Abstract
Background
Leprosy is a rare but serious mycobacterial infection. Immigration from areas where the disease is endemic has resulted in the importation of leprosy into countries where it is not endemic and where physicians and health care workers have little or no experience in diagnosis and therapy. In this study we characterized leprosy patients seen in a tropical disease unit that manages most of the reported leprosy cases in Canada.
Methods
We reviewed the clinical records of all 184 leprosy patients who were referred to the Tropical Disease Unit at Toronto General Hospital between 1979 and 2002 and abstracted demographic and clinical information.
Results
Patients were more likely to be male (122 or 66.3%) and of Indian (44 or 23.9%), Filipino (49 or 26.6%) or Vietnamese (37 or 20.1%) origin. Patients experienced symptoms for a mean of 4.8 years before referral to the Tropical Disease Unit. Most had no family history of leprosy (152/172 or 88.4%). Most patients presented with either borderline tuberculoid (80 or 43.5%) or borderline lepromatous (37 or 20.1%) disease. On average, patients presented with 5.8 skin lesions. Upper- and lower-extremity nerve dysfunction was common at presentation, with up to one-third of patients demonstrating either sensory or motor loss. A significantly greater lag time to presentation was observed in patients who emigrated from low-prevalence regions (p < 0.001).
Interpretation
Leprosy is a chronic infectious disease that is associated with serious morbidity if left untreated. Leprosy is uncommon in developed countries, but it is important for physicians to have a high index of suspicion when a foreign-born patient presents with chronic dermatitis and peripheral nerve involvement.
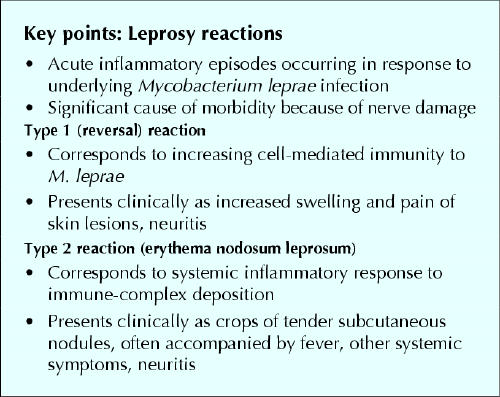
Leprosy, or Hansen's disease, is a chronic and complex infectious illness, dependent in large part upon the individual's immunologic response to the causative organism, Mycobacterium leprae.1 The spectrum of illness ranges from tuberculoid (or paucibacillary) disease, characterized by relatively few (no more than 5) skin lesions and a marked cell-mediated immune response to M. leprae, to lepromatous (or multibacillary) disease, which exhibits multiple lesions and little cell-mediated immunity to M. leprae (see Fig. 1 in companion article2 on page 71). At the tuberculoid pole, skin lesions are typically few in number, anesthetic and hypopigmented, whereas at the lepromatous pole, the lesions are widely disseminated, sensate and minimally hypopigmented.1 Nerve damage can occur at any point in the clinical spectrum; it may occur early in tuberculoid disease, but tends to be more insidious in lepromatous leprosy.1 Patients can also present with acute inflammatory responses to M. leprae, called leprosy reactions, which can result in marked nerve damage and subsequent limb disability and deformity. The 2 common types of leprosy reactions are type 1 or reversal reactions, mediated by an upgrade in cellular immune responses to the bacterium, and type 2 or erythema nodosum leprosum (ENL), which corresponds to a systemic response to immune complex deposition created by dead and dying M. leprae. Reactions should be viewed as medical emergencies. Although few physicians in developed countries have expertise in leprosy, prompt recognition and treatment are essential to limit morbidity and loss of quality of life.1
We report here on a number of imported cases of leprosy in Canada. The objective was to review the demographic characteristics, presentation and outcome of patients presenting in an urban centre. This report underscores the importance of accurate diagnosis and appropriate management of patients with leprosy.
