laminar flow was similar to treatment with βCD, an agent depleting membrane cholesterol. In contrast, oscillatory flow increased the level of membrane cholesterol as early as 30 min, an effect mimicked by cholesterol treatment. We then stimulated ECs with βCD or cholesterol and found that βCD decreased but cholesterol increased the level of ATPSβ in the plasma membrane. Reciprocally, βCD augmented and cholesterol decreased the content of ATPSβ in mitochondria (Fig. 2B). To investigate further the effects of membrane cholesterol on the flow-induced ATPSβ translocation, we pretreated ECs with βCD or cholesterol before exposure to flow. As shown in Fig. 2C, cholesterol and βCD could block the effects of laminar and oscillatory flow, respectively, on ATPSβ translocation. Therefore, the cholesterol content in ECs changed by the applied flow patterns may cause the translocation of ATPSβ between plasma membrane and mitochondria.
Figure 2. Endothelial membrane cholesterol plays an important role in flow-induced ATPSβ translocation.
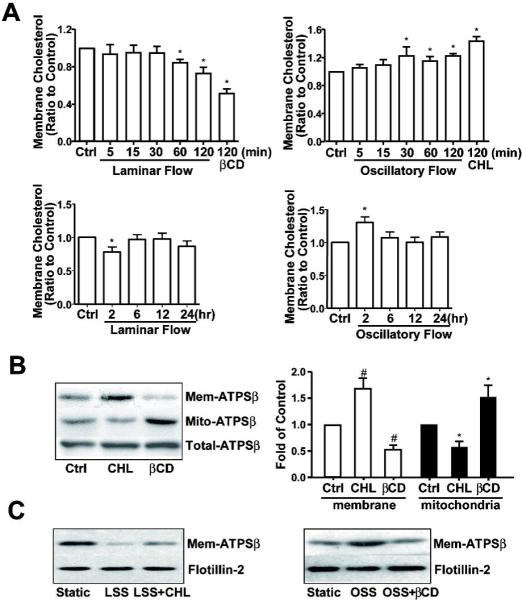
(A) BAECs were subjected to laminar flow or oscillatory flow for different times. βCD (5 mM, 2 hr) or cholesterol (CHL, 30 μg/ml, 2 hr) was applied as positive controls. The level of membrane cholesterol was determined with the same amount of protein, which was then normalized to that in the static control, set as 1. Data represent the mean±SEM from 3 independent experiments. * P<0.05 versus control; (B) BAECs were stimulated with CHL (30 μg/ml, 2 hr) or βCD (5 mM, 2 hr). Proteins from membrane or mitochondrial fractions and whole-cell lysates were isolated, and ATPSβ was detected by western blot analysis. Graph shows the ratio of membrane or mitochondrial ATPSβ to total ATPSβ, with that of static controls set as 1. The data are averaged from 3 independent experiments. # P<0.05 versus membrane control; *P<0.05 versus mitochondrion control. (C) In the presence of cholesterol or βCD, BAECs were subjected to laminar flow or oscillatory flow. Proteins from membrane fraction were isolated, and ATPSβ was detected by western blot analysis. Data were representative from 3 independent experiments.
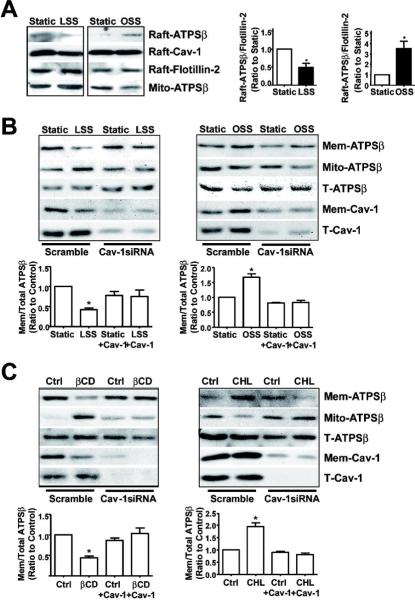
Translocation of ATPSβ depends on Cav-1
Ecto-ATPSβ is present in endothelial caveolae, and our previous study demonstrated that ecto-ATPSβ was associated with Cav-1 in caveolae after cholesterol loading.11 Because cholesterol loading mimicked oscillatory flow in driving ATPSβ translocation, we investigated the role of Cav-1 in this translocation in ECs responding to laminar versus oscillatory flow. As shown in Fig. 3A, laminar flow induced the migration of both ATPSβ and Cav-1 from lipid rafts. However, oscillatory flow caused an opposite effect and drove ATPSβ and Cav-1 into lipid rafts. To further delineate the role of Cav-1 in ecto-ATPSβ migration, we knocked down Cav-1 by siRNA and blocked the laminar flow-induced ATPSβ translocation (Fig. 3B). Moreover, βCD or cholesterol-induced translocation of ATPSβ in ECs was inhibited by Cav-1 siRNA (Fig. 3C).
