Abstract
The ehrlichioses have been subject to increasing interest from veterinary and public health perspectives, but experimental studies of these diseases and their etiologic agents can be challenging. Ehrlichia canis, the primary etiologic agent of canine monocytic ehrlichiosis, is relatively well characterized and offers unique advantages and opportunities to study interactions between a monocytotropic pathogen and both its vertebrate and invertebrate hosts. Historically, advances in tick-borne disease control strategies have typically followed explication of tick-pathogen-vertebrate interactions, thus it is reasonable to expect novel, more sustainable approaches to control of these diseases as the transmission of their associated infections are investigated at the molecular through ecological levels. Better understanding of the interactions between E. canis and its canine and tick hosts would also elucidate similar interactions for other Ehrlichia species as well as the potential roles of canine sentinels, reservoirs and models of tick-borne zoonoses. This article summarizes natural exposure studies and experimental investigations of E. canis in the context of what is understood about biological vectors of tick-borne Anaplasmataceae.
Keywords: Ehrlichia canis, canine monocytic ehrlichiosis, host surveys, biological transmission, tick biology, Metastriata, Prostriata
Introduction
Ehrlichia canis is the primary etiologic agent of canine monocytic ehrlichiosis (CME), also known as tropical canine pancytopenia, a relatively well characterized cosmopolitan tick-borne disease of dogs (Ewing, 1969; Groves et al., 1975; Kordick et al., 1999; Lewis et al., 1977; Mathew et al., 1996; Smith et al., 1976). E. canis invades and develops in canine monocytes and macrophages, which can lead to leukopenia, thrombocytopenia, fever, depression and anorexia. Some closely related pathogens, including E. ewingii, E. chaffeensis, Anaplasma phagocytophilum and Neorickettsia risticii, can cause atypical disease in dogs (Dawson and Ewing, 1992; Kakoma et al., 1994; Rikihisa, 1991). However, E. canis is responsible for the most common and clinically severe form of canine ehrlichiosis.
The ehrlichioses have been subject to increasing interest from veterinary and public health perspectives (Olano and Walker, 2002; Paddock and Childs, 2003; Rikihisa, 1991; Skotarczak, 2003; Walker and Dumler, 1996). Members of the genus Ehrlichia, which, like the closely related genus Anaplasma, is classified in the family Anaplasmataceae of the order Rickettsiales (Dumler et al., 2001), and all currently defined Ehrlichia species are biologically transmitted by ticks of the family Ixodidae. The objective of this review is to summarize current understanding of the biological transmission of E. canis based on natural exposure studies and experimental investigations with the canine model in the context of what is understood about biological vectors of tick-borne Anaplasmataceae.
Canine Monocytic Ehrlichiosis
CME canis can be divided into acute, subclinical and chronic phases. These phases were characterized with experimental infections of purpose-bred dogs infected by needle inoculation with E. canis-infected cells (Buhles et al., 1974; Buhles et al., 1975; de Castro et al., 2004; Ewing, 1969; Ewing and Buckner, 1965; Huxsoll et al., 1972; Reardon and Pierce, 1981), and this disease is reviewed in considerable detail elsewhere (Ewing, 1969; Harrus et al., 1999; Neer and Harrus, 2006; Skotarczak, 2003; Woody and Hoskins, 1991). The acute phase of experimental CME begins approximately 10 days, with a range of 8-20 days, after infection with E. canis, and can involve anemia, anorexia, ataxia, conjunctivitis, depression, fever, leukopenia, ocular discharge, thrombocytopenia and vomiting that end with dogs undergoing partial recovery, with clinical signs subsiding, approximately 20 to 30 days after infection. Acute CME is usually followed by a subclinical phase that can last from months to years (Baneth et al., 1996; Botros et al., 1995; Harrus et al., 1998; Waner et al., 1997). As its name suggests, there are no prominent clinical signs during this phase of the disease, but hematologic signs such as leukopenia, anemia and thrombocytopenia can still occur, with mild thrombocytopenia as the most consistent finding under experimental conditions (Waner et al., 1997).
The chronic phase of CME can be mild or severe, with recurrent clinical and hematologic signs that include pancytopenia, hemorrhage, monocytosis, lymphocytosis and weight loss (Ewing, 1969). In a retrospective study of 19 fatal cases diagnosed with chronic CME in Greece, Mylonakis et al. (2004) reported pancytopenia, bone marrow hypoplasia, anorexia, depression, severe bleeding tendencies, hypoalbuminemia and increased serum alanine aminotransferase. PCR testing of bone marrow detected E. canis in 13 cases and A. phagocytophilum, A. platys or Leishmania infantum in individual cases, but co-infections were not detected.
Ante mortem diagnosis of CME is reliant on direct or indirect detection of E. canis by microscopy, serology, cell culture or PCR. Microscopy can be very time consuming, and requires considerable experience and skill because both false positives and false negatives are common problems with this method. Currently available serologic methods are useful in determining if a host was exposed to an Ehrlichia spp., and these methods are also tractable for epidemiological, experimental and some immunological studies, but these tests are not necessarily useful for detecting active infections or if a host is clear of the organism after treatment (Iqbal et al., 1994). Moreover, antigenic cross-reactivity between E. chaffeensis, E. ewingii and other Ehrlichia spp. can affect specificity when defined antigens are not used for serologic assays (Perez et al., 1996; Rikihisa et al., 1992; Wen et al., 1995). The recent availability of in-clinic ELISA testing for E. canis is promising. O'Connor et al. (2006), however, noted a poor sensitivity of detection with a commercially available ELISA when compared to IFA testing of canine sera with low titers (1:80 to 1:160), but good correlation between these methods was reported for samples with titers outside of this range. The authors suggested that this poor correlation at low antibody titers might be due to high IFA sensitivity or cross-reactivity with related anaplasmal species. While serology is not always indicative of active infection, isolating E. canis by inoculating cell lines with host tissues, buffy coats, mononuclear cell fractions or peripheral blood does allow demonstration of active infection and specific identification of the pathogen (Aguirre et al., 2004a; Iqbal et al., 1994; Iqbal and Rikihisa, 1994; Keysary et al., 1996; Nyindo et al., 1971). Cultivation methods can also be sensitive, but they are technically difficult and require a relatively long, laborious and costly process.
PCR assays have contributed considerably to our understanding of anaplasmal agents and prompted new questions regarding the infectious cycle of E. canis by allowing direct detection of low numbers of organisms in naturally and experimentally infected hosts. These assays usually involve amplification of genomic DNA, so expression of a specific gene is not required for pathogen detection in various hosts and tissues. E. canis-specific PCR assays were developed and widely used to amplify fragments of the E. canis 16S rRNA gene (16S rDNA) from both vertebrate and invertebrate hosts (Fenollar and Raoult, 2004; Sparagano et al., 1999). Another PCR assay was developed to amplify an outer membrane protein-1 (omp-1) paralog, p30 (Stich et al., 2002), and a sensitive single-step p30-based assay was used to amplify E. canis DNA out of single tick extracts (Bremer et al., 2005; Stich et al., 2002). The increased sensitivity provided by the p30-based assay could be because species-specific nucleic acid sequences, like those encoding outer membrane proteins, provide greater ranges of specific targets for primer design (Stich et al., 2002; Wagner et al., 2004). Conventional PCR requires DNA isolation, amplification and gel electrophoresis, but there is work toward real time PCR assays that could be more sensitive, specific and faster without the gel electrophoresis step (Branger et al., 2004; Doyle et al., 2005; Labruna et al., 2007; Ndip et al., 2005). Although these assays can be challenging to implement, to our knowledge PCR is currently the most feasible approach to identify an active infection during all three phases of CME.
E. canis host surveys
Domestic dogs
CME canis has been described around the world, but appears to be particularly prevalent in tropical regions. The first published description of canine infection with E. canis was from Algeria in 1935 (Donatien and Lestoquard, 1935). Studies regarding the distribution of E. canis and other related organisms can be complicated by difficulties associated with sensitive, specific detection of these parasites, including cross-reactivity of closely related pathogens and occasional detection of seronegative but PCR-positive, PCR-negative but seropositive or PCR-negative but culture-positive hosts.
Serologic and molecular studies detected evidence of E. canis among African dogs throughout the continent, including Sudan (Inokuma et al., 2006b), Zimbabwe (Kelly et al., 2004; Matthewman et al., 1993), Cameroon (Ndip et al., 2005), Ivory Coast and Gabon (Davoust et al., 2006), and Tunisia, Senegal and Chad (Brouqui et al., 1991). Positive test rates in these studies ranged from 32% (Ndip et al., 2005) to 80.8% (Inokuma et al., 2006b). Botros et al. (1995) used the E. canis IFA test to survey 252 dogs from five military kennels and 122 privately owned dogs in Egypt, and reported an overall seroprevalance of 33%, with 29% among military and 41% among privately owned dogs, respectively. Dogs infested with R. sanguineus had a seroprevalance of 44%, while 31% of non-infested dogs were seropositive for E. canis. There is also serologic evidence of E. canis and E. chaffeensis, both of which are monocytotropic, among dogs in South Africa (Pretorius and Kelly, 1998), which is further complicated by the detection of an E. ruminantium-like pathogen in dogs with clinical signs consistent with ehrlichiosis (Allsopp and Allsopp, 2001). Several studies from southern Africa have provided important research milestones and insights into the natural history of the Anaplasmataceae, and it appears that future investigations in this region are destined to do the same.
Prior to an outbreak among military working dogs in Southeast Asia in 1967, naturally occurring canine ehrlichiosis was widely considered a mild disease characterized by fever, vomiting and naso-ocular discharge. During the Vietnam War, a highly hemorrhagic form of E. canis caused epizootic losses of military working dogs (Nims et al., 1971). Serological, molecular and microscopic evidence of E. canis infections has since been reported in dogs from many Asian countries such as China, Israel, Korea, Japan, Malaysia and Thailand (Baneth et al., 1996; Chae et al., 2003; Inokuma et al., 1999; Rajamanickam et al., 1985; Suksawat et al., 2001; Wen et al., 2003). Reports describing the seroprevalence of E. canis infections include 30% of stray and pet dogs in Israel, 13.6% of dogs in Japan, 0.2% of dogs in Malaysia and 22% of stray dogs in Thailand, with a 14.1% prevalence of E. canis detected by microscopic examination in Bangkok and surrounding areas (Jittapalapong et al., 2003). Most seropositive dogs in Thailand had higher titers to E. canis than to E. chaffeensis or A. phagocytophlium, thus E. canis appeared to be the more prevalent anaplasmal species among dogs in Bangkok. In a study of 150 randomly selected dogs from Japan, seroprevalence of E. canis was estimated at 10% (Watanabe et al., 2006), but these authors noted considerable cross reactivity between E. canis, E. chaffeensis, E. muris and an Ehrlichia sp. isolated from Ixodes ovatus ticks (IOE). Notably, the latter two pathogens are indigenous to Japan (Shibata et al., 2000; Wen et al., 1995), and E. canis was not detected in any of 1,211 Japanese ticks examined with 16S rDNA-based nested PCR (Inokuma et al., 2003).
The European range of E. canis is primarily throughout areas bordering the Mediterranean Sea (Spain, southern France, Italy and Turkey), with isolated reports noted in central Europe (Trotz-Williams and Trees, 2003). Seroprevalence among dogs in northeastern Spain was found to be 16.7% (Solano-Gallego et al., 2006b), and the organism was successfully cultivated from a naturally infected dog in that region (Aguirre et al., 2004a). A study of canine sera collected in northern Sardinia noted that 50% of the samples tested were IFA-positive, with a rate of 62.5% among samples from 13-60 month old dogs (Cocco et al., 2003). In France, Jouret-Gourjault et al. (2006) intravenously inoculated four naïve Beagles with the E. canis Borgo 89 strain isolated from sick dog in Corsica, and observed clinical CME comparable to that reported for other strains with one case of unapparent infection. Marsilio et al. (2006) used a citrate synthase gene-based PCR assay to detect E. canis in three of 14 blood samples from seropositive dogs in Italy, and amplicon sequence of two of these samples revealed 99.2% identity to the Oklahoma strain. Solano-Gallego et al. (2006b) estimated the prevalence of E. canis with real-time PCR from blood of Italian dogs, and reported prevalence in northern, central and southern Italy of 2.9%, 8% and 9.7%, respectively. Unver et al. (2005) reported infection in 3 dogs from Ankara, Turkey, by PCR assay; analysis of a 1,388 bp 16S rRNA sequence found this strain to be identical to E. ovina and to a strain isolated from dogs in Venezuela, with 99.9% similarity to the Oklahoma strain. A similar study by Unver et al. (2003) demonstrated 99.9% identity between Oklahoma and Venezuelan strains to one sequenced from a naturally infected Japanese dog. A serologic survey of Swiss dogs found reactive antibodies in 2.2% of the samples tested, the authors suggested that the dogs in question were likely exposed while traveling outside the country, because the range of R. sanguineus in this area is thought to be south of the Alps (Pusterla et al., 1998).