Methods
We evaluated records for all patients with leprosy who presented to the Tropical Disease Unit of the Toronto General Hospital between 1979 and 2002. This unit has managed over 80% of reported leprosy cases nationwide,3 and its referral basis is primarily general practitioners and specialists across Ontario. Two of us (A.K.B. and J.D.C.) reviewed charts for all patients with leprosy (confirmed at the Tropical Disease Unit clinically and by skin biopsy, demonstration of acid-fast bacilli on skin biopsy or smear, or both).1,4 We abstracted descriptive information, including demographic data, country of origin, clinical presentation, sensory and motor dysfunction, disability, and past treatment and reactions. Demographic, clinical and follow-up data were extracted and entered into a relational database (Microsoft Access, Microsoft, Seattle) through use of a codifying form. Data were analyzed with descriptive statistics and χ2 analysis for comparing proportions of categorical variables. For multiple comparisons of proportions of categorical variables, the χ2 distribution via contingency tables was employed. Means for continuous variables were compared with Student's t test. For subsamples with unequal variance the Kruskal–Wallis one-way analysis of variance was applied.
This study was approved by the Research Ethics Board of the Toronto General Hospital.
Results
From 1979 to 2002, leprosy was diagnosed in a total of 184 patients at the Tropical Disease Unit (Table 1). The mean age at presentation was 39.9 years (Table 2). The patients were predominantly male and had immigrated from a total of 23 different countries. The