Figure 3. ATPSβ translocation depends on caveolin-1.
(A) BAECs were subjected to laminar flow or oscillatory flow for 2 hr. Sucrose gradient ultracentrifugation was used for isolating lipid rafts (fractions 4–5) and mitochondrial fractions (fractions 8–10). Lipid raft and mitochondrial fractions were examined by western blot analysis with anti-ATPSβ, anti-Cav-1, and anti-Flotillin-2 antibodies. The images are representative of 3 independent experiments. (B,C) BAECs were transfected with scramble or Cav-1 siRNA for 48 hr, then subjected to different flow patterns, βCD, or CHL for 2 hr. The level of ATPSβ and Cav-1 was detected by western blot analysis. Graph shows the ratio of membrane ATPSβ to total ATPSβ. Data were from 3 independent experiments with static control set as 1. *P<0.05. T: total; Raft: lipid raft; Mem: membrane; Mito: mitochondria.
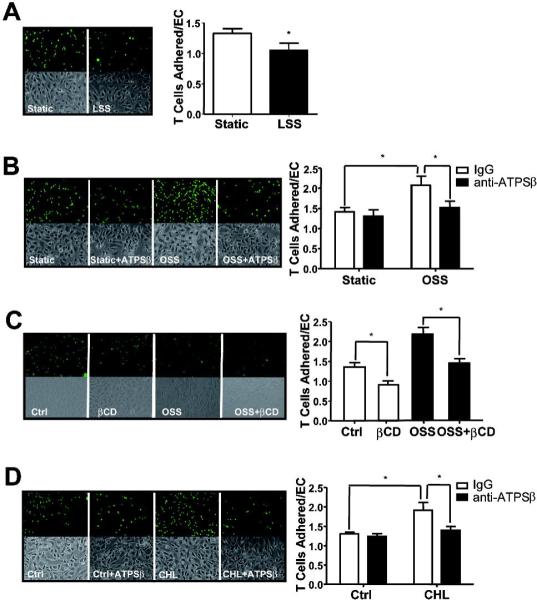
Ecto-ATPSβ affects the adhesion of γ/δ T lymphocytes to ECs
Given that ecto-ATPSβ binds to the TCR of γ/δ T cells,4 we compared the adhesion of human γ/δ T cells to HUVECs exposed to laminar or oscillatory flow. Compared with static controls, laminar flow significantly decreased but oscillatory flow greatly increased the adhesion of γ/δ T cells (Fig. 4 A,B). To test whether the oscillatory flow-enhanced γ/δ T-cell attachment was due, at least in part, to an increase in ecto-ATPSβ level, we pretreated HUVECs with a blocking antibody against ATPSβ. As expected, the blockade of ecto-ATPSβ abolished the γ/δ T-cell attachment imposed by oscillatory flow (Fig. 4B).
Figure 4. γ/δ T-cell adhesion to ECs is mediated through endothelial ecto-ATPSβ.
HUVECs were subjected to (A) laminar flow (LSS), (B) oscillatory flow (OSS), (C) βCD for 1 hr, or (D) CHL treatment for 2 hr. ECs were then incubated with BCECF-labeled γ/δ T cells for 30 min. In the blocking experiment, HUVECs were pre-treated with anti-ATPSβ antibody (50 μg/ml) for 30 min before γ/δ T-cell incubation. Adherent T cells were counted using fluorescence microscopy. The representative result on the left shows the attached cells (green) and phase-contrast images. The graphs on the right show the number of bound lymphocytes per EC. The results represent the mean±SEM from 3 independent experiments. *P<0.05.