It is tempting to speculate that E. canis spread from the Old to the New World with its primary tick host, R. sanguineus. However, additional studies, perhaps involving robust molecular characterization of E. canis strains within both dogs and ticks on all the major continents, are needed to address this question (Pinyoowong et al., 2008). Bool and Sutmoller (1957) described the first New World infection of dogs with E. canis in Aruba. Since that initial report, others have characterized the occurrence of E. canis throughout South, Central and North America. Brazilian studies have estimated seroreactivity among privately owned dogs at 19.8-23% (Labarthe et al., 2003; Trapp et al., 2006), and PCR detected rates of 1.4-3.5% in non-thrombocytopenic dogs, 20-26.8% in mildly thrombocytopenic dogs and 63.1% in dogs with platelet counts less than 100,000/μl (Bulla et al., 2004; Dagnone et al., 2003; Macieira et al., 2005). Recently, Labruna et al. (2007) submitted the first report of canine infection in the Amazon region of Brazil, which involved PCR findings and sequence analysis of amplicons from 4 of 5 dogs examined (Labruna et al., 2007). In another study, PCR analysis of 55 canine blood samples from Venezuela found an infection rate of approximately 33%; a 1,408 bp 16S rDNA sequence from the Venezuelan dog Ehrlichia (VDE) strain cultured from one of the samples was identical to that cultured from a human blood sample reported in the same study and 99.9% identical to the Oklahoma strain of E. canis (Unver et al., 2001b). A study of 120 dogs admitted to vet clinics in the Yucatan, Mexico, found 44.1% of dogs seropositive with ELISA testing (Rodriguez-Vivas et al., 2005). Although there is limited information regarding the prevalence of E. canis in Canada, a recent article noted a relatively low seroprevalence in southern Ontario and Quebec compared to most regions of the United States (Gary et al., 2006).
The first description of E. canis in North America was in the U.S., from a dog in Oklahoma that was co-infected with Babesia canis (Ewing, 1963). Interestingly, at that time the author could not conclude whether observed intraleukocytic inclusion bodies were an undescribed schizogenous phase of B. canis or an infection with a second organism, but it was in the course of careful investigation of B. canis reproduction in experimentally inoculated dogs that leukocytic inclusions were serendipitously observed from a negative control animal, in the absence of intraerythrocytic piroplasms, and subsequent work confirmed that canine erythrocytes and leukocytes were infected with different parasites in these cases (Ewing, 1964; Ewing and Buckner, 1965). Many cases have since been recognized in Oklahoma and the southern U.S. The Oklahoma Animal Disease Diagnostic Lab found that 53% of 259 samples submitted for testing were seropositive for E. canis from the period of June 1986 to July 1987 (Rodgers et al., 1989). Stockham et al. (1992) found that 25 of 31 dogs presenting clinical signs of polyarthritis in Missouri had E. canis sera titers from 1:20 to 1:5120, with thrombocytopenia being the most common hematologic abnormality. In a survey of blood samples from 65 tick-infested dogs from northern Oklahoma, 3.1% were PCR-positive for E. canis, while 10.8% were seropositive (Murphy et al., 1998). A case report from the same region described concurrent infection of an Oklahoma dog displaying neurologic signs with both E. canis and E. ewingii (Meinkoth et al., 1998). Hoskins et al. (1988) performed serologic testing on both individually owned clinically ill thrombocytopenic dogs and apparently healthy dogs in south Louisiana, finding 25.9% of the ill dogs and 20.3% of the healthy dogs seroreactive. Further west, a retrospective study of sera collected in 1975 from 339 dogs in Phoenix, Arizona revealed a seroreactivity rate of 12% among all dogs, with German Shepherd dogs demonstrating the highest degree of exposure (21%) (Stephenson and Ristic, 1978). A later study of 105 canine sera from Texas and Arizona revealed a seroconversion rate of 76% with 44% of the samples testing PCR-positive with a nested 16S rDNA-based assay; three IFA-negative samples were PCR-positive and 37 of the IFA-positive samples were PCR-negative (Wen et al., 1997).
Surveys of dogs in the eastern U.S. also revealed the presence of E. canis in that region. Stephenson et al. (1975) recognized an early domestic case of CME in a Collie cross from Virginia, presenting epistaxis and thrombocytopenia, with a serum titer of 1:20 based on IFA. Five of 30 IFA-negative canine blood samples from Ohio were PCR-positive for E. canis (Wen et al., 1997). Suksawat et al. (2000) tested sera from 1,845 sick dogs admitted to the North Caroline State Veterinary Teaching Hospital, finding 44 IFA-positive dogs, 36 of which were also positive by Western immunoblotting (Suksawat et al., 2000). Among 90 adult dogs admitted to an animal shelter in eastern Tennessee, 11% were seroreactive, but all tested PCR-negative and possible exposure to another anaplasmal species was not excluded (Seaman et al., 2004). The first recognized case of CME in the northeastern U.S. was in a dog in Connecticut, which led to recognition of the risk for CME in higher latitudes than previously expected (Magnarelli et al., 1990), and a 11.7% seroprevalence was reported from a retrospective study of 60 canine sera collected in 1986 in Connecticut and New York (Magnarelli and Anderson, 1993). Sampling in Rhode Island revealed a 2.9% seroprevalence for E. canis among 277 dogs in animal shelters (Hinrichsen et al., 2001). A large study found that 11% of military working dogs worldwide and 57% of civilian dogs in the U.S. were IFA-positive for E. canis (Keefe et al., 1982); the high seroprevalence among civilian dogs was skewed by practitioners submitting samples from dogs with clinical signs of CME.
Other mammalian hosts
Some investigators have observed natural or experimental E. canis infections of vertebrates other than domestic dogs. There is evidence that wild canids are susceptible to both E. canis infection and CME. An outbreak associated with a severe R. sanguineus infestation and significant mortality ascribed to CME was observed among wolves, dogs and wolf-dog crosses in a Florida zoo (Harvey et al., 1979). Coyotes, red and gray foxes were susceptible to infection by blood inoculation from dogs, and the gray fox was shown capable of providing an infectious blood meal for R. sanguineus larvae (Amyx and Huxsoll, 1973; Ewing et al., 1964). These experimental observations were supported by a report of 36% seropositivity among foxes in Israel (Fishman et al., 2004). Although a survey by Pusterla et al. (1999) failed to detect antibodies to E. canis in any of 1,550 sera samples from red foxes in Switzerland, this is not an area known to be enzootic for E. canis among the domestic canine population. Jackals sampled in Kenya and Israel displayed seroconversion at levels of 2% and 35.8%, respectively (Alexander et al., 1994; Waner et al., 1999). Inokuma et al. (2006a) identified an E. canis titer in 1 of 187 raccoon sera examined in Japan; these authors noted the potential exposure of this host prior to its import into Japan. Field testing and experimental trials failed to identify E. canis infections among capybara, deer and lemurs (Dawson et al., 1994; Labruna et al., 2007; Yabsley et al., 2004). The high similarity between 16S rDNA gene sequences of E. canis and E. ovina (Bekker et al., 2002; Unver et al., 2005), the latter of which naturally infects sheep, suggests that it would be interesting to investigate the susceptibility of their respective hosts to experimental infection.
Feline ehrlichiosis has been increasingly recognized worldwide since the first case due to natural infection was reported in France (Charpentier and Groulade, 1986). There appears to be at least two forms of feline ehrlichiosis or anaplasmosis, one attributed to E. canis, with inclusions in mononuclear cells, and the other to A. phagocytophilum, which is associated with inclusions in neutrophils (Foley et al., 1999; Lappin et al., 2004; Lewis et al., 1975). Despite an early report of failure to experimentally infect cats with E. canis (Ewing, 1969), the pathogenic role of E. canis in cats has received increasing acceptance, predominantly on the basis of the serologic evidence of natural infection and the infrequent detection of morulae within feline leukocytes. Clinical signs of feline ehrlichiosis include anemia, anorexia, dehydration, fever, lethargy, tachypnea and thrombocytopenia (Breitschwerdt et al., 2002; Tarello, 2005), and such cases are usually identified in geographic regions that are enzootic for canine, equine or human anaplasmosis. In Thailand, E. canis-like infections were described in stray cats of Bangkok (Jittapalapong and Jansawan, 1993). In 2003, seropositive rates for E. canis, E. chaffeensis and A. phagocytophilum were 4.9, 5.2 and 2.4 %, respectively among sera taken from 287 stray cats in monasteries around Bangkok (Jittapalapong et al., 2004). Studies in Spain and the U.S. reported seroconversion among cats to E. canis, with the more extensive testing in Spain describing rates of 10.6 to 17.9% (Aguirre et al., 2004b; Boulay et al., 1994; Ortuno et al., 2005; Solano-Gallego et al., 2006a). Breitschwerdt et al. (2002) examined blood and sera samples from 3 cats, one from North Carolina and two from Ontario, Canada, displaying clinical evidence of ehrlichiosis (Breitschwerdt et al., 2002). Partial 16S rDNA sequences amplified from these cats were identical to one another and to a canine isolate of E. canis. Filoni et al. (2006) identified one seropositive Brazilian puma of 18 tested. Experimental tick transmission studies with canine and feline isolates are needed to determine whether cats are susceptible to infection with E. canis or a closely related species.
Human monocytic ehrlichiosis (HME) was initially thought to be caused by E. canis due to serological cross-reactivity and the affinity of both pathogens for monocytes and macrophages (Maeda et al., 1987). However, this hypothesis was challenged (Ewing et al., 1987) and eventually refuted through analysis of ecological, genetic and vector competence differences between E. canis and the ultimate etiologic agent of HME: E. chaffeensis (Dawson et al., 1991; Ewing et al., 1995; Rikihisa, 1991). Differences were observed between 16S rDNA sequences of these species, E. canis is enzootic throughout the U.S. while E. chaffeensis appears to be primarily restricted to southern states, and E. chaffeensis is transmitted by Amblyomma americanum while this tick species is not considered capable of transmitting E. canis. Although E. canis is not considered an agent of HME in the U.S., newer evidence indicates that a Venezuelan strain of E. canis might be transmitted to human beings (Perez et al., 2006; Perez et al., 1996). This recently described strain, named Venezuelan human Ehrlichia (VHE), is associated with human ehrlichiosis in that region and has a 16S rDNA sequence that is 99% identical to that of E. canis. Further work is needed to confirm whether VHE is indeed E. canis, and, importantly, if VHE is tick-transmissible among dogs.
Invertebrate hosts
Less attention has been devoted to characterizing E. canis within its tick hosts. Both R. sanguineus and Dermacentor variabilis were demonstrated to be competent experimental vectors of the parasite (Bremer et al., 2005; Groves et al., 1975; Johnson et al., 1998; Smith et al., 1976). E. canis has been detected in unattached and feeding ticks in Africa (Loftis et al., 2006), Europe and the Americas. Ewing et al. (1990) found no evidence of E. canis infection in an Otobius megnini found feeding on a child with serologic evidence of ehrlichial infection, and an attempt to infect dogs with this soft tick was unsuccessful (Ewing et al., 1990). Magnarelli et al. (1991) noted organisms in hemocytes of 9.7% of nymphal and adult Ixodes scapularis dammini collected from Connecticut, and that these bacteria reacted with fluorescein-conjugated antiserum to E. canis. A similar result was reported for 6.9% of the D. variabilis collected. However, although they shared antigens with E. canis, the specific identity of these rickettsiae was not determined. In 1998, (Murphy et al.) reported use of a 16S rDNA-based PCR assay to detect E. canis in two pools of R. sanguineus males that were collected from dogs that were seropositive for E. canis. Similarly, Unver et al. (2001c) also found PCR evidence of E. canis in pools of R. sanguineus collected from Venezuela, and Christova et al. (2003) noted that 7% of metastriate ticks collected from Turkey and Albania contained Anaplasmataceae, with E. canis in one of 28 individual R. sanguineus from Albania. Wielinga et al. (2006) used a 16S rDNA-based reverse line blot technique to identify amplicons consistent with E. canis in three of 1600 (0.19%) unattached Dutch I. ricinus, which, interestingly, suggests that E. canis or a closely related species (e.g., an E. muris- or IOE-like species) may infect prostriate ticks in that region.
Ticks and the transmission of E. canis
Biology of ixodid ticks
Ticks of the family Ixodidae biologically transmit members of the closely related rickettsial genera Ehrlichia and Anaplasma. Ixodid ticks have four development stages: egg, larva, nymph and adult (Sonenshine, 1991). Larvae and nymphs require a blood meal before molting into the next stage, and adult stage females require a blood meal prior to oviposition. Female ixodid ticks die after oviposition, but the fate of ixodid males after copulation is not as certain and varies with the biology and reproductive behaviors among groups within this family. Most authorities agree that the Ixodidae consists of five subfamilies or tribes. All but one tribe fall into a group called the Metastriata, while the more ‘primitive’ Prostriata contains the remaining tribe (the Ixodinae) (Barker and Murrell, 2004; Sonenshine, 1991).