most highly represented regions were the Indian subcontinent (58 patients or 31.5%, including 44 patients or 23.9% from India), the Philippines (49 or 26.6%) and Vietnam (37 or 20.1%). The one Canadian-born patient contracted leprosy while studying in India.
Table 1
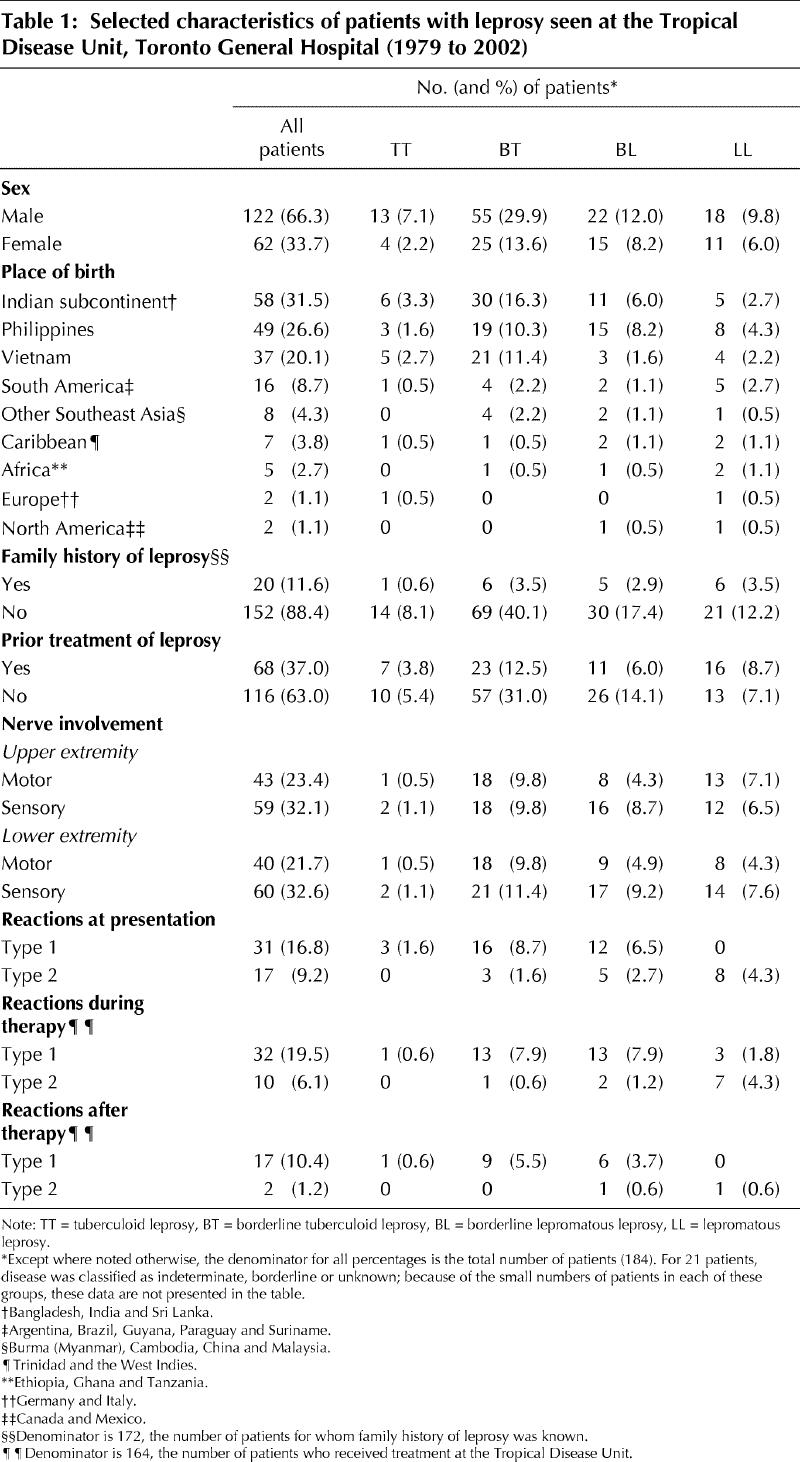
Table 2
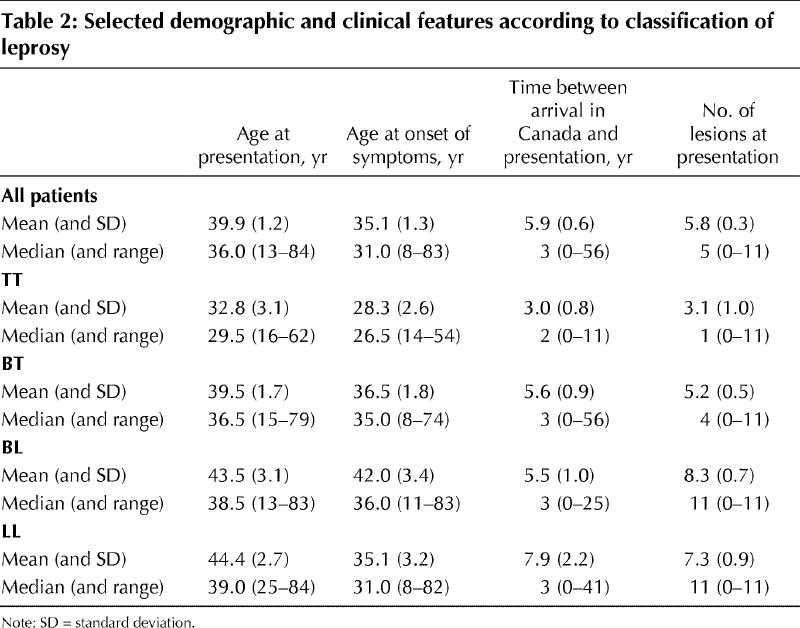
Patients experienced symptoms for a mean of 4.8 years before presentation to the Tropical Disease Unit (Table 2). Greater lag time to presentation was predicted by emigration from a low-prevalence region rather than a region where leprosy is endemic (p < 0.001). Patients from low-prevalence countries (fewer than 0.5 cases per 10 000 population) such as Sri Lanka, Guyana and Trinidad had a mean 4.7-year lag time to diagnosis, whereas those from higher-prevalence countries such as India, Vietnam and the Philippines had a mean lag time of 1.5 years. Patients with lepromatous disease (lepromatous leprosy [LL] or borderline lepromatous leprosy [BL]) were older at presentation than those with tuberculoid disease (borderline tuberculoid leprosy [BL] or tuberculoid leprosy [TT]) (p = 0.048). Mean age at presentation was 32.8 years for TT patients and 44.4 years for LL patients (Table 2). At presentation, patients were most likely to be classified as having BT (80 patients or 43.5%), BL (37 or 20.1%) or LL (29 or 15.8%) disease. Of note, patients originally from the Philippines were more likely to have lepromatous leprosy (LL or BL) at presentation (23/49 patients or 47%) than those from Vietnam (7/37 or 19%) or India (11/44 or 25%) (χ2 contingency table = 8.49, p < 0.025).