Because cholesterol depletion or enrichment with βCD or cholesterol treatment caused similar effects as those by laminar or oscillatory flow, respectively, on ATPSβ translocation, we investigated the adhesion of γ/δ T cells to HUVECs treated with βCD or cholesterol. Indeed, γ/δ T lymphocytes decreased their adhesion to the βCD-treated ECs as compared with untreated controls (Fig. 4C). However, the γ/δ T-cell adhesion increased on pretreatment with cholesterol (Fig. 4D). Similar to oscillatory flow, anti-ATPSβ antibody treatment abolished the cholesterol-enhanced γ/δ T-cell adhesion (Fig. 4D). Therefore, laminar and oscillatory flow had opposite effects on the adhesion of γ/δ T cells, which was mainly due to the level of ecto-ATPSβ in the EC membrane.
Binding of ecto-ATPSβ and γ/δ TCR activated both endothelium and T lymphocytes
When the TCR of lymphocytes binds to its ligands present in the target cells, the cognate cells are activated. Upon binding to ecto-ATPSβ, the activated γ/δ T cells release various pro-inflammatory cytokines, including IL-8, IFNγ and TNFα,17 which are hallmarks of γ/δ T-cell activation. Consistent with results from our adhesion assay, incubation of ECs with cholesterol increased the levels of TNFα and IFNγ in the media (Fig. 5A). When ECs were co-treated with antibody against ATPSβ and cholesterol, TNFα secretion was prevented (Fig. 5B).
Figure 5. γ/δ T lymphocytes and ECs were activated by their interaction.
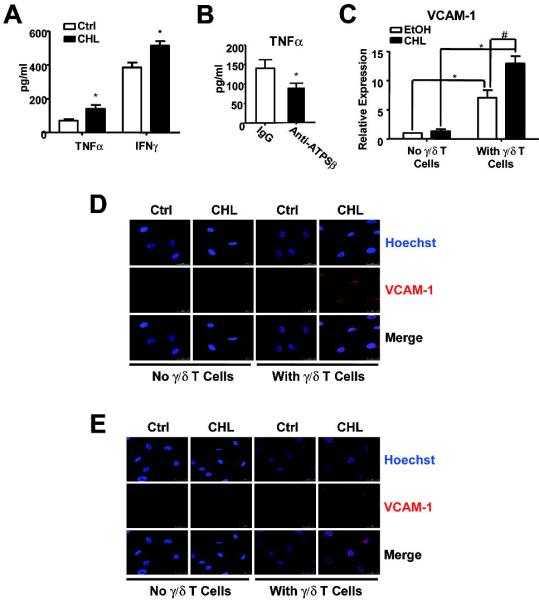
(A) HUVECs were pretreated with cholesterol for 2 hr and then coincubated with γ/δ T cells for 24 hr. (B) HUVECs were pre-treated with cholesterol for 2 hr and then coincubated with γ/δ T cells in the presence of IgG or anti-ATPSβ antibody (50 μg/ml) for 24 hr. Supernatant was collected, and the levels of TNFα and IFNγ were determined by ELISA. Graphs show amount of TNFα or IFNγ secreted to the media with T cells under different conditions. *P<0.05 versus controls. (C,D) HUVECs were pretreated with ethanol or cholesterol for 2 hr, and then incubated with or without γ/δ T cells for 24 hr. The VCAM-1 expression in ECs was analyzed by real-time PCR (C) and immunostaining (D). (E) The collected co-cultured medium was incubated with new HUVECs for 24 hr, VCAM-1 expression was analyzed by immunostaining with anti-VCAM-1 antibody and Hoechst 33258. Pseudo color images show the merge of VCAM-1 (red) and nucleus (blue). Results are representative from 3 independent experiments.
TNFα released from activated γ/δ T cells can induce an inflammatory response of ECs such as increased expression of VCAM-1.27,28 Thus, we detected VCAM-1 expression in ECs incubated with γ/δ T cells. Both real-time PCR and immunofluorescence experiments showed VCAM-1 expression enhanced by cholesterol pretreatment combined with the addition of γ/δ T cells (Fig. 5 C,D). However, VCAM-1 expression did not increase in ECs treated with cholesterol alone (Fig. 5C). This effect of VCAM-1 upregulation in ECs was confirmed by using conditioned media collected from the co-culture of γ/δ T cells and cholesterol-pretreated ECs (Fig. 5E).
