Abstract
Purpose
Patients 16 to 21 years of age with acute lymphoblastic leukemia (ALL) have an inferior outcome compared with younger children, leading some medical oncologists to advocate allogeneic stem-cell transplantation in first remission for these patients. We examined outcome for young adults with ALL enrolled onto the Children's Cancer Group (CCG) 1961 study between 1996 and 2002.
Patients and Methods
CCG 1961 entered patients with ALL 1 to 21 years of age with initial WBC count ≥ 50,000/μL and/or age ≥ 10 years. Randomly assigned therapies evaluated the impact of postinduction treatment intensification on outcome. We examined outcome and prognostic factors for 262 young adults with ALL.
Results
Five-year event-free and overall survival rates for young adult patients are 71.5% (SE, 3.6%) and 77.5% (SE, 3.3%), respectively. Rapid responder patients (< 25% bone marrow blasts on day 7) randomly assigned to augmented therapy had 5-year event-free survival of 81.8% (SE, 7%), as compared with 66.8% (SE, 6.7%) for patients receiving standard therapy (P = .07). One versus two interim maintenance and delayed intensification courses had no significant impact on event-free survival. WBC count more than 50,000/μL was an adverse prognostic factor.
Conclusion
Young adult patients with ALL showing a rapid response to induction chemotherapy benefit from early intensive postinduction therapy but do not benefit from a second interim maintenance and delayed intensification phase. Given the excellent outcome with this chemotherapy, there seems to be no role for the routine use of allogeneic stem-cell transplantation in first remission for young adults with ALL.
INTRODUCTION
Historically, young adults 16 to 21 years of age with acute lymphoblastic leukemia (ALL) have had lower event-free and overall survival compared with younger patients.1–4 This is in part related to the higher incidence of T-cell and Philadelphia chromosome–positive (Ph-positive) ALL and a lower frequency of t(12;21) and hyperdiploidy seen in this age group.
In the Children's Cancer Group (CCG) 1882 study (1989 to 1995), we showed that postinduction intensification improved outcome for National Cancer Institute high-risk patients who showed a slow early marrow response (> 25% bone marrow blasts on day 7) to four-drug induction therapy.5 Postinduction intensification included the addition of vincristine and L-asparaginase to consolidation and reconsolidation courses, the use of “Capizzi” methotrexate (vincristine and methotrexate on day 1 and L-asparaginase on day 2) instead of oral mercaptopurine and oral methotrexate during interim maintenance phases, and the addition of a second interim maintenance and delayed intensification phase.
In 1996, the CCG opened an ALL study (CCG 1961) that included patients 1 to 9 years of age and WBCs ≥ 50,000/μL or patients 10 to 21 years of age. The study was designed to address whether postinduction intensification would improve outcome for rapid early responder (RER) patients and whether introducing idarubicin instead of doxorubicin into the initial part of delayed intensification courses would benefit patients with a slow early marrow response (SER). Patients eligible for a matched sibling donor transplantation in first remission included those with either Ph-positive ALL or extreme hypodiploidy (< 44 chromosomes).
Overall, the CCG 1961 study showed that RER patients randomly assigned to augmented-intensity arms had a significantly improved event-free survival compared with patients receiving standard intensity arms (81.2% v 71.7%; P < .0001).6 There was no significant difference in event-free survival for patients randomly assigned to one or two interim maintenance and delayed intensification phases. The idarubicin versus doxorubicin randomization for SER patients has not been analyzed at the present time.
We analyzed outcomes (both overall and by treatment) and prognostic factors for 262 young adult patients with ALL 16 to 21 years of age treated on this trial.
PATIENTS AND METHODS
The CCG 1961 protocol opened in September 1996 and closed in May 2002. Eligibility criteria included age ≥ 10 years through 21 years of age or age 1 through 9 years of age and presenting WBCs ≥ 50,000/μL. Criteria for diagnosis and the details of therapy have been published previously.6 Induction therapy consisted of vincristine 1.5 mg/m2/wk for 4 weeks, daunorubicin 25 mg/m2/wk for 4 weeks, prednisone 60 mg/m2/d for 28 days, Escherichia coli L-asparaginase 6,000 U/m2 intramuscularly three times a week for nine doses, and intrathecal cytarabine on day 0 and intrathecal methotrexate on days 7 and 28. All patients had a bone marrow aspirate performed on day 7. Patients who had ≤ 25% blasts on day 7 were considered to be RERs, whereas those with more than 25% blasts were considered to be SERs.