There are notable differences in reproduction between prostriates and metastriates (Kiszewski et al., 2001). In contrast to prostriates, most metastriate males require a blood meal prior to sexual maturation and copulate on the vertebrate host (El Said and Swiderski, 1983; Homsher and Sonenshine, 1972, 1976; Kiszewski et al., 2001; Londt and Van Der Bijl, 1977; Londt and Spickett, 1976; Oliver and Dotson, 1993; Sonenshine, 1991; Valero et al., 1997). In addition, metastriate male ticks are attracted to feeding sites by aggregation pheromones and to feeding females by sex pheromones (Leahy and Booth, 1983; Norval et al., 1989; Norval et al., 1996; Sobbhy et al., 1994; Sonenshine, 1985), suggesting means for interhost transfer and intermittent feedings on multiple vertebrate hosts. Thus, metastriate males may remain in the environment, potentially transmitting pathogens to multiple vertebrate hosts without the need for additional acquisition or molting periods.
Most ixodid species are three-host ticks with immature stages that detach from their hosts after each blood meal before molting to the next stage. One-host ticks, such as the Rhipicephalus subgenus Boophilus, seek out their hosts as larvae and remain on the same vertebrate host between molts. Although each feeding stage of R. sanguineus, the primary vector of E. canis, will readily feed on canine hosts, these are three-host ticks because engorged immature stages detach and molt away from the host.
Biological Transmission Scenarios
Each feeding tick stage or blood meal represents an opportunity to acquire and transmit pathogens (Figure 1). The majority of tick-borne pathogens are biologically transmitted, which means that these organisms also infect the invertebrate vector, where they multiply and/or develop prior to transmission to another vertebrate host. Several adverbs are used to describe pathogen passage in ticks: “transstadial” or “interstadial” describe passage of tick-borne pathogens between development stages of the same tick, while “intrastadial” describes maintenance of an infection within the same tick development stage. Thus, horizontal transmission by ticks can occur transstadially when an immature tick stage acquires the pathogen while taking a blood meal and the tick transmits the organism during a subsequent development stage, and intrastadial transmission can occur when ticks both acquire and transmit a pathogen during the same development stage. Vertical transmission by ticks is primarily thought to occur after transovarian passage from a female tick to larvae. Thus, transovarian passage is also a form of transmission between the invertebrate hosts themselves. Although transovarian passage has been reported for tick-borne members of the rickettsial family Rickettsiaceae, this phenomenon is considered rare among the Anaplasmataceae (Groves et al., 1975; Long et al., 2003; Potgeiter, 1981; Stich et al., 1989).
Figure 1. Pathogen acquisition and transmission scenarios.
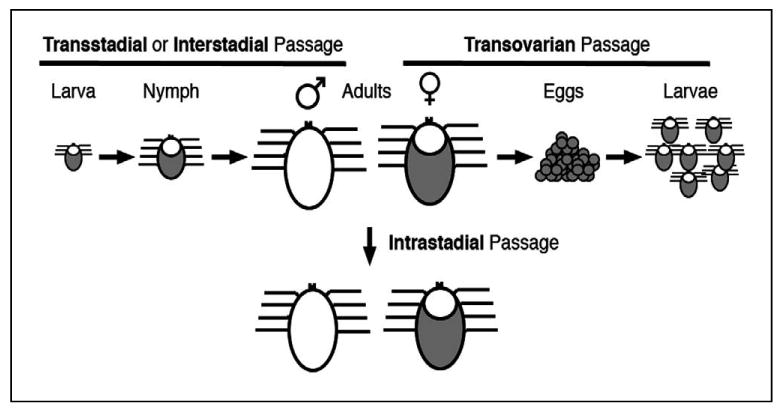
Transstadial (a.k.a. Interstadial) transmission occurs when a pathogen is acquired by an immature tick stage (i.e., a larva or a nymph) and is transmitted by a subsequent developmental stage. Transovarial transmission occurs when a pathogen is transmitted by a generation subsequent to the one that acquires the pathogen. Intrastadial transmission occurs when the same tick stage both acquires and transmits a pathogen.
The relevance of each tick transmission scenario is in large part dependent on the preferred host for each tick species and development stage. Transovarian passage is always important, especially with one-host tick species. Transstadial transmission is most important with two- or three-host ticks that have two or more stages that feed on vertebrates that are susceptible to infection with the pathogen in question. Intrastadial transmission is important with tick species that may transfer between hosts during a particular development stage. For example, intrastadial transmission could be important with adult male metastriates because they have been shown to move among and to feed on different hosts under experimental conditions, and because they are thought to persist throughout the tick season. The intrastadial transmission scenario could be even more important when interhost transfer may occur (e.g., utilization of gregarious vertebrate hosts) or when immature stages do not feed on hosts that are susceptible to infection with the pathogen. Notably, a recent report documented movement of adult R. sanguineus, especially male ticks, among dogs with experimentally pre-established infestations (Little et al., 2007). These results indicate that previously fed adult R. sanguineus will undergo interhost transfer under ad libitum conditions, and confirmed the potential importance of intrastadial transmission scenarios of E. canis with its primary vector (Bremer et al., 2005). Further investigation is needed to determine the frequency of interhost transfer of male ticks that parasitize both canine and human hosts.
Tick surveys
Reports that distinguish tick stages and sexes collected in surveys often underscore the importance of differences in the biology and behavioral ecology among tick tribes. For example, surveys of unattached questing ticks provide evidence that equivalent numbers of female and male ixodid offspring reach maturity. Among the surveys summarized in Table 1, the average yields of unattached adult prostriate female and male ticks were 39 and 61%, respectively, while unattached metastriate female and male yields averaged 53 and 47%, respectively. However, there was no statistical difference, according to a one-tailed Student's T-test, between the mean percentages of unattached female and male ticks of either the prostriates (P>0.2) or metastriates (P>0.1) among these reports. None of the surveys of unattached ticks reported any test to determine whether these ticks had previously fed during their adult stage.
Table 1. Summary of surveys reporting questing three-host tick sexes.
| Tick species | Collection Method | Adult Ticks Collected | Source | |
|---|---|---|---|---|
| Males (%) | Females (%) | |||
| Ixodes ricinus | CO2 Trap | 381 (41) | 553 (59) | (Gray, 1985) |
| Drag | 156 (54) | 135 (46) | ||
| I. scapularis dammini | CO2 Trap | 1.1 (88) | 1.6 (13) | (Solberg et al., 1992)a |
| Drag | 0.7 (83) | 0.1 (17) | ||
| Human host | 0.5 (36) | 0.1 (64) | ||
| Amblyomma americanum | CO2 Trap | 89.9 (48) | 160.6 (52) | |
| Drag | 6.1 (38) | 6.6 (62) | ||
| Human host | 3.2 (44) | 5.2 (56) | ||
| A. cajennense | Drag | 253 (42) | 212 (58) | (Oliveira et al., 2000)b |
| Rhipicephalus spp. | Drag | 36 (61) | 49 (39) | (Spickett et al., 1992) |
| Drag | 116 (54) | 98 (46) | (Zieger et al., 1998a) | |
| Dermacentor variabilis | Drag | 549 (41) | 705 (59) | (Harlan and Foster, 1990) |
| Haemaphysalis leachi | Drag | 31 (54) | 20 (46) | (Spickett et al., 1992) |
Data are reported as means of ticks collected at four survey times
Data presented are estimates (by this author) of the total values reported for a 2 yr period
There is evidence that metastriate males persist on hosts longer than females under natural conditions. Table 2 provides a summary of studies where burdens of adult female and male ticks were reported for various mammalian hosts. Relative percentages of male and female ticks collected from different hosts in separate studies are also presented due to substantial differences in numbers of ticks collected, host species, environmental factors, seasons of collection and experimental designs. Despite the production of equal proportions of female and male offspring, adult male metastriate ticks outnumber females in the majority of these reports where ticks where collected from vertebrate hosts. An interesting observation is made when the numbers of metastriate ticks reported in Table 2 are broken down into the three tribes represented in these studies (Rhipicephalinae, Amblyomminae and Haemaphysalinae): on average male ticks make up 65% of the adult ticks collected for each tribe. In addition, the differences between percent female and male ticks collected from hosts for each metastriate tribe were statistically different (P<0.001 for rhipicephalines and amblyommines, and P< 0.01 for haemaphysalines).
Table 2. Summary of surveys reporting three-host tick sexes attached to mammalsa.
| Tick Species | Hosts | Adult Ticks Collected | Source | |
|---|---|---|---|---|
| Males (%) | Females (%) | |||
| Haemaphysalinae | ||||
| Haemaphysalis leachi | Dogs | 1184 (58) | 843 (42) | (Horak, 1995)b |
| 2426 (39) | 3720 (61) | (Horak et al., 1987) c | ||
| Caracals | 57 (63) | 34 (37) | ||
| H. spinulosa | 60 (75) | 20 (25) | ||
| Rhipicephalinae | ||||
| Rhipicephalus spp. | Zebra | 816 (66) | 420 (34) | (Zieger et al., 1998b) d |
| Bushpig | 12 (45) | 14.5 (55) | ||
| Impala | 28.9 (73) | 10.6 (27) | ||
| Tsessebe | 66.7 (65) | 36.6 (35) | ||
| Hartebeest | 685 (79) | 186 (21) | ||
| Eland | 3389 (69) | 1488 (31) | ||
| Greater kudu | 866 (49) | 892 (51) | ||
| Sable | 62 (81) | 15 (19) | ||
| Water-buck | 1161 (63) | 688.1 (37) | ||
| Kafue lechwe | 280 (76) | 87 (24) | ||
| R. sanguineus | Dogs | 525 (56) | 408 (44) | (Horak, 1995)b |
| 585 (71) | 244 (29) | (L'Hostis et al., 1998) | ||
| R. simus | 131 (67) | 64 (33) | ||
| 612 (45) | 739 (55) | (Horak et al., 1987) c | ||
| Leopard | 375 (72) | 146 (28) | ||
| R. appendiculatus | 2481 (57) | 1854 (43) | ||
| R. pravus | Scrub Hares | 191 (78) | 55 (22) | (Horak et al., 1993) |
| 101 (81) | 24 (19) | (Horak et al., 1995b) | ||
| R. kochi | 93 (76) | 29 (24) | ||
| R. turanicus | 72 (73) | 26 (27) | ||
| Dermacentor variabilis | White-tailed deer and Feral Swine | 94 (51) | 89 (49) | (Allan et al., 2001) |
| Raccoons | 51 (58) | 37 (42) | (Pung et al., 1994) | |
| 96 (47) | 110 (53) | (Ouellette et al., 1997) | ||
| Amblyomminae | ||||
| Amblyomma americanum | 51 (59) | 36 (41) | ||
| White-tailed deer | 73 (53) | 65 (47) | (Allan et al., 2001) | |
| A. maculatum | and Feral Swine | 516 (64) | 293 (36) | |
| A. variegatum | Hartebeest | 24 (80) | 6 (20) | (Zieger et al., 1998b) d |
| Eland | 30 (70) | 13 (30) | ||
| Ixodinae | ||||
| Ixodes scapularis | White-tailed deer and Feral Swine | 314 (29) | 780 (71) | (Allan et al., 2001) |
| Raccoons | 3 (11) | 24 (89) | (Pung et al., 1994) | |
| I. texanus | 0 (0) | 135 (100) | (Ouellette et al., 1997) | |
| I. pilosus | Dogs | 38 (23) | 130 (77) | (Horak et al., 1987) c |
| I. rubicundis | Caracals | 132 (36) | 232 (64) | |
Data represents ≥80 total adult ticks (rows 1-25) or ≥15 adult ticks per host (rows 26-37).
Domestic dogs diagnosed with canine babesiosis (a tick-borne disease) in South Africa.
Collected every 2 to 4 weeks from 4 dogs over 3 years; single collection from 50 caracals and 2 leopards.
Data is reported as mean number of ticks per host.
Interestingly, there is not a difference between the percentage of Haemaphysalinae males and females reported in one investigation summarized in Table 2 (Horak et al., 1987). At first glance this observation may suggest that these male and female ticks persisted on dogs at the same rate in this study. However, this is not necessarily the case when one considers that these results represented total tick numbers removed from four dogs every 2 to 4 weeks over the course of three years, thus these values are closer to the total number of ticks that attached to these dogs rather than the number of ticks on the hosts at a single time point. Therefore, although this observation supports the idea that equivalent numbers of metastriate female and male ticks feed in nature, this particular study design was not conducive with estimating the persistence of ticks over time because the ticks were periodically removed from the same hosts.