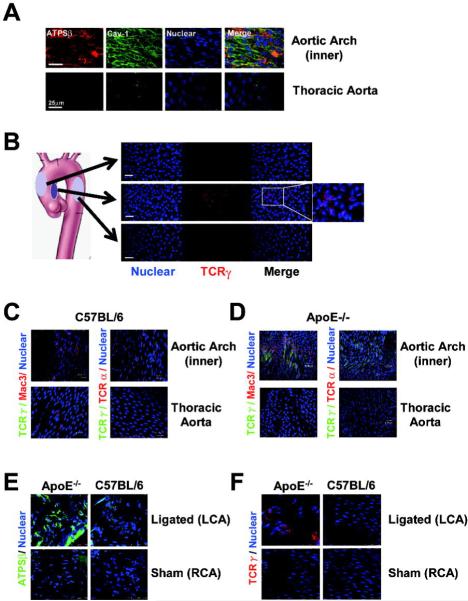
Disturbed flow induced γ/δ T cells attaching to the vascular wall in vivo
To confirm in vivo the findings obtained from in vitro experiments, we investigated the distribution of ATPSβ and γ/δ T cells in mouse aorta with hyperlipidemia. At the inner curvature of the aortic arch, blood flow patterns are disturbed and the flows on the outer curvature and thoracic aorta are relatively laminar.3,29 To induce hyperlipidemia, 8-week-old male ApoE−/− mice were fed a high-fat diet for 1 week, then aortas were isolated for en face immunostaining for ATPSβ and Cav-1. Confocal microscopy showed that ATPSβ and Cav-1 expression on the EC membrane was enhanced in the curvature of the aortic arch, when compared with the thoracic aorta (Fig. 6A). As a functional consequence of the ATPSβ surface expression, TCRγ-positive cells were increased in the inner curvature of the aortic arch, which indicates an increase in the adhesion of γ/δ T lymphocytes to the vessel wall, not seen in the outer curvature or thoracic aorta (Fig. 6B).
Figure 6. Disturbed flow enhanced endothelial ATPSβ membrane translocation and γ/δ T cells binding to the aortic endothelium in vivo.
Following partial ligation of carotid arteries, 8-week-old male ApoE−/− mice (n=6) were fed a high-fat diet and C57BL/6 mice a normal diet for 1 week. The aortic arch, thoracic aorta and left (partially ligated) and right (sham operation) carotid arteries were isolated for en face immunofluorescence analysis. (A) Representative images of ATPSβ (red), Cav-1 (green), and nucleus (blue) from different aortic regions of ApoE−/− mice. (B) Representative images of TCRγ (red) and nucleus (blue) in different regions of aorta of ApoE−/− mice. (C,D) Representative images of TCRγ (green), Mac3 (red, upper panel), and TCRα (red, lower panel) with their nuclei in blue from distinct aortic segments of C57BL/6 and ApoE−/− mice. (E, F) Representative images of ATPSβ (green), TCRγ (red) and nucleus (blue) from different ligated and sham-treated carotid arteries of ApoE−/− and C57BL/6 mice.
Given that macrophages and conventional α/β T lymphocytes are associated with endothelial activation and atherogenesis, the adhesion of Mac3- or TCRα-positive cells on the surface of endothelium of mouse aorta was assayed in C57BL/6 and ApoE−/− mice. In C57BL/6 mice, we could not detect any T-cell subpopulation adhered to the thoracic aorta. However, a weak staining of Mac3- and TCRγ-positive cells was found in the inner curvature of the aortic arch (Fig. 6C). Compared with control mice, ApoE−/− mice fed a high-fat diet showed significantly increased adhesion of Mac3- or TCRγ-positive cells but not TCRα-positive cells in the inner curvature of the aortic arch (Fig. 6D). To further investigate the effect of disturbed flow on the adhesion of γ/δ T cells to endothelium, we used an animal model of changed arterial shear stress and accelerated atherogenesis after partial ligation of carotid arteries of ApoE−/− mice followed by a 1-week high-fat diet. Reducing shear stress by partial ligation markedly promoted ecto-ATPSβ expression in endothelium and γ/δ T-lymphocyte adhesion in ApoE−/− mice fed a high fat diet (Fig. 6E,F). In control C57BL/6 mice, partial ligation led to a moderate increase in ecto-ATPSβ expression (Fig. 6E) but not γ/δ T-lymphocyte adhesion on the endothelium (Fig. 6F).