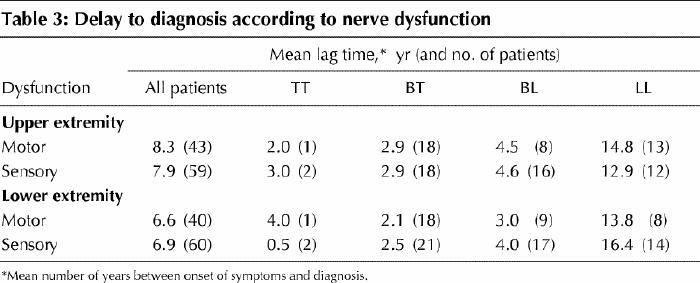
One-third of patients exhibited upper- or lower-extremity sensory nerve dysfunction, and one-quarter had motor dysfunction on presentation (Table 1). Of those with upper-extremity nerve dysfunction, generalized sensory loss was most common (31/180 or 17.2% of the main sample), whereas motor involvement was most likely in the ulnar distribution (15/180 or 8.3% of the main sample), followed by the combination of median and ulnar distribution (12/180 or 6.7% of the main sample). Similarly, lower-extremity sensory loss, present in 33.1% of patients, was most likely to be generalized (42/181 or 23.2% of the main sample), whereas motor dysfunction, present in 22.3% of patients, was most likely to be in the distribution of the common peroneal nerve (29/179 or 16.2% of the main sample). Table 3 highlights that lag time to presentation was greater among those with nerve dysfunction at the lepromatous pole.
Table 3
On the basis of World Health Organization recommendations,1,4 most patients with paucibacillary leprosy (indeterminate leprosy, TT or BT) were treated with dapsone plus rifampin (67/103 or 65.0%); for a minority (12/103 or 11.7%) clofazimine was added. The majority of patients with multibacillary disease (borderline leprosy [BB], BL or LL) were treated with dapsone, rifampin and clofazimine triple therapy (42/73 or 57.5%). The median duration of treatment for paucibacillary and multibacillary patients was 6 and 24 months respectively.
At presentation, 48 patients (25.5%) were undergoing a leprosy reaction, with most suffering a type 1 reversal reaction (Table 1). During treatment, 42 patients experienced a reaction, and following treatment cessation an additional 19 patients experienced a reaction; in both situations, the majority were type 1 or reversal reactions (Table 1). Mean duration of follow-up for the sample was 47.9 (median 44, range 1 to 132) months. Of patients completing therapy at the Tropical Disease Unit, true relapse was documented clinically and histologically in only 2 individuals, but 17 patients were lost to follow-up before the recommended 5 years.
Interpretation
In this study, patients from the most highly represented countries (India, the Philippines and Vietnam) differed significantly with respect to clinical presentation. While the proportion of patients from the Philippines with lepromatous disease (BL or LL) roughly equaled those with tuberculoid disease (TT or BT), the disease distribution among patients from India and Vietnam was skewed, with lepromatous leprosy occurring less frequently than tuberculoid disease. Our data are supported by the findings of other studies conducted within these countries, where leprosy is endemic,5,6,7,8 and underscore the potential of genetic susceptibilities within these populations. Presentation with lepromatous disease has particular public health implications, in that high bacillary loads render these patients more contagious. Studies in the United States have reported transmission of imported leprosy to nonimmigrant American residents,9 and prolonged or intimate contact with individuals with lepromatous leprosy increases the risk of transmission. Therefore, a number of Canadian household contacts of patients with lepromatous leprosy included in this series were investigated and treated with 6 months of standard 2-drug therapy on the assumption that they might have acquired M. leprae infection from this close contact.
The positive correlation in this study between age and shift toward the lepromatous end of the disease spectrum is interesting. A possible explanation is that debilitating nerve involvement occurs later in lepromatous disease than in tuberculoid illness, and patients may thus delay seeking care because they perceive their symptoms as benign. This possibility is supported by the shorter lag time to presentation among patients with paucibacillary (tuberculoid) disease than among those with multibacillary (lepromatous) disease (in particular, the median lag time to diagnosis was 8 years among those with LL disease but just 3 years among those with TT disease). In addition, those born outside high-prevalence areas had a greater lag time from onset of symptoms to presentation and might therefore have had more encounters or more opportunities for encounters with the health care system before accurate diagnosis. This greater lag time suggests that a lack of disease awareness and recognition may contribute to delay in diagnosis and treatment. However, additional prospective studies are needed to establish whether this is in fact the case.