Conversely, the percentage of prostriate (Ixodinae) female ticks (80%) collected from mammalian hosts was greater (P< 0.001) than the percent males found on these hosts, which supports the paradigm that male Ixodes spp. may not be important pathogen vectors in nature. This observation meshes well with prostriate biology, because these ticks are often nidicolous and mate on or off of the host (Kiszewski et al., 2001; Sonenshine, 1991), so there is a conceivable advantage for males of many prostriate species to remain off the host.
These summaries support the paradigm that equivalent numbers of female and male ixodids are produced under natural conditions (Table 1) and that the ratio of the sexes found on hosts is reflective of where the males of the different subfamilies mate and that metastriate males can persist on hosts after mating (Table 2). These hypotheses require additional testing, in part through more surveys that distinguish tick development stages in addition to their species and infection status.
Intrastadial Transmission
There is considerable evidence of anaplasmal pathogen transmission through interhost transfer of ticks. Experimental studies have provided evidence for an epizootiological role of three-host male ticks as intrastadial vectors of A. marginale after interhost transfer (Andrew and Norval, 1989; Coan et al., 1987; Kocan et al., 1992; Stiller and Coan, 1995; Stiller et al., 1987; Stiller et al., 1989; Zaugg et al., 1986). For example, one study involved the release of differentially marked ticks on to an A. marginale-infected calf that was penned with a naive calf. Of the ticks recovered from these calves, 30.8% were found to have transferred between hosts (Coan et al., 1987; Stiller and Coan, 1995). In addition, the naive calf developed clinical anaplasmosis 25 days after being penned with the infected calf; 21 days after the first two males from the infected calf were found to have transferred. It was also demonstrated in another study that intrastadially infected D. andersoni male ticks could continually transmit A. marginale to multiple hosts without any apparent loss of virulence (Kocan et al., 1992). A. hebraeum males placed on a host infected with E. ruminantium were found to transfer between hosts sharing the same stall in sufficient numbers to transmit a lethal dose of this pathogen (Andrew and Norval, 1989). Furthermore, epidemiological investigations have suggested that one-host tick species such as R. (subgenus Boophilus) microplus and D. albipictus were associated with natural transmission of A. marginale (Bock et al., 1999; Ewing et al., 1997), which corroborates at least one report of experimental transmission (Aguirre et al., 1994). Intrastadial or transstadial transmission after interhost transfer are the most likely scenarios in such cases because transovarial transmission is considered unlikely for Anaplasmataceae (Bram and Roby, 1970; Long et al., 2003; Potgeiter, 1981; Stich et al., 1989) and because R. Boophilus microplus has been reported to transfer between bovine hosts (Mason and Norval, 1981). A unique finding of a questing R. Boophilus decoloratus adult further supports the idea that interhost transfer of one-host ixodid ticks may occur under natural conditions (Spickett et al., 1991). Further investigations are needed to evaluate the importance of (1) interhost transfer of one-host ticks and (2) intrastadial transmission scenarios for zoonotic agents and their tick vectors that parasitize both canine and human hosts.
Experimental transmission of E. canis
Several interesting studies were focused on tick transmission of E. canis. Seminal work demonstrated that E. canis could be transstadially transmitted between dogs with R. sanguineus nymphs or adults (Groves et al., 1975; Lewis et al., 1977; Smith et al., 1976). These investigations leave no doubt that R. sanguineus is an important vector of E. canis, because all three feeding stages of this tick species readily attach to canine hosts, thus E. canis is likely to be transstadially transmitted by R. sanguineus in nature. This tick species is particularly problematic due to its somewhat unique ability to persistently infest buildings and kennels (Dantas-Torres, 2008; Harvey et al., 1979). E. canis probably multiplies within the midgut cells, hemocytes and salivary glands of its tick host (Smith et al., 1976); however, more extensive investigation of the parasite's development in ticks is needed. Another finding from early studies was that transstadial transmission of E. canis could not be demonstrated when ticks were allowed to acquisition feed on dogs in the subclinical or chronic phase of CME (Lewis et al., 1977), thus it is often assumed that dogs cannot serve as infection reservoirs once past the acute phase of CME. However, although difficult to detect, E. canis infections can persist in dogs long after the acute phase of CME, possibly with cyclic rickettsemia and recrudescent disease, so the potential of such hosts as sources of tick infection should not be disregarded until the aforementioned report is confirmed by additional, exhaustive, experimental and epidemiological investigations.
One E. canis strain, the Oklahoma isolate, was reportedly non-transmissible by R. sanguineus, presumably due to extensive passage in a mammalian cell line (Mathew et al., 1996). Interestingly, a subsequent study demonstrated infection of adult stage R. sanguineus with the same Oklahoma E. canis strain, but transmission by these ticks was not reported (Unver et al., 2001a). Taken together these two studies suggest that the Oklahoma strain might be infective for but not transmissible by R. sanguineus, which could provide a novel opportunity to explore events between acquisition and transmission of this pathogen by R. sanguineus.
At least one investigation demonstrated that D. variabilis is also capable of transstadially transmitting E. canis under experimental conditions (Johnson et al., 1998). Epidemiological implications of this finding are difficult to estimate because, while D. variabilis adult stages readily feed on dogs, the immature stages have a reported predilection for natural infestation of smaller mammals (Apperson et al., 1993; Clark et al., 2001; Gage et al., 1992; Jackson et al., 1975; Kollars, 1996; Sonenshine et al., 1966). Although there are a few reports of D. variabilis nymphs attached to canids in nature (Bishopp and Trembley, 1945; Bloemer and Zimmerman, 1988; Brillhart et al., 1994; Kollars, 1993; Kollars et al., 2000), intrastadial transmission of E. canis would probably be more important when this tick species is involved. Further work is warranted to determine if male D. variabilis are capable of intrastadial transmission of E. canis and ad libitum interhost transfer.
As previously mentioned, male R. sanguineus were reported capable of both intrastadial and transstadial transmission of E. canis under experimental conditions (Bremer et al., 2005). These transmission scenarios took place in the absence of female ticks, indicating that copulation was not required for multiple feedings or pathogen acquisition and transmission by the male ticks. The absence of an intermittent molt in the intrastadial transmission cycle would mean a shorter requisite time period between pathogen acquisition and transmission. Cross sectional surveys of natural canine tick burdens indicated that the majority of adult R. sanguineus collected were male (Horak et al., 1995a; L'Hostis et al., 1998), supporting the paradigm that male R. sanguineus, like other metastriates, may persist after their female counterparts have left the host. Moreover, a recent report indicated that adult male R. sanguineus will transfer among canine hosts under experimental conditions (Little et al., 2007), confirming the potential role of this tick stage in the transmission of pathogens.
Another observation was that milder acute phase CME was associated with transmission of E. canis by male ticks compared to intravenous inoculation with the same strain in carrier blood (Bremer et al., 2005). Several factors may contribute to the severity of CME, which include strain of the agent, infective dose, and age, sex, immunocompetence, concomitant disease and breed susceptibility of the canine host (Rikihisa, 1991). Other reports associated with experimental tick transmission of E. canis indicated more severe clinical signs when feeding large numbers of transstadially infected male and female ticks (Johnson et al., 1998; Mathew et al., 1996). Therefore more severe acute CME might result with large tick numbers or the presence of immature or female ticks. At least one study demonstrated severity of disease associated with subcutaneous inoculation with different amounts of E. canis in frozen stabilate (Gaunt et al., 1996). However, it is difficult to estimate infectious organism numbers in peripheral monocytes in blood from an E. canis carrier (with leukopenia) compared to ticks allowed to acquisition feed on experimental hosts during acute CME and presumably peak rickettsemia. Further work is needed to determine whether clinical differences after tick versus needle inoculation could be due to infective dose. Another consideration is that female ticks, which were included in other investigations, might affect the severity of disease. Female ixodid ticks process considerably more host blood than males, which in turn requires elimination of greater volumes of water via saliva, a known medium of pathogen transmission and vertebrate immunomodulation (Sonenshine, 1991). Therefore it is possible that female tick feeding could result in transmission of larger pathogen numbers or a more pronounced pharmacological effect on the vertebrate host (Bergman et al., 1995; Stich et al., 1993; Wikel et al., 1994). Regardless of the mechanism(s) underlying the differences in severity of clinical signs, these results underscore the relevance of experimental tick transmission in characterization and development of control measures for E. canis and other tick-borne pathogens, especially those characterized mainly through needle-inoculation of experimental hosts.
Concluding remarks
Although the complex triad of canid-E. canis-R. sanguineus interactions are relatively well characterized compared to other Ehrlichia spp., there is still a great deal to learn about the infectious cycle of this pathogen and the etiology of CME. The role of different R. sanguineus development stages in natural transmission of E. canis is difficult to estimate, because all stages of this tick species have a strong preference for canine hosts. However, in the absence of an intermittent molt, the intrastadial transmission cycle would require considerably less time from pathogen acquisition to transmission. Further investigations are needed to determine if E. canis-infected male ticks can transmit the pathogen to multiple hosts. If so, R. sanguineus males could remain in the environment, transmitting E. canis to multiple canine hosts without any acquisition or molting period, and subsequent vertebrate hosts would be more rapidly exposed to the pathogen. Control of the adult male tick populations would then be important for limiting outbreaks of CME, and the logistics of those treatments, including timing and areas treated, would have to be evaluated. More questions remain regarding the effects of tick burdens, stages and copulation on acquisition and transmission of E. canis and the effect of immature and female ticks on the severity of subsequent disease. Although D. variabilis transmits E. canis transstadially, intrastadial transmission might be more important because it appears to be the most likely natural transmission scenario between dogs by this tick species. Attempts to answer these and future questions regarding this enigmatic transmission and disease model is expected to advance our understanding of the infectious cycle of E. canis, to augment understanding of the ehrlichioses and to stimulate interest in the potential roles of dogs as models, sentinels and sources of tick-borne pathogens.
Acknowledgments
The authors thank Dr. Hans Klompen of The Ohio State University for helpful comments regarding tick taxonomy.
The senior author is supported by NIH grant RO1 AI47932.