Discussion
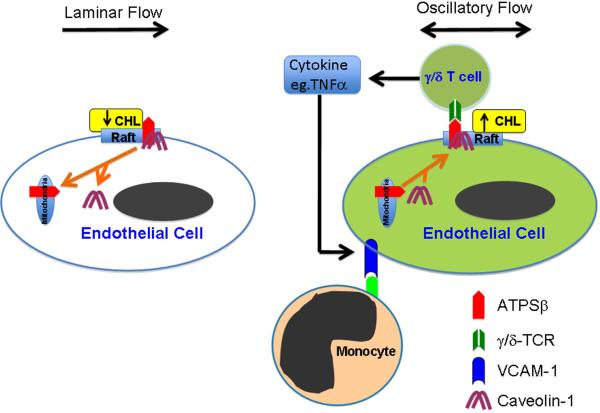
The focal nature of atherosclerotic lesions is largely due to the distinct effects of local flow patterns predisposing other atherogenic events. Steady laminar flow is atheroprotective by counteracting hyperlipidemia and inflammation, but disturbed flow is atheroprone and aggravates these pathological factors. In this study, steady laminar flow reduced ecto-ATPSβ adhesion on the EC membrane in vitro and in vivo, which resulted from the depletion of membrane cholesterol. In contrast, oscillatory flow enhanced the localization of ecto-ATPSβ at the EC membrane. We showed that Cav-1 plays a central role in determining the ecto-ATPSβ distribution. The functional consequence of increased level of ecto-ATPSβ on the EC membrane was the enhanced adhesion of γ/δ T lymphocytes to ECs, which in turn induced the release of cytokines, including IFNγ and TNFα, by the attached γ/δ T cells and hence elevated the expression of VCAM-1 in ECs. This model of action is presented in Figure 7.
Figure 7. A proposed model of ATPSβ translocation in response to different flow patterns.
Laminar flow causes the translocation of ATPSβ/Cav-1 from membrane lipid rafts to mitochondria, which depends on membrane cholesterol level. Oscillatory flow increases membrane cholesterol, leading to ATPSβ translocation from mitochondria to membrane rafts, together with Cav-1. Oscillatory flow increases membrane ATPSβ, which can interact with TCR of γ/δ T cells, leading to the release of cytokines (e.g., TNFα) by the activated T cells. The secreted cytokines further enhance the VCAM-1 expression in ECs, which causes the adhesion of monocytes.
In response to oscillatory flow, the increase in ATPSβ bound to the membrane lasted for 24 hr (Fig. 1). Thus, the effect of oscillatory flow on ATPSβ translocation was sustained. This notion is consistent with results from en face staining demonstrating a higher level of ecto-ATPSβ in ECs under disturbed flow than under laminar flow (Fig. 6). Yamamoto et al. showed an increase in ecto-ATPSβ level that led to ATP release within minutes after the onset of a step flow administered to ECs.30 This experimental condition could be viewed as the EC response to a rapid change in flow environment. In contrast, results seen in Fig. 1 and 6 represent the regulation of ATPSβ by physiological or pathophysiological flow conditions.
Our previous work demonstrated that cholesterol incubation enhanced ecto-ATPSβ translocation in ECs. The involved mechanism was cholesterol increasing the lipid raft content, which drove the migration of a complex of ATPSβ and Cav-1 from mitochondria to lipid rafts.11 These effects could be blocked by cytochalasin B, which suggests that this process depends on the actin-based cytoskeleton.11 The effect of laminar versus oscillatory flow in determining the localization of ATPSβ was similar to that with cholesterol or βCD incubation. Thus, we hypothesize that the flow-induced ecto-ATPSβ translocation depends on cholesterol content. βCD and cholesterol blocked the distinct effects of oscillatory and laminar flows on ATPSβ translocation. Furthermore, Cav-1 knockdown blocked both flow- and cholesterol-induced ATPSβ translocation. Thus, the intracellular ATPSβ translocation would be highly associated with lipid rafts (e.g., caveolae) and Cav-1 protein level. However, we found that the distinct effects of different flows on membrane cholesterol content were greater in short-term experiments (Fig. 2A) as on ecto-ATPSβ translocation (Fig. 1). The intracellular cholesterol homeostasis may be exquisitely regulated and depends on the balance between cholesterol synthesis and influx, cholesterol ester formation, and translocation to the plasma membrane for efflux. In flow channel experiments, ECs are adapted to the change of membrane cholesterol to maintain homeostasis after prolonged exposure of the applied shear stress. Therefore, the dynamic change of membrane translocation of cholesterol seems more important in initiating the cascade of ATPSβ translocation. More importantly, our in vivo experiments showed that disturbed flow patterns promoted ecto-ATPSβ expression in endothelium, with increased adhesion of γ/δ T-lymphocytes. This result also suggests that the change in membrane cholesterol content by oscillatory flow promotes ATPSβ translocation. When synergistic with hypercholesterolemia, this atheroprone effect leads to dysfunctional endothelium.