At the time of presentation, sensory loss was apparent in one-third of patients and motor dysfunction in one- quarter. As Table 3 illustrates, those at the lepromatous end of the spectrum tended to have symptoms, including motor and sensory neuropathy, for a longer period before diagnosis. Clearly, this type of nerve involvement is an important cause of disability and lost productivity. However, these deficits are often ameliorable with multidrug therapy and corticosteroids. That a minority of patients in this study were managed differently from the current treatment guidelines emphasizes the changes in recommendations over time, the complexity of the disease and the occasional need for individualized, case-based therapy.
Leprosy reactions are common among patients undergoing multidrug therapy, but care is needed in distinguishing reactions from relapse, because their management is different.2
In summary, the diagnosis of leprosy should be considered in cases of chronic dermatitis with peripheral nerve involvement in foreign-born individuals, as well as those who have undertaken protracted travel abroad. Leprosy can be a complicated and challenging disease to manage. If suspected, referral to a tropical disease expert or a dermatologist with expertise in leprosy is warranted.
Figure.
β See related article page 71
Footnotes
This article has been peer reviewed.
Contributors: Jason D. Correia and Jay S. Keystone contributed to the study design, data acquisition and analysis, and were involved in revising the manuscript. Andrea K. Boggild and Kevin C. Kain contributed to the study design, data acquisition, analysis and interpretation, and were primarily responsible for writing the manuscript.
Acknowledgement: We thank Stephen Retchford for his invaluable contribution to database design and implementation throughout this project.
Competing interests: None declared.
Correspondence to: Dr. Kevin C. Kain, Toronto General Hospital, 200 Elizabeth St., ES-9-412, Toronto ON M5G 2C4; fax 416 595-5826; Kevin.Kain@uhn.on.ca
References
- 1.Bryceson A, Pfaltzgraff RE. Clinical pathology, symptoms and signs. In: Hastings RC, editor. Leprosy. Medicine in the tropics. 3rd ed. Edinburgh: Churchill-Livingstone; 1990. p. 11-55.
- 2.Boggild AK, Keystone JS, Kain KC. Leprosy: a primer for Canadian physicians. CMAJ 2004;170(1):71-8. [PMC free article] [PubMed]
- 3.Reported cases of leprosy in Canada by year, 1989-1998. In: Notifiable diseases on-line: leprosy. Ottawa: Health Canada, Population and Public Health Branch; 2000. Available: dsol-smed.hc-sc.gc.ca /dsol-smed/ndis/disease2 /lepr_e.html (accessed 2003 Nov 28).
- 4.WHO Expert Committee on Leprosy. 7th report. no. 874 of Technical Report series. Geneva: World Health Organization; 1998. [PubMed]
- 5.Balagon MV, Cellona RV, Fajardo TT Jr, Abalos RM, Walsh DS, Villahermosa LG, et al. Detection of new leprosy cases at a walk-in skin clinic in Cebu City, Philippines, highlights surveillance. Int J Dermatol 1999; 38 (10): 796-7. [DOI] [PubMed]
- 6.Alcais A, Sanchez FO, Thuc NV, Lap VD, Oberti J, Lagrange PH, et al. Granulomatous reaction to intradermal injection of lepromin (Mitsuda reaction) is linked to the human NRAMP1 gene in Vietnamese leprosy sibships. J Infect Dis 2000;181(1):302-8. [DOI] [PubMed]
- 7.Bhattacharya SN, Sehgal VN. Leprosy in India. Clin Dermatol 1999;17:159-70. [DOI] [PubMed]
- 8.de Vries RRP, Ottenhoff THM. Immunogenetics of leprosy. In: Hastings RC, editor. Leprosy. 2nd ed. Edinburgh: Churchill Livingstone; 1994. p. 113-21.
- 9.Worth RM, Bomgaars MR. Immigration and leprosy in Hawaii, 1960–1981. Int J Lepr Other Mycobact Dis 1982;50(3):335-41. [PubMed]