Footnotes
None of the authors have a financial or personal relationship with other people or organizations that could inappropriately influence or bias this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre DH, Gaido AB, Vinabal AE, De Echaide ST, Guglielmone AA. Transmission of Anaplasma marginale with adult Boophilus microplus ticks fed as nymphs on calves with different levels of rickettsaemia. Parasite. 1994;1:405–407. doi: 10.1051/parasite/1994014405. [DOI] [PubMed] [Google Scholar]
- Aguirre E, Sainz A, Dunner S, Amusategui I, Lopez L, Rodriguez-Franco F, Luaces I, Cortes O, Tesouro MA. First isolation and molecular characterization of Ehrlichia canis in Spain. Vet Parasitol. 2004a;125:365–372. doi: 10.1016/j.vetpar.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Aguirre E, Tesouro MA, Amusategui I, Rodriguez-Franco F, Sainz A. Assessment of feline ehrlichiosis in central Spain using serology and a polymerase chain reaction technique. Ann N Y Acad Sci. 2004b;1026:103–105. doi: 10.1196/annals.1307.013. [DOI] [PubMed] [Google Scholar]
- Alexander KA, Kat PW, Wayne RK, Fuller TK. Serologic survey of selected canine pathogens among free-ranging jackals in Kenya. J Wildl Dis. 1994;30:486–491. doi: 10.7589/0090-3558-30.4.486. [DOI] [PubMed] [Google Scholar]
- Allan SA, Simmons LA, Burridge MJ. Ixodid ticks on white-tailed deer and feral swine in Florida. J Vector Ecol. 2001;26:93–102. [PubMed] [Google Scholar]
- Allsopp MT, Allsopp BA. Novel Ehrlichia genotype detected in dogs in South Africa. J Clin Microbiol. 2001;39:4204–4207. doi: 10.1128/JCM.39.11.4204-4207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyx HL, Huxsoll DL. Red and gray foxes--potential reservoir hosts for Ehrlichia canis. J Wildl Dis. 1973;9:47–50. doi: 10.7589/0090-3558-9.1.47. [DOI] [PubMed] [Google Scholar]
- Andrew HR, Norval RA. The role of males of the bont tick (Amblyomma hebraeum) in the transmission of Cowdria ruminantium (heartwater) Vet Parasitol. 1989;34:15–23. doi: 10.1016/0304-4017(89)90159-3. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Levine JF, Evans TL, Braswell A, Heller J. Relative utilization of reptiles and rodents as hosts by immature Ixodes scapularis (Acari: Ixodidae) in the coastal plain of North Carolina, USA. Exp Appl Acarol. 1993;17:719–731. doi: 10.1007/BF00051830. [DOI] [PubMed] [Google Scholar]
- Baneth G, Waner T, Koplah A, Weinstein S, Keysary A. Survey of Ehrlichia canis antibodies among dogs in Israel. Vet Rec. 1996;138:257–259. doi: 10.1136/vr.138.11.257. [DOI] [PubMed] [Google Scholar]
- Barker SC, Murrell A. Systematics and evolution of ticks with a list of valid genus and species names. Parasitology. 2004;129(Suppl):S15–36. doi: 10.1017/s0031182004005207. [DOI] [PubMed] [Google Scholar]
- Bekker CP, de Vos S, Taoufik A, Sparagano OA, Jongejan F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet Microbiol. 2002;89:223–238. doi: 10.1016/s0378-1135(02)00179-7. [DOI] [PubMed] [Google Scholar]
- Bergman DK, Ramachandra RN, Wikel SK. Dermacentor andersoni: salivary gland proteins suppressing T-lymphocyte responses to concanavalin A in vitro. Exp Parasitol. 1995;81:262–271. doi: 10.1006/expr.1995.1117. [DOI] [PubMed] [Google Scholar]
- Bishopp FC, Trembley HL. Distribution and hosts of certain North American ticks. J Parasitology. 1945;31:1–42. [Google Scholar]
- Bloemer SR, Zimmerman RH. Ixodid ticks on the coyote and gray fox at land between the lakes, Kentucky-Tennessee, and implications for tick dispersal. J Med Entomol. 1988;25:5–8. doi: 10.1093/jmedent/25.1.5. [DOI] [PubMed] [Google Scholar]
- Bock RE, Kingston TG, De Vos AJ. Effect of breed of cattle on innate resistance to infection with Anaplasma marginale transmitted by Boophilus microplus. Aust Vet J. 1999;77:748–751. doi: 10.1111/j.1751-0813.1999.tb12920.x. [DOI] [PubMed] [Google Scholar]
- Bool PH, Sutmoller P. Ehrlichia canis infections in dogs on Aruba (Netherlands Antilles) J Am Vet Med Assoc. 1957;130:418–420. [PubMed] [Google Scholar]
- Botros BA, Elmolla MS, Salib AW, Calamaio CA, Dasch GA, Arthur RR. Canine ehrlichiosis in Egypt: sero-epidemiological survey. Onderstepoort J Vet Res. 1995;62:41–43. [PubMed] [Google Scholar]
- Boulay RP, Lappin MR, Holland CH, Thrall MA, Baker D, O'Neil S. Clinical ehrlichiosis in a cat. J Am Vet Med Assoc. 1994;204:1475–1478. [PubMed] [Google Scholar]
- Bram RA, Roby TO. Attempted hereditary transmission of Anaplasma marginale Theiler with Dermacentor albipictus (Packard) J Parasitol. 1970;56:620–621. [PubMed] [Google Scholar]
- Branger S, Rolain JM, Raoult D. Evaluation of antibiotic susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis, and Anaplasma phagocytophilum by real-time PCR. Antimicrob Agents Chemother. 2004;48:4822–4828. doi: 10.1128/AAC.48.12.4822-4828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Abrams-Ogg AC, Lappin MR, Bienzle D, Hancock SI, Cowan SM, Clooten JK, Hegarty BC, Hawkins EC. Molecular evidence supporting Ehrlichia canis-like infection in cats. J Vet Intern Med. 2002;16:642–649. doi: 10.1111/j.1939-1676.2002.tb02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer WG, Schaefer JJ, Wagner ER, Ewing SA, Rikihisa Y, Needham GR, Jittapalapong S, Moore DL, Stich RW. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet Parasitol. 2005;131:95–105. doi: 10.1016/j.vetpar.2005.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillhart DB, Fox LB, Upton SJ. Ticks (Acari: Ixodidae) collected from small and medium-sized Kansas mammals. J Med Entomol. 1994;31:500–504. doi: 10.1093/jmedent/31.3.500. [DOI] [PubMed] [Google Scholar]
- Brouqui P, Davoust B, Haddad S, Vidor E, Raoult D. Serological evaluation of Ehrlichia canis infections in military dogs in Africa and Reunion Island. Vet Microbiol. 1991;26:103–105. doi: 10.1016/0378-1135(91)90046-i. [DOI] [PubMed] [Google Scholar]
- Buhles WC, Jr, Huxsoll DL, Hildebrandt PK. Tropical canine pancytopenia: role of aplastic anaemia in the pathogenesis of severe disease. J Comp Pathol. 1975;85:511–521. doi: 10.1016/0021-9975(75)90118-8. [DOI] [PubMed] [Google Scholar]
- Buhles WC, Jr, Huxsoll DL, Ristic M. Tropical canine pancytopenia: Clinical, hematologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J Infect Dis. 1974;130:357–367. doi: 10.1093/infdis/130.4.357. [DOI] [PubMed] [Google Scholar]
- Bulla C, Kiomi Takahira R, Pessoa Araujo J, Jr, AparecidaTrinca L, Souza Lopes R, Wiedmeyer CE. The relationship between the degree of thrombocytopenia and infection with Ehrlichia canis in an endemic area. Vet Res. 2004;35:141–146. doi: 10.1051/vetres:2003038. [DOI] [PubMed] [Google Scholar]
- Chae JS, Kim CM, Kim EH, Hur EJ, Klein TA, Kang TK, Lee HC, Song JW. Molecular epidemiological study for tick-borne disease (Ehrlichia and Anaplasma spp.) surveillance at selected U.S. military training sites/installations in Korea. Ann N Y Acad Sci. 2003;990:118–125. doi: 10.1111/j.1749-6632.2003.tb07349.x. [DOI] [PubMed] [Google Scholar]
- Charpentier F, Groulade P. Un cas d'ehrlichiose probable chez le chat. Buulletin de l' Academie Veterinaire de France. 1986;59:287–290. [Google Scholar]
- Christova I, Van De Pol J, Yazar S, Velo E, Schouls L. Identification of Borrelia burgdorferi sensu lato, Anaplasma and Ehrlichia species, and spotted fever group rickettsiae in ticks from Southeastern Europe. Eur J Clin Microbiol Infect Dis. 2003;22:535–542. doi: 10.1007/s10096-003-0988-1. [DOI] [PubMed] [Google Scholar]
- Clark KL, Oliver JH, Jr, Grego JM, James AM, Durden LA, Banks CW. Host associations of ticks parasitizing rodents at Borrelia burgdorferi enzootic sites in South Carolina. J Parasitol. 2001;87:1379–1386. doi: 10.1645/0022-3395(2001)087[1379:HAOTPR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Coan ME, Zaugg JL, Stiller D. Probable anaplasmosis transmission by natural intrastadial interhost transfer of adult 3-host ticks. Abstr Proc 8th Ann West Conf Food Animal Vet Med; Boise, ID. 1987. p. 34. [Google Scholar]
- Cocco R, Sanna G, Cillara MG, Tola S, Ximenes L, Pinnaparpaglia ML, Masala G. Ehrlichiosis and rickettsiosis in a canine population of Northern Sardinia. Ann N Y Acad Sci. 2003;990:126–130. doi: 10.1111/j.1749-6632.2003.tb07350.x. [DOI] [PubMed] [Google Scholar]
- Dagnone AS, de Morais HS, Vidotto MC, Jojima FS, Vidotto O. Ehrlichiosis in anemic, thrombocytopenic, or tick-infested dogs from a hospital population in South Brazil. Vet Parasitol. 2003;117:285–290. doi: 10.1016/j.vetpar.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173–185. doi: 10.1016/j.vetpar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Davoust B, Bourry O, Gomez J, Lafay L, Casali F, Leroy E, Parzy D. Surveys on seroprevalence of canine monocytic ehrlichiosis among dogs living in the Ivory Coast and Gabon and evaluation of a quick commercial test kit dot-ELISA. Ann N Y Acad Sci. 2006;1078:464–469. doi: 10.1196/annals.1374.086. [DOI] [PubMed] [Google Scholar]
- Dawson JE, Anderson BE, Fishbein DB, Sanchez JL, Goldsmith CS, Wilson KH, Duntley CW. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JE, Childs JE, Biggie KL, Moore C, Stallknecht D, Shaddock J, Bouseman J, Hofmeister E, Olson JG. White-tailed deer as a potential reservoir of Ehrlichia spp. J Wildl Dis. 1994;30:162–168. doi: 10.7589/0090-3558-30.2.162. [DOI] [PubMed] [Google Scholar]
- Dawson JE, Ewing SA. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am J Vet Res. 1992;53:1322–1327. [PubMed] [Google Scholar]
- de Castro MB, Machado RZ, de Aquino LP, Alessi AC, Costa MT. Experimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings. Vet Parasitol. 2004;119:73–86. doi: 10.1016/j.vetpar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Donatien A, Lestoquard F. Existence in Algerie dume Rickettsia du chien. Bull Soc Pathol Exot. 1935;28:418–419. [Google Scholar]
- Doyle CK, Labruna MB, Breitschwerdt EB, Tang YW, Corstvet RE, Hegarty BC, Bloch KC, Li P, Walker DH, McBride JW. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J Mol Diagn. 2005;7:504–510. doi: 10.1016/S1525-1578(10)60581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- El Said AM, Swiderski Z. Copulation and spermatophore formation in Hyalomma dromedarii. J Egypt Soc Parasitol. 1983;13:59–62. [PubMed] [Google Scholar]
- Ewing SA. Observation on leukocytic inclusion bodies from dogs infected with Babesia canis. J Am Vet Med Assoc. 1963;143:503–506. [PubMed] [Google Scholar]
- Ewing SA. Correspondence. J Am Vet Med Assoc. 1964;144:4. [Google Scholar]
- Ewing SA. Canine ehrlichiosis. Adv Vet Sci Comp Med. 1969;13:331–353. [PubMed] [Google Scholar]
- Ewing SA, Buckner RG. Manifestations of babesiosis, ehrlichiosis, and combined infections in the dog. Am J Vet Res. 1965;26:815–828. [PubMed] [Google Scholar]
- Ewing SA, Bucker RG, Springer BC. The coyote, a potential host for Babesia canis and Ehrlichia sp. J Parasitol. 1964;50:704. [PubMed] [Google Scholar]
- Ewing SA, Dawson JE, Kocan AA, Barker RW, Warner CK, Panciera RJ, Fox JC, Kocan KM, Blouin EF. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- Ewing SA, Harkess JR, Kocan KM, Barker RW, Fox JC, Tyler RD, Cowell RL, Morton RB. Failure to transmit Ehrlichia canis (Rickettsiales: Ehrlichieae) with Otobius megnini (Acari: Argasidae) J Med Entomol. 1990;27:803–806. doi: 10.1093/jmedent/27.5.803. [DOI] [PubMed] [Google Scholar]
- Ewing SA, Johnson EM, Kocan KM. Human infection with Ehrlichia canis [letter] N Engl J Med. 1987;317:899–900. doi: 10.1056/NEJM198710013171412. [DOI] [PubMed] [Google Scholar]
- Ewing SA, Panciera RJ, Kocan KM, Ge NL, Welsh RD, Olson RW, Barker RW, Rice LE. A winter outbreak of anaplasmosis in a nonendemic area of Oklahoma: a possible role for Dermacentor albipictus. J Vet Diagn Invest. 1997;9:206–208. doi: 10.1177/104063879700900219. [DOI] [PubMed] [Google Scholar]