In addition to many inflammatory cells such as macrophages and α/β T cells, lymphocytes bearing γ/δ TCR are also involved in atherogenesis.15 Although γ/δ T cells represent a small portion of CD3+ cells in human peripheral blood (<5%), these cells account for a higher percentage (10%~15%) among infiltrated T lymphocytes in early lesions.31 Compared with α/β CD4+ and CD8+ T cells, γ/δ T cells have a higher potency to transmigrate endothelium16. ATPSβ on the tumor-cell surface binds to γ/δ TCR, which activates γ/δ T cells.12,32 Our results showed that oscillatory flow or cholesterol incubation potentiated the adhesion of γ/δ T cells to ECs, whereas laminar flow or βCD attenuated this association. The effect of oscillatory flow or cholesterol was reversed by the blockade of ecto-ATPSβ, which suggests that ecto-ATPSβ mediated the γ/δ T-cell-EC interaction (Fig. 4). In addition to changes in γ/δ T-cell adhesion, the increase in mitochondrial ATPSβ with a complementary decrease in ecto-ATPSβ may also benefit mitochondrial biogenesis in ECs. This scenario could be due to the involvement of ATPase in ATP production and electron transport in mitochondria.
The activation of γ/δ T cells is manifested by the release of various pro-inflammatory cytokines, including TNFα, IFNγ, and IL-8.17 The expression of adhesion molecules such as VCAM-1, a marker of endothelial activation, can be induced by these cytokines. Our ELISA experiments revealed that γ/δ T cells coincubated with cholesterol-treated ECs increased the release of TNFα and IFNγ into the co-cultured medium, as compared with in the absence of cholesterol (Fig. 5A). The release of those cytokines would be due to the increase in the membrane ATPSβ and the activation of γ/δ T lymphocytes, because a blocking antibody against ATPSβcould attenuate the release of TNFα (Fig. 5B). Importantly, TNFα was undetectable in γ/δ T cells (data not shown). Conversely, co-culture of γ/δ T cells with βCD-treated HUVECs reduced the release of TNFα (data not shown). Furthermore, immunofluorescence assay and RT-PCR showed that VCAM-1 expression was increased in ECs pretreated with cholesterol and prolonged co-culture with γ/δ T cells (Fig. 5C). Such an elevated expression of VCAM-1 in ECs was not seen in the absence of γ/δ T cells, which indicates that the interaction of γ/δ T cells caused the activation of ECs. Further, this effect was induced by cytokines released in the medium because of VCAM-1 upregulation seen in ECs incubated with the conditioned medium (Fig. 5E). In line with the in vitro study, in vivo, the inner curve of the aortic arch, which is presumably under disturbed flow, showed increased membrane ATPSβ level and γ/δ T lymphocyte adhesion (Fig. 6A,B). Importantly, ApoE−/− mice with hypercholesterolemia but not control C57BL/6 mice showed increased ATPSβ-mediated adhesion of γ/δ T lymphocytes (Fig. 6C,D). Our results agree with previous reports that VCAM-1 and ICAM-1 are highly expressed in the inner curve of the aortic arch,25,26 but are reduced or absent in the outer curve or thoracic aorta.