- Fenollar F, Raoult D. Molecular genetic methods for the diagnosis of fastidious microorganisms. Apmis. 2004;112:785–807. doi: 10.1111/j.1600-0463.2004.apm11211-1206.x. [DOI] [PubMed] [Google Scholar]
- Filoni C, Catao-Dias JL, Bay G, Durigon EL, Jorge RS, Lutz H, Hofmann-Lehmann R. First evidence of feline herpesvirus, calicivirus, parvovirus, and Ehrlichia exposure in Brazilian free-ranging felids. J Wildl Dis. 2006;42:470–477. doi: 10.7589/0090-3558-42.2.470. [DOI] [PubMed] [Google Scholar]
- Fishman Z, Gonen L, Harrus S, Strauss-Ayali D, King R, Baneth G. A serosurvey of Hepatozoon canis and Ehrlichia canis antibodies in wild red foxes (Vulpes vulpes) from Israel. Vet Parasitol. 2004;119:21–26. doi: 10.1016/j.vetpar.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Foley JE, Foley P, Jecker M, Swift PK, Madigan JE. Granulocytic ehrlichiosis and tick infestation in mountain lions in California. J Wildl Dis. 1999;35:703–709. doi: 10.7589/0090-3558-35.4.703. [DOI] [PubMed] [Google Scholar]
- Gage KL, Hopla CE, Schwan TG. Cotton rats and other small mammals as hosts for immature Dermacentor variabilis (Acari: Ixodidae) in central Oklahoma. J Med Entomol. 1992;29:832–842. doi: 10.1093/jmedent/29.5.832. [DOI] [PubMed] [Google Scholar]
- Gary AT, Webb JA, Hegarty BC, Breitschwerdt EB. The low seroprevalence of tick-transmitted agents of disease in dogs from southern Ontario and Quebec. Can Vet J. 2006;47:1194–1200. [PMC free article] [PubMed] [Google Scholar]
- Gaunt SD, Corstvet RE, Berry CM, Brennan B. Isolation of Ehrlichia canis from dogs following subcutaneous inoculation. J Clin Microbiol. 1996;34:1429–1432. doi: 10.1128/jcm.34.6.1429-1432.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS. A carbon dioxide trap for prolonged sampling of Ixodes ricinus L. populations. Exp Appl Acarol. 1985;1:35–44. doi: 10.1007/BF01262198. [DOI] [PubMed] [Google Scholar]
- Groves MG, Dennis GL, Amyx HL, Huxsoll DL. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus) Am J Vet Res. 1975;36:937–940. [PubMed] [Google Scholar]
- Harlan HJ, Foster WA. Micrometeorologic factors affecting field host-seeking activity of adult Dermacentor variabilis (Acari: Ixodidae) J Med Entomol. 1990;27:471–479. doi: 10.1093/jmedent/27.4.471. [DOI] [PubMed] [Google Scholar]
- Harrus S, Waner T, Aizenberg I, Foley JE, Poland AM, Bark H. Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J Clin Microbiol. 1998;36:73–76. doi: 10.1128/jcm.36.1.73-76.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrus S, Waner T, Bark H, Jongejan F, Cornelissen AW. Recent advances in determining the pathogenesis of canine monocytic ehrlichiosis. J Clin Microbiol. 1999;37:2745–2749. doi: 10.1128/jcm.37.9.2745-2749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JW, Simpson CF, Gaskin JM, Sameck JH. Ehrlichiosis in wolves, dogs, and wolf-dog crosses. J Am Vet Med Assoc. 1979;175:901–905. [PubMed] [Google Scholar]
- Hinrichsen VL, Whitworth UG, Breitschwerdt EB, Hegarty BC, Mather TN. Assessing the association between the geographic distribution of deer ticks and seropositivity rates to various tick-transmitted disease organisms in dogs. J Am Vet Med Assoc. 2001;218:1092–1097. doi: 10.2460/javma.2001.218.1092. [DOI] [PubMed] [Google Scholar]
- Homsher PJ, Sonenshine DE. Spermatogenesis in Dermacentor variabilis (Say) in relation to duration of attachment and the presence of chromosomal aberrations in stocks injected with radioactive glucose (Acarina: Ixodidae) J Med Entomol. 1972;9:171–177. doi: 10.1093/jmedent/9.2.171. [DOI] [PubMed] [Google Scholar]
- Homsher PJ, Sonenshine DE. The effect of presence of females on spermatogenesis and early mate seeking behavior in two species of Dermacentor ticks (Acari : Ixodidae) Acarologia. 1976;18:226–233. [PubMed] [Google Scholar]
- Horak IG. Ixodid ticks collected at the Faculty of Veterinary Science, Onderstepoort, from dogs diagnosed with Babesia canis infection. J S Afr Vet Assoc. 1995;66:170–171. [PubMed] [Google Scholar]
- Horak IG, Boomker J, Flamand JR. Parasites of domestic and wild animals in South Africa. XXXIV. Arthropod parasites of nyalas in north-eastern KwaZulu-Natal. Onderstepoort J Vet Res. 1995a;62:171–179. [PubMed] [Google Scholar]
- Horak IG, Guillarmod AJ, Moolman LC, de Vos V. Parasites of domestic and wild animals in South Africa. XXII. Ixodid ticks on domestic dogs and on wild carnivores. Onderstepoort J Vet Res. 1987;54:573–580. [PubMed] [Google Scholar]
- Horak IG, Spickett AM, Braack LE, Penzhorn BL. Parasites of domestic and wild animals in South Africa. XXXII. Ixodid ticks on scrub hares in the Transvaal. Onderstepoort J Vet Res. 1993;60:163–174. [PubMed] [Google Scholar]
- Horak IG, Spickett AM, Braack LE, Penzhorn BL, Bagnall RJ, Uys AC. Parasites of domestic and wild animals in South Africa. XXXIII. Ixodid ticks on scrub hares in the north-eastern regions of northern and eastern Transvaal and of KwaZulu-Natal. Onderstepoort J Vet Res. 1995b;62:123–131. [PubMed] [Google Scholar]
- Hoskins JD, Breitschwerdt EB, Gaunt SD, French TW, Burgdorfer W. Antibodies to Ehrlichia canis, Ehrlichia platys, and spotted fever group rickettsiae in Louisiana dogs. J Vet Intern Med. 1988;2:55–59. doi: 10.1111/j.1939-1676.1988.tb02793.x. [DOI] [PubMed] [Google Scholar]
- Huxsoll DL, Amyx HL, Hemelt IE, Hildebrandt PK, Nims RM, Gochenour WS., Jr Laboratory studies of tropical canine pancytopenia. Exp Parasitol. 1972;31:53–59. doi: 10.1016/0014-4894(72)90046-x. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Beppu T, Okuda M, Shimada Y, Sakata Y. Epidemiological survey of Anaplasma platys and Ehrlichia canis using ticks collected from dogs in Japan. Vet Parasitol. 2003;115:343–348. doi: 10.1016/s0304-4017(03)00238-3. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Makino T, Kabeya H, Nogami S, Fujita H, Asano M, Inoue S, Maruyama S. Serological survey of Ehrlichia and Anaplasma infection of feral raccoons (Procyon lotor) in Kanagawa Prefecture, Japan. Vet Parasitol. 2006a doi: 10.1016/j.vetpar.2006.11.002. in press. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Ohno K, Yamamoto S. Serosurvey of Ehrlichia canis and Hepatozoon canis infection in dogs in Yamaguchi Prefecture, Japan. J Vet Med Sci. 1999;61:1153–1155. doi: 10.1292/jvms.61.1153. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Oyamada M, Davoust B, Boni M, Dereure J, Bucheton B, Hammad A, Watanabe M, Itamoto K, Okuda M, Brouqui P. Epidemiological Survey of Ehrlichia canis and Related Species Infection in Dogs in Eastern Sudan. Ann N Y Acad Sci. 2006b;1078:461–463. doi: 10.1196/annals.1374.085. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Chaichanasiriwithaya W, Rikihisa Y. Comparison of PCR with other tests for early diagnosis of canine ehrlichiosis. J Clin Microbiol. 1994;32:1658–1662. doi: 10.1128/jcm.32.7.1658-1662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal Z, Rikihisa Y. Reisolation of Ehrlichia canis from blood and tissues of dogs after doxycycline treatment. J Clin Microbiol. 1994;32:1644–1649. doi: 10.1128/jcm.32.7.1644-1649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JO, DeFoliart GR, Sonenshine DE, Stout J. Relationships of immature Dermacentor variabilis (Say) (Acari: Ixodidae) with the white-footed mouse, Peromyscus leucopus, in southwestern Wisconsin. J Med Entomol. 1975;12:409–412. doi: 10.1093/jmedent/12.4.409. [DOI] [PubMed] [Google Scholar]
- Jittapalapong S, Jansawan W. Preliminary Study of Blood Parasites in Cats in Bangkok. Kaset J (Natural Sciences) 1993;27:330–333. [Google Scholar]
- Jittapalapong S, Nimsuphan B, Stich RW, Maruyama S, Bruqui P, Inokuma H. Detection of antibodies against Ehrlichia canis, Ehrlichia chaffeensis and Anaplasma phagocytophila in stray dogs in Bangkok, Thailand. The 28th World Small Animal Veterinary Association; Bangkok, Thailand. October 24-27.2003. [Google Scholar]
- Jittapalapong S, Nimsuphan B, Stich RW, Maruyama S, Bruqui P, Inokuma H. Detection of antibodies against Ehrlichia canis, Ehrlichia chaffeensis and Anaplasma phagocytophila in stray cats in Bangkok, Thailand. Joint International Tropical Medicine Meeting; Bangkok, Thailand. Nov 29-Dec 1, 2004.2004. [Google Scholar]
- Johnson EM, Ewing SA, Barker RW, Fox JC, Crow DW, Kocan KM. Experimental transmission of Ehrlichia canis (Rickettsiales: Ehrlichieae) by Dermacentor variabilis (Acari: Ixodidae) Vet Parasitol. 1998;74:277–288. doi: 10.1016/s0304-4017(97)00073-3. [DOI] [PubMed] [Google Scholar]
- Jouret-Gourjault S, Parzy D, Davoust B. Experimental infections in dogs with Ehrlichia canis strain Borgo 89. Ann N Y Acad Sci. 2006;1078:470–475. doi: 10.1196/annals.1374.132. [DOI] [PubMed] [Google Scholar]
- Kakoma I, Hansen RD, Anderson BE, Hanley TA, Sims KG, Liu L, Bellamy C, Long MT, Baek BK. Cultural, molecular, and immunological characterization of the etiologic agent for atypical canine ehrlichiosis. J Clin Microbiol. 1994;32:170–175. doi: 10.1128/jcm.32.1.170-175.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe TJ, Holland CJ, Salyer PE, Ristic M. Distribution of Ehrlichia canis among military working dogs in the world and selected civilian dogs in the United States. J Am Vet Med Assoc. 1982;181:236–238. [PubMed] [Google Scholar]
- Kelly PJ, Eoghain GN, Raoult D. Antibodies reactive with Bartonella henselae and Ehrlichia canis in dogs from the communal lands of Zimbabwe. J S Afr Vet Assoc. 2004;75:116–120. doi: 10.4102/jsava.v75i3.465. [DOI] [PubMed] [Google Scholar]
- Keysary A, Waner T, Rosner M, Warner CK, Dawson JE, Zass R, Biggie KL, Harrus S. The first isolation, in vitro propagation, and genetic characterization of Ehrlichia canis in Israel. Vet Parasitol. 1996;62:331–340. doi: 10.1016/0304-4017(95)00866-7. [DOI] [PubMed] [Google Scholar]
- Kiszewski AE, Matuschka FR, Spielman A. Mating strategies and spermiogenesis in ixodid ticks. Annu Rev Entomol. 2001;46:167–182. doi: 10.1146/annurev.ento.46.1.167. [DOI] [PubMed] [Google Scholar]
- Kocan KM, Goff WL, Stiller D, Claypool PL, Edwards W, Ewing SA, Hair JA, Barron SJ. Persistence of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in male Dermacentor andersoni (Acari: Ixodidae) transferred successively from infected to susceptible calves. J Med Entomol. 1992;29:657–668. doi: 10.1093/jmedent/29.4.657. [DOI] [PubMed] [Google Scholar]
- Kollars TM., Jr Ticks (Acari: Ixodidae) infesting medium-sized wild mammals in southwestern Tennessee. J Med Entomol. 1993;30:896–900. doi: 10.1093/jmedent/30.5.896. [DOI] [PubMed] [Google Scholar]
- Kollars TM., Jr Interspecific differences between small mammals as hosts of immature Dermacentor variabilis (Acari: Ixodidae) and a model for detection of high risk areas of Rocky Mountain spotted fever. J Parasitol. 1996;82:707–710. [PubMed] [Google Scholar]
- Kollars TM, Jr, Oliver JH, Jr, Masters EJ, Kollars PG, Durden LA. Host utilization and seasonal occurrence of Dermacentor species (Acari: Ixodidae) in Missouri, USA. Exp Appl Acarol. 2000;24:631–643. doi: 10.1023/a:1026566301325. [DOI] [PubMed] [Google Scholar]
- Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, Bradley JM, Rumbough R, McPherson JT, MacCormack JN. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hostis M, Rose-Rosette F, Thomas N, Fourgeaud P. Tick infestation of feral dogs in Martinique. Ann N Y Acad Sci. 1998;849:395–397. doi: 10.1111/j.1749-6632.1998.tb11079.x. [DOI] [PubMed] [Google Scholar]
- Labarthe N, de Campos Pereira M, Barbarini O, McKee W, Coimbra CA, Hoskins J. Serologic prevalence of Dirofilaria immitis, Ehrlichia canis, and Borrelia burgdorferi infections in Brazil. Vet Ther. 2003;4:67–75. [PubMed] [Google Scholar]
- Labruna MB, McBride JW, Camargo LM, Aguiar DM, Yabsley MJ, Davidson WR, Stromdahl EY, Williamson PC, Stich RW, Long SW, Camargo EP, Walker DH. A preliminary investigation of Ehrlichia species in ticks, humans, dogs, and capybaras from Brazil. Vet Parasitol. 2007;143:189–195. doi: 10.1016/j.vetpar.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Lappin MR, Breitschwerdt EB, Jensen WA, Dunnigan B, Rha JY, Williams CR, Brewer M, Fall M. Molecular and serologic evidence of Anaplasma phagocytophilum infection in cats in North America. J Am Vet Med Assoc. 2004;225:893–896. 879. doi: 10.2460/javma.2004.225.893. [DOI] [PubMed] [Google Scholar]
- Leahy MG, Booth KS. Attraction of metastriate ticks (Acari: Ixodidae) to the sex pheromone 2,6-dichlorophenol and to substituted phenols. J Med Entomol. 1983;20:104–105. doi: 10.1093/jmedent/20.1.104. [DOI] [PubMed] [Google Scholar]
- Lewis GE, Jr, Huxsoll DL, Ristic M, Johnson AJ. Experimentally induced infection of dogs, cats, and nonhuman primates with Ehrlichia equi, etiologic agent of equine ehrlichiosis. Am J Vet Res. 1975;36:85–88. [PubMed] [Google Scholar]
- Lewis GE, Jr, Ristic M, Smith RD, Lincoln T, Stephenson EH. The brown dog tick Rhipicephalus sanguineus and the dog as experimental hosts of Ehrlichia canis. Am J Vet Res. 1977;38:1953–1955. [PubMed] [Google Scholar]
- Little SE, Hostetler J, Kocan KM. Movement of Rhipicephalus sanguineus adults between co-housed dogs during active feeding. Vet Parasitol. 2007;150:139–145. doi: 10.1016/j.vetpar.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Loftis AD, Reeves WK, Szumlas DE, Abbassy MM, Helmy IM, Moriarity JR, Dasch GA. Rickettsial agents in Egyptian ticks collected from domestic animals. Exp Appl Acarol. 2006;40:67–81. doi: 10.1007/s10493-006-9025-2. [DOI] [PubMed] [Google Scholar]
- Londt GH, Van Der Bijl EB. The life cycle of the two-host tick Rhipicephalus evertsi evertsi Neumann, 1897, under laboratory conditions (Acarina: Ixodidae) Onderstepoort J Vet Res. 1977;44:21–28. [PubMed] [Google Scholar]
- Londt JG, Spickett AM. Gonad development and gametogenesis in Boophilus decoloratus (Koch, 1844) (Acarina Metastriata Ixodidae) Onderstepoort J Vet Res. 1976;43:79–96. [PubMed] [Google Scholar]
- Long SW, Zhang X, Zhang J, Ruble RP, Teel P, Yu XJ. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 2003;40:1000–1004. doi: 10.1603/0022-2585-40.6.1000. [DOI] [PubMed] [Google Scholar]
- Macieira DB, Messick JB, Cerqueira AM, Freire IM, Linhares GF, Almeida NK, Almosny NR. Prevalence of Ehrlichia canis infection in thrombocytopenic dogs from Rio de Janeiro, Brazil. Vet Clin Pathol. 2005;34:44–48. doi: 10.1111/j.1939-165x.2005.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Maeda K, Markowitz N, Hawley RC, Ristic M, Cox D, McDade JE. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- Magnarelli LA, Anderson JF. Serologic evidence of canine and equine ehrlichiosis in northeastern United States. J Clin Microbiol. 1993;31:2857–2860. doi: 10.1128/jcm.31.11.2857-2860.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli LA, Andreadis TG, Stafford KC, Holland CJ. Rickettsiae and Borrelia burgdorferi in ixodid ticks. J Clin Microbiol. 1991;29:2798–2804. doi: 10.1128/jcm.29.12.2798-2804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli LA, Litwin HJ, Holland CJ, Anderson JF, Ristic M. Canine ehrlichiosis in Connecticut. J Clin Microbiol. 1990;28:366–367. doi: 10.1128/jcm.28.2.366-367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsilio F, Di Martino B, Meridiani I, Bianciardi P. Direct identification of Ehrlichia canis by a novel polymerase chain reaction method and molecular analysis of the citrate synthase (gltA) gene from various Italian strains. J Vet Diagn Invest. 2006;18:215–217. doi: 10.1177/104063870601800215. [DOI] [PubMed] [Google Scholar]
- Mason CA, Norval RAI. The transfer of Boophilus microplus(Acarina: Ixodidae) from infested to uninfested cattle under field conditions. Vet Parasitol. 1981;8:185–188. [Google Scholar]
- Mathew JS, Ewing SA, Barker RW, Fox JC, Dawson JE, Warner CK, Murphy GL, Kocan KM. Attempted transmission of Ehrlichia canis by Rhipicephalus sanguineus after passage in cell culture. Am J Vet Res. 1996;57:1594–1598. [PubMed] [Google Scholar]
- Matthewman LA, Kelly PJ, Bobade PA, Tagwira M, Mason PR, Majok A, Brouqui P, Raoult D. Infections with Babesia canis and Ehrlichia canis in dogs in Zimbabwe. Vet Rec. 1993;133:344–346. doi: 10.1136/vr.133.14.344. [DOI] [PubMed] [Google Scholar]
- Meinkoth JH, Ewing SA, Cowell RL, Dawson JE, Warner CK, Mathew JS, Bowles M, Thiessen AE, Panciera RJ, Fox C. Morphologic and molecular evidence of a dual species ehrlichial infection in a dog presenting with inflammatory central nervous system disease. J Vet Intern Med. 1998;12:389–393. doi: 10.1111/j.1939-1676.1998.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. 1998;79:325–339. doi: 10.1016/s0304-4017(98)00179-4. [DOI] [PubMed] [Google Scholar]
- Mylonakis ME, Koutinas AF, Breitschwerdt EB, Hegarty BC, Billinis CD, Leontides LS, Kontos VS. Chronic canine ehrlichiosis (Ehrlichia canis): a retrospective study of 19 natural cases. J Am Anim Hosp Assoc. 2004;40:174–184. doi: 10.5326/0400174. [DOI] [PubMed] [Google Scholar]
- Ndip LM, Ndip RN, Esemu SN, Dickmu VL, Fokam EB, Walker DH, McBride JW. Ehrlichial infection in Cameroonian canines by Ehrlichia canis and Ehrlichia ewingii. Vet Microbiol. 2005;111:59–66. doi: 10.1016/j.vetmic.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Neer TM, Harrus S. Canine monocytotrpoic ehrlichiosis and neorickettsiosis (E. canis, E. chaffeensis, E. ruminantium, N. sennetsu, and N. risticii infections) In: Greene CE, editor. Infectious Diseases of the Dog and Cat Saunders. Elsevier; St. Louis, Missouri: 2006. pp. 203–216. [Google Scholar]
- Nims RM, Ferguson JA, Walker JL, Hildebrandt PK, Huxsoll DL, Reardon MJ, Varley JE, Kolaja GJ, Watson WT, Shroyer EL, Elwell PA, Vacura GW. Epizootiology of tropical canine pancytopenia in Southeast Asia. J Am Vet Med Assoc. 1971;158:53–63. [PubMed] [Google Scholar]
- Norval RA, Andrew HR, Yunker CE. Pheromone-mediation of host-selection in bont ticks (Amblyomma hebraeum koch) Science. 1989;243:364–365. doi: 10.1126/science.2911745. [DOI] [PubMed] [Google Scholar]
- Norval RA, Sonenshine DE, Allan SA, Burridge MJ. Efficacy of pheromone-acaricide-impregnated tail-tag decoys for controlling the bont tick, Amblyomma hebraeum (Acari: Ixodidae), on cattle in Zimbabwe. Exp Appl Acarol. 1996;20:31–46. doi: 10.1007/BF00051475. [DOI] [PubMed] [Google Scholar]
- Nyindo MB, Ristic M, Huxsoll DL, Smith AR. Tropical canine pancytopenia: in vitro cultivation of the causative agent--Ehrlichia canis. Am J Vet Res. 1971;32:1651–1658. [PubMed] [Google Scholar]
- O'Connor TP, Hanscom JL, Hegarty BC, Groat RG, Breitschwerdt EB. Comparison of an indirect immunofluorescence assay, western blot analysis, and a commercially available ELISA for detection of Ehrlichia canis antibodies in canine sera. Am J Vet Res. 2006;67:206–210. doi: 10.2460/ajvr.67.2.206. [DOI] [PubMed] [Google Scholar]
- Olano JP, Walker DH. Human ehrlichioses. Med Clin North Am. 2002;86:375–392. doi: 10.1016/s0025-7125(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Oliveira PR, Borges LM, Lopes CM, Leite RC. Population dynamics of the free-living stages of Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) on pastures of Pedro Leopoldo, Minas Gerais State, Brazil. Vet Parasitol. 2000;92:295–301. doi: 10.1016/s0304-4017(00)00322-8. [DOI] [PubMed] [Google Scholar]
- Oliver JH, Dotson EM. Hormonal control of molting and reproduction in ticks. Am Zool. 1993;33:384–396. [Google Scholar]
- Ortuno A, Gauss CB, Garcia F, Gutierrez JF. Serological evidence of Ehrlichia spp. exposure in cats from northeastern Spain. J Vet Med B Infect Dis Vet Public Health. 2005;52:246–248. doi: 10.1111/j.1439-0450.2005.00849.x. [DOI] [PubMed] [Google Scholar]
- Ouellette J, Apperson CS, Howard P, Evans TL, Levine JF. Tick-raccoon associations and the potential for Lyme disease spirochete transmission in the coastal plain of North Carolina. J Wildl Dis. 1997;33:28–39. doi: 10.7589/0090-3558-33.1.28. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Childs JE. Ehrlichia chaffeensis: a Prototypical Emerging Pathogen. Clin Microbiol Rev. 2003;16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann N Y Acad Sci. 2006;1078:110–117. doi: 10.1196/annals.1374.016. [DOI] [PubMed] [Google Scholar]
- Perez M, Rikihisa Y, Wen B. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J Clin Microbiol. 1996;34:2133–2139. doi: 10.1128/jcm.34.9.2133-2139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyoowong D, Jittapalapong S, Suksawat F, Stich RW, Thamchaipenet A. Molecular characterization of Thai Ehrlichia canis and Anaplasma platys strains detected in dogs. Infect Genet Evol. 2008;8:433–438. doi: 10.1016/j.meegid.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Potgeiter FT. Tick transmission of anaplasmosis in South Africa. International Conference on Tick Biology and Control; Grahamstown, South Africa. 1981. p. 222. [Google Scholar]
- Pretorius AM, Kelly PJ. Serological survey for antibodies reactive with Ehrlichia canis and E. chaffeensis in dogs from the Bloemfontein area, South Africa. J S Afr Vet Assoc. 1998;69:126–128. doi: 10.4102/jsava.v69i4.840. [DOI] [PubMed] [Google Scholar]
- Pung OJ, Durden LA, Banks CW, Jones DN. Ectoparasites of opossums and raccoons in southeastern Georgia. J Med Entomol. 1994;31:915–919. doi: 10.1093/jmedent/31.6.915. [DOI] [PubMed] [Google Scholar]
- Pusterla N, Deplazes P, Braun U, Lutz H. Serological evidence of infection with Ehrlichia spp. in red foxes (Vulpes vulpes) in Switzerland. J Clin Microbiol. 1999;37:1168–1169. doi: 10.1128/jcm.37.4.1168-1169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusterla N, Pusterla JB, Deplazes P, Wolfensberger C, Muller W, Horauf A, Reusch C, Lutz H. Seroprevalence of Ehrlichia canis and of canine granulocytic Ehrlichia infection in dogs in Switzerland. J Clin Microbiol. 1998;36:3460–3462. doi: 10.1128/jcm.36.12.3460-3462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamanickam C, Wiesenhutter E, Zin FM, Hamid J. The incidence of canine haematozoa in Peninsular Malaysia. Vet Parasitol. 1985;17:151–157. doi: 10.1016/0304-4017(85)90101-3. [DOI] [PubMed] [Google Scholar]
- Reardon MJ, Pierce KR. Acute experimental canine ehrlichiosis. I. Sequential reaction of the hemic and lymphoreticular systems. Vet Pathol. 1981;18:48–61. doi: 10.1177/030098588101800106. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, Ewing SA, Fox JC, Siregar AG, Pasaribu FH, Malole MB. Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J Clin Microbiol. 1992;30:143–148. doi: 10.1128/jcm.30.1.143-148.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers SJ, Morton RJ, Baldwin CA. A serological survey of Ehrlichia canis, Ehrlichia equi, Rickettsia rickettsii, and Borrelia burgdorferi in dogs in Oklahoma. J Vet Diagn Invest. 1989;1:154–159. doi: 10.1177/104063878900100212. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vivas RI, Albornoz RE, Bolio GM. Ehrlichia canis in dogs in Yucatan, Mexico: seroprevalence, prevalence of infection and associated factors. Vet Parasitol. 2005;127:75–79. doi: 10.1016/j.vetpar.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Seaman RL, Kania SA, Hegarty BC, Legendre AM, Breitschwerdt EB. Comparison of results for serologic testing and a polymerase chain reaction assay to determine the prevalence of stray dogs in eastern Tennessee seropositive to Ehrlichia canis. Am J Vet Res. 2004;65:1200–1203. doi: 10.2460/ajvr.2004.65.1200. [DOI] [PubMed] [Google Scholar]