Disturbed flow, hypercholesterolemia, and vascular inflammation are important pathogenic factors leading to atherosclerosis. Hence, our work provides a novel mechanism of the synergistic effect of these features in atherogenesis. Local flow patterns predispose other risk factors such as hyperlipidemia to result in the focal nature of atherosclerosis. In atherosclerosis-susceptible regions of ApoE−/− mice (e.g., aortic root and inner curvature of the aortic arch), disturbed blood flow enhances lesion development in part through ATPSβ-mediated γ/δ T-cell adhesion. Similar results were obtained from a model in which the flow pattern was changed by partial ligation of mouse carotid arteries (Fig. 6E, F). With this animal model, Nam at el reported marked shear stress reduction as well as significant endothelial dysfunction and atherogenesis in ligated carotid arteries in ApoE−/− mice fed a high-fat diet. However, atheroprotective flow spares atherogenesis in the straight part of vessels under hyperlipidemic conditions (e.g., thoracic aorta and unligated sham-treated carotid artery of ApoE−/− mice). In control C57BL/6 mice, partial ligation led to a moderate increase in ecto-ATPSβ expression (Fig. 6E) but not adhesion of γ/δ T-lymphocytes on intima (Fig. 6F). This finding suggests a synergistic effect between disturbed flow and hypercholesterolemia on the adhesion of γ/δ T cells to dysfunctional endothelium in the atheroprone areas in vivo.
Novelty and Significance.
What Is Known?
Shear stress resulted from blood flow plays a key role in the focal nature of atherosclerosis in human patients and various animal models.
Endothelial dysfunction caused by athero-prone pattern of flow and high level of plasma cholesterol is one of the earliest vascular events leading to atherogenesis. Macrophages and T lymphocytes are the two major hematopoietic cell types infiltrating the vessel wall during atherosclerosis.
Cell membrane-bound ATP synthase β chain (ATPSβ) in tumor cells could be recognized by γ/δ T lymphocytes.
What New Information Does This Article Contribute?
In vitro, athero-protective flow decreases the level of membrane-bound ATPSβ, whereas athero-prone flow increases the level of cell membrane-bound ATPSβ.
High level of cholesterol and athero-prone flow induce the translocation of ATPSβ from mitochondria to membrane caveolae in endothelial cells, which causes the adhesion of γ/δ T cells.
In vivo, the γ/δ T cell adhesion increases in the lesion prone areas in the arterial tree.
Endothelial dysfunction, affected by the imposed athero-prone flow, is one of the earliest events leading to atherosclerosis. The infiltration of T lymphocytes into the vessel wall also contributes to atherogenesis. However, the detrimental effect synergized by γ/δ T lymphocytes and endothelial dysfunction under flow is largely unknown. We found that athero-prone flow increased ATPSβ and cholesterol on the endothelial membrane. Consequently, endothelial cells become more inflammatory to attract γ/δ T lymphocytes. This study provides the first evidence that disturbed flow and hypercholesterolemia synergistically promote the activation of γ/δ T-lymphocyte through the membrane translocation of ATPSβ in endothelial cells, which is a novel mechanism of endothelial activation.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by grants from the Major National Basic Research Grant of China (2010CB912504) (Y.Z.); the National Natural Science Foundation of China (30630032), (30821001) (Y.Z.); the “111” plan of China, and HL89940 and HL105318 from the National Institutes of Health (J.S.).
Non-standard Abbreviations and Acronyms
- ECs
Endothelial cells
- ATPSβ
ATP synthase β chain
- bCD
β-cyclodextrin
- ApoE−/−
apolipoprotein E knockout
- TCR
T-cell receptor
- HUVECs
Human umbilical vein endothelial cells
- BAECs
Bovine aortic endothelial cells
- Cav-1
caveolin-1
- LSS
laminar flow
- OSS
oscillatory flow
- VCAM-1
vascular cell adhesion molecule 1
Footnotes
Disclosure: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–24. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 3.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champagne E, Martinez LO, Collet X, Barbaras R. Ecto-F1Fo ATP synthase/F1 ATPase: metabolic and immunological functions. Curr Opin Lipidol. 2006;17:279–284. doi: 10.1097/01.mol.0000226120.27931.76. [DOI] [PubMed] [Google Scholar]
- 5.Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezón E, Champagne E, Pineau T, Georgeaud V, Walker JE, Tercé F, Collet X, Perret B, Barbaras R. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- 6.Beisiegel U, Weber W, Havinga JR, Ihrke G, Hui DY, Wernette-Hammond ME, Turck CW, Innerarity TL, Mahley RW. Apolipoprotein E-binding proteins isolated from dog and human liver. Arteriosclerosis. 1988;8:288–297. doi: 10.1161/01.atv.8.3.288. [DOI] [PubMed] [Google Scholar]
- 7.Mahley RW, Hui DY, Innerarity TL, Beisiegel U. Chylomicron remnant metabolism. Role of hepatic lipoprotein receptors in mediating uptake. Arteriosclerosis. 1989;9:I14–8. [PubMed] [Google Scholar]
- 8.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moser TL, Stack MS, Asplin I, Enghild JJ, Højrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burwick NR, Wahl ML, Fang J, Zhong Z, Moser TL, Li B, Capaldi RA, Kenan DJ, Pizzo SV. An Inhibitor of the F1 subunit of ATP synthase (IF1) modulates the activity of angiostatin on the endothelial cell surface. J Biol Chem. 2005;280:1740–1745. doi: 10.1074/jbc.M405947200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Chen Z, Wang X, Shyy JY, Zhu Y. Cholesterol loading increases the translocation of ATP synthase beta chain into membrane caveolae in vascular endothelial cells. Biochim Biophys Acta. 2006;1761:1182–1190. doi: 10.1016/j.bbalip.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Estève JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 14.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 15.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 16.Galea P, Brezinschek R, Lipsky PE, Oppenheimer-Marks N. Phenotypic characterization of CD4−/alpha beta TCR+ and gamma delta TCR+ T cells with a transendothelial migratory capacity. J Immunol. 1994;153:529–542. [PubMed] [Google Scholar]
- 17.Dyugovskaya L, Lavie P, Lavie L. Phenotypic and functional characterization of blood gammadelta T cells in sleep apnea. Am J Respir Crit Care Med. 2003;168:242–249. doi: 10.1164/rccm.200210-1226OC. [DOI] [PubMed] [Google Scholar]
- 18.Zhu M, Fu Y, Hou Y, Wang N, Guan Y, Tang C, Shyy JY, Zhu Y. Laminar shear stress regulates liver X receptor in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:527–533. doi: 10.1161/ATVBAHA.107.143487. [DOI] [PubMed] [Google Scholar]
- 19.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 20.Guo D, Chien S, Shyy JY. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ Res. 2007;100:564–571. doi: 10.1161/01.RES.0000259561.23876.c5. [DOI] [PubMed] [Google Scholar]
- 21.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Liao HL, Wang N, Yuan Y, Ma KS, Verna L, Stemerman MB. Lipoprotein promotes caveolin-1 and Ras translocation to caveolae: role of cholesterol in endothelial signaling. Arterioscler Thromb Vasc Biol. 2000;20:2465–2470. doi: 10.1161/01.atv.20.11.2465. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez E, Nagiel A, Lin AJ, Golan DE, Michel T. Small interfering RNA-mediated down-regulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J Biol Chem. 2004;279:40659–40669. doi: 10.1074/jbc.M407051200. [DOI] [PubMed] [Google Scholar]
- 24.Schilbach K, Geiselhart A, Handgretinger R. Induction of proliferation and augmented cytotoxicity of gammadelta T lymphocytes by bisphosphonate clodronate. Blood. 2001;97:2917–2918. doi: 10.1182/blood.v97.9.2917. [DOI] [PubMed] [Google Scholar]
- 25.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 26.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–43. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 28.Stannard AK, Riddell DR, Sacre SM, Tagalakis AD, Langer C, von Eckardstein A, Cullen P, Athanasopoulos T, Dickson G, Owen JS. Cell-derived apolipoprotein E (ApoE) particles inhibit vascular cell adhesion molecule-1 (VCAM-1) expression in human endothelial cells. J Biol Chem. 2001;276:46011–46016. doi: 10.1074/jbc.M104812200. [DOI] [PubMed] [Google Scholar]
- 29.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto K, Shimizu N, Obi S, Kumagaya S, Taketani Y, Kamiya A, Ando J. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1646–53. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- 31.Wick G, Romen M, Amberger A, Metzler B, Mayr M, Falkensammer G, Xu Q. Atherosclerosis, autoimmunity, and vascular-associated lymphoid tissue. FASEB J. 1997;11:1199–1207. doi: 10.1096/fasebj.11.13.9367355. [DOI] [PubMed] [Google Scholar]
- 32.Vantourout P, Martinez LO, Fabre A, Collet X, Champagne E. Ecto-F1-ATPase and MHC-class I close association on cell membranes. Mol Immunol. 2008;45:485–492. doi: 10.1016/j.molimm.2007.05.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.