- Shibata S, Kawahara M, Rikihisa Y, Fujita H, Watanabe Y, Suto C, Ito T. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J Clin Microbiol. 2000;38:1331–1338. doi: 10.1128/jcm.38.4.1331-1338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotarczak B. Canine ehrlichiosis. Ann Agric Environ Med. 2003;10:137–141. [PubMed] [Google Scholar]
- Smith RD, Sells DM, Stephenson EH, Ristic MR, Huxsoll DL. Development of Ehrlichia canis, causative agent of canine ehrlichiosis, in the tick Rhipicephalus sanguineus and its differentiation from a symbiotic Rickettsia. Am J Vet Res. 1976;37:119–126. [PubMed] [Google Scholar]
- Sobbhy H, Aggour MG, Sonenshine DE, Burridge MJ. Cholesteryl esters on the body surfaces of the camel tick, Hyalomma dromedarii (Koch, 1844) and the brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) Exp Appl Acarol. 1994;18:265–280. doi: 10.1007/BF00132316. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L, Hegarty B, Espada Y, Llull J, Breitschwerdt E. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet Microbiol. 2006a;118:274–277. doi: 10.1016/j.vetmic.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L, Trotta M, Razia L, Furlanello T, Caldin M. Molecular Survey of Ehrlichia canis and Anaplasma phagocytophilum from Blood of Dogs in Italy. Ann N Y Acad Sci. 2006b;1078:515–518. doi: 10.1196/annals.1374.101. [DOI] [PubMed] [Google Scholar]
- Solberg VB, Neidhardt K, Sardelis MR, Hildebrandt C, Hoffmann FJ, Boobar LR. Quantitative evaluation of sampling methods for Ixodes dammini and Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1992;29:451–456. doi: 10.1093/jmedent/29.3.451. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Pheromones and other semiochemicals of the acari. Annu Rev Entomol. 1985;30:1–28. doi: 10.1146/annurev.en.30.010185.000245. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of Ticks. Oxford University Press; New York: 1991. [Google Scholar]
- Sonenshine DE, Atwood EL, Lamb JT., Jr The ecology of ticks transmitting Rocky Mountain spotted fever in a study area in Virginia. Ann Entomol Soc Am. 1966;59:1234–1262. doi: 10.1093/aesa/59.6.1234. [DOI] [PubMed] [Google Scholar]
- Sparagano OA, Allsopp MT, Mank RA, Rijpkema SG, Figueroa JV, Jongejan F. Molecular detection of pathogen DNA in ticks (Acari: Ixodidae): a review. Exp Appl Acarol. 1999;23:929–960. doi: 10.1023/a:1006313803979. [DOI] [PubMed] [Google Scholar]
- Spickett AM, Horak IG, Braack LE, van Ark H. Drag-sampling of free-living ixodid ticks in the Kruger National Park. Onderstepoort J Vet Res. 1991;58:27–32. [PubMed] [Google Scholar]
- Spickett AM, Horak IG, Van Niekerk A, Braack LE. The effect of veld-burning on the seasonal abundance of free-living ixodid ticks as determined by drag-sampling. Onderstepoort J Vet Res. 1992;59:285–292. [PubMed] [Google Scholar]
- Stephenson EH, Clothier ER, Ristic M. Ehrlichia canis infection in a dog in Virginia. J Am Vet Med Assoc. 1975;167:71–72. [PubMed] [Google Scholar]
- Stephenson EH, Ristic M. Retrospective study of an Ehrlichia canis epizootic around Phoenix, Arizona. J Am Vet Med Assoc. 1978;172:63–65. [PubMed] [Google Scholar]
- Stich RW, Kocan KM, Palmer GH, Ewing SA, Hair JA, Barron SJ. Transstadial and attempted transovarial transmission of Anaplasma marginale by Dermacentor variabilis. Am J Vet Res. 1989;50:1377–1380. [PubMed] [Google Scholar]
- Stich RW, Rikihisa Y, Ewing SA, Needham GR, Grover DL, Jittapalapong S. Detection of Ehrlichia canis in canine carrier blood and in individual experimentally infected ticks with a p30-based PCR assay. J Clin Microbiol. 2002;40:540–546. doi: 10.1128/JCM.40.2.540-546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich RW, Sauer JR, Bantle JA, Kocan KM. Detection of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in secretagogue-induced oral secretions of Dermacentor andersoni (Acari: Ixodidae) with the polymerase chain reaction. J Med Entomol. 1993;30:789–794. doi: 10.1093/jmedent/30.4.789. [DOI] [PubMed] [Google Scholar]
- Stiller D, Coan ME. Recent developments in elucidating tick vector relationships for anaplasmosis and equine piroplasmosis. Vet Parasitol. 1995;57:97–108. doi: 10.1016/0304-4017(94)03114-c. [DOI] [PubMed] [Google Scholar]
- Stiller D, Coan ME, Goff WL, Johnson LW, McGuire TC. The importance and putative role of Dermacentor spp. males in anaplasmosis epidemiology: transmission of Anaplasma marginale to cattle by ad libitum interhost transfer of D. andersoni males under semi-natural conditions. Eighth National Veterinary Hemoparasite Disease Conference; St. Louis, MO. 1989. p. 209. [Google Scholar]
- Stiller D, Johnson LW, Kuttker KL. Experimental transmission of Anaplasma ovis by Dermacentor albipictus and Dermacentor variabilis male ticks. Abstr Proc 8th Ann West Conf Food Animal Vet Med; Boise, ID. 1987. p. 36. [Google Scholar]
- Stockham SL, Schmidt DA, Curtis KS, Schauf BG, Tyler JW, Simpson ST. Evaluation of granulocytic ehrlichiosis in dogs of Missouri, including serologic status to Ehrlichia canis, Ehrlichia equi and Borrelia burgdorferi. Am J Vet Res. 1992;53:63–68. [PubMed] [Google Scholar]
- Suksawat J, Hegarty BC, Breitschwerdt EB. Seroprevalence of Ehrlichia canis, Ehrlichia equi, and Ehrlichia risticii in sick dogs from North Carolina and Virginia. J Vet Intern Med. 2000;14:50–55. doi: 10.1892/0891-6640(2000)014<0050:socear>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Suksawat J, Pitulle C, Arraga-Alvarado C, Madrigal K, Hancock SI, Breitschwerdt EB. Coinfection with three Ehrlichia species in dogs from Thailand and Venezuela with emphasis on consideration of 16S ribosomal DNA secondary structure. J Clin Microbiol. 2001;39:90–93. doi: 10.1128/JCM.39.1.90-93.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarello W. Microscopic and clinical evidence for Anaplasma (Ehrlichia) phagocytophilum infection in Italian cats. Vet Rec. 2005;156:772–774. doi: 10.1136/vr.156.24.772. [DOI] [PubMed] [Google Scholar]
- Trapp SM, Dagnone AS, Vidotto O, Freire RL, Amude AM, de Morais HS. Seroepidemiology of canine babesiosis and ehrlichiosis in a hospital population. Vet Parasitol. 2006;140:223–230. doi: 10.1016/j.vetpar.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Trotz-Williams LA, Trees AJ. Systematic review of the distribution of the major vector-borne parasitic infections in dogs and cats in Europe. Vet Rec. 2003;152:97–105. doi: 10.1136/vr.152.4.97. [DOI] [PubMed] [Google Scholar]
- Unver A, Ohashi N, Tajima T, Stich RW, Grover D, Rikihisa Y. Transcriptional analysis of p30 major outer membrane multigene family of Ehrlichia canis in dogs, ticks, and cell culture at different temperatures. Infect Immun. 2001a;69:6172–6178. doi: 10.1128/IAI.69.10.6172-6178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unver A, Perez M, Orellana N, Huang H, Rikihisa Y. Molecular and antigenic comparison of Ehrlichia canis isolates from dogs, ticks, and a human in Venezuela. J Clin Microbiol. 2001b;39:2788–2793. doi: 10.1128/JCM.39.8.2788-2793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unver A, Rikihisa Y, Borku K, Ozkanlar Y, Hanedan B. Molecular detection and characterization of Ehrlichia canis from dogs in Turkey. Berl Munch Tierarztl Wochenschr. 2005;118:300–304. [PubMed] [Google Scholar]
- Unver A, Rikihisa Y, Kawahara M, Yamamoto S. Analysis of 16S rRNA gene sequences of Ehrlichia canis, Anaplasma platys, and Wolbachia species from canine blood in Japan. Ann N Y Acad Sci. 2003;990:692–698. doi: 10.1111/j.1749-6632.2003.tb07445.x. [DOI] [PubMed] [Google Scholar]
- Unver AM, Perez M, Orellana N, Huang H, Rikihisa Y. Molecular and antigenic comparison of Ehrlichia canis isolates from dogs, ticks, and a human in Venezuela. J Clin Microbiol. 2001c;39:2788–2793. doi: 10.1128/JCM.39.8.2788-2793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero A, Hueli LE, Diaz-Saez V. Spermatogenesis in the Ixodid tick Haemaphysalis (Herpetobia) sulcata (Acarina: Ixodidae) J Parasitol. 1997;83:212–214. [PubMed] [Google Scholar]
- Wagner ER, Bremer WG, Rikihisa Y, Ewing SA, Needham GR, Unver A, Wang X, Stich RW. Development of a p28-based PCR assay for Ehrlichia chaffeensis. Mol Cell Probes. 2004;18:111–116. doi: 10.1016/j.mcp.2003.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DH, Dumler JS. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waner T, Baneth G, Strenger C, Keysary A, King R, Harrus S. Antibodies reactive with Ehrlichia canis, Ehrlichia phagocytophila genogroup antigens and the spotted fever group rickettsial antigens, in free-ranging jackals (Canis aureus syriacus) from Israel. Vet Parasitol. 1999;82:121–128. doi: 10.1016/s0304-4017(99)00002-3. [DOI] [PubMed] [Google Scholar]
- Waner T, Harrus S, Bark H, Bogin E, Avidar Y, Keysary A. Characterization of the subclinical phase of canine ehrlichiosis in experimentally infected beagle dogs. Vet Parasitol. 1997;69:307–317. doi: 10.1016/s0304-4017(96)01130-2. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Oikawa T, Hiraoka H, Kaneko N, Itamoto K, Mizuno T, Okuda M, Inokuma H. Experimental inoculation of Beagle dogs with Ehrlichia species detected from Ixodes ovatus. Vet Parasitol. 2006;136:147–154. doi: 10.1016/j.vetpar.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Wen B, Cao W, Pan H. Ehrlichiae and ehrlichial diseases in china. Ann N Y Acad Sci. 2003;990:45–53. doi: 10.1111/j.1749-6632.2003.tb07335.x. [DOI] [PubMed] [Google Scholar]
- Wen B, Rikihisa Y, Mott J, Fuerst PA, Kawahara M, Suto C. Ehrlichia muris sp. nov., identified on the basis of 16S rRNA base sequences and serological, morphological, and biological characteristics. Int J Syst Bacteriol. 1995;45:250–254. doi: 10.1099/00207713-45-2-250. [DOI] [PubMed] [Google Scholar]
- Wen B, Rikihisa Y, Mott JM, Greene R, Kim HY, Zhi N, Couto GC, Unver A, Bartsch R. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J Clin Microbiol. 1997;35:1852–1855. doi: 10.1128/jcm.35.7.1852-1855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielinga PR, Gaasenbeek C, Fonville M, de Boer A, de Vries A, Dimmers W, Akkerhuis Op Jagers G, Schouls LM, Borgsteede F, van der Giessen JW. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in The Netherlands. Appl Environ Microbiol. 2006;72:7594–7601. doi: 10.1128/AEM.01851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK, Ramachandra RN, Bergman DK. Tick-induced modulation of the host immune response. Int J Parasitol. 1994;24:59–66. doi: 10.1016/0020-7519(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Woody BJ, Hoskins JD. Ehrlichial diseases of dogs. Vet Clin North Am Small Anim Pract. 1991;21:75–98. doi: 10.1016/s0195-5616(91)50009-7. [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, Norton TM, Powell MR, Davidson WR. Molecular and serologic evidence of tick-borne Ehrlichiae in three species of lemurs from St. Catherines Island, Georgia, USA. J Zoo Wildl Med. 2004;35:503–509. doi: 10.1638/03-116. [DOI] [PubMed] [Google Scholar]
- Zaugg JL, Stiller D, Coan ME, Lincoln SD. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am J Vet Res. 1986;47:2269–2271. [PubMed] [Google Scholar]
- Zieger U, Horak IG, Cauldwell AE. Dynamics of free-living ixodid ticks on a game ranch in the Central Province, Zambia. Onderstepoort J Vet Res. 1998a;65:49–59. [PubMed] [Google Scholar]
- Zieger U, Horak IG, Cauldwell AE, Uys AC. Ixodid tick infestations of wild birds and mammals on a game ranch in central province, Zambia. Onderstepoort J Vet Res. 1998b;65:113–124. [PubMed] [Google Scholar]