RER patients who achieved remission were randomly assigned to standard- or increased-intensity postinduction therapy blocks and one or two interim maintenance and delayed intensification phases. In increased-intensity arms, patients received additional vincristine and pegylated asparaginase courses during consolidation and delayed intensification phases and vincristine, intravenous methotrexate without rescue, and pegylated asparaginase during interim maintenance phases. The postinduction regimens have been published.6
Patients randomly assigned to two delayed intensification phases received dexamethasone on days 1 through 7 and 14 to 21 of each course in an effort to reduce the high incidence of osteonecrosis seen in 16- to 21-year-old patients treated on CCG 1882.7 All patients randomly assigned to the intensified postinduction therapy received PEG-asparaginase after induction. SER patients received augmented postinduction treatment and two interim maintenance and delayed intensification courses and were randomly assigned to receive idarubicin or doxorubicin during the initial part of each delayed intensification course. SER patients received 18-Gy cranial radiation. Maintenance therapy lasted 2 years for girls and 3 years for boys, beginning from the first interim maintenance phase. Patients with Ph-positive ALL or CNS leukemia (≥ 5 WBCs/μL of CSF containing lymphoblasts or clinical evidence of CNS involvement) were excluded from random assignment.
This protocol was approved by the National Cancer Institute and institutional review boards of the participating institutions. Informed consent was obtained from the patients, their parents, or both as deemed appropriate according to United States Department of Health and Human Services guidelines.
Study Design and Statistical Analysis
RER patients were randomly assigned in a 2 × 2 factorial design to one of four regimens: standard intensity with one interim maintenance and delayed intensification (control), standard intensity and two interim maintenance and delayed intensification phases, augmented intensity with one interim maintenance and delayed intensification phase, and augmented intensity with two interim maintenance and delayed intensification phases.
Outcome analyses used life-table methods and associated statistics. The primary end points examined were event-free survival and overall survival from the time of random assignment. Events included induction failure, induction death, relapse at any site, death in remission, or a second malignant neoplasm, whichever occurred first. Patients who had not had an event were censored at the time of the last contact. Life-table estimates were calculated by the Kaplan-Meier method, and SEs of the estimate were obtained by the method of Peto. The log-rank test was used to compare survival curves between groups. Tests for interaction effects of the treatment components were performed with Cox regression methods. Five-year estimates of survival rates are presented in this report, unless otherwise stated.
RESULTS
Patients
Data used for this report were frozen as of May 2006. Two hundred sixty-two (12.7%) of the 2,057 eligible patients enrolled onto CCG 1961 were 16 to 21 years of age at diagnosis. Among these patients, 177 patients were classified as RER, 75 were SER, and 10 had no day 7 marrow evaluation performed. The ratio of young adult patients with a RER:SER was similar to that seen among all patients enrolled onto CCG 1961 (70:30 v 71:29, respectively). Of the patients who achieved remission, 164 of the RER patients and 53 of the SER patients were randomly assigned. The demographic features for patients 16 to 21 years of age are shown in Table 1. For 144 patients who had adequate, centrally reviewed karyotypes, 10 patients had t(9;22)(q34;q11), two patients had t(4;11)(q21;q23), and one patient had hypodiploid ALL. Ten patients had hyperdiploidy with trisomies of chromosomes 4, 10, and 17. Nine patients had a t(1;19)(q23;p13). TEL-AML 1 fusion gene testing was not performed on this study.
Table 1.
Children's Cancer Group 1961 Young Adult Demographic Features
| Feature | No. of Patients |
|---|---|
| WBC count, cells/μL | |
| < 50,000 | 193 |
| ≥ 50,000 | 69 |
| Sex | |
| Male | 172 |
| Female | 90 |
| Race/ethnicity | |
| White | 187 |
| Hispanic | 36 |
| Black | 22 |
| Other | 11 |
| Unknown | 6 |
| Lineage | |
| B cell | 174 |
| T cell | 47 |
| Not determined | 41 |
| CNS status | |
| CNS-1 | 229 |
| CNS-2 | 21 |
| CNS-3 | 12 |
| Age, years | |
| 16-17 | 209 |
| 18-21 | 53 |
Outcome of Treatment
In the young adult subgroup, 71 patients had an adverse event (Table 2).
Table 2.
Children's Cancer Group 1961 Young Adult Patients: Events by Early Marrow Response
| Event Type | RER | SER | No Day 7 Evaluation |
|---|---|---|---|
| Induction death | 3 | 3 | 0 |
| Remission death | 6 | 1 | 0 |
| Induction failure | 1 | 3 | 2 |
| M3 ± EMD | 28 | 9 | 1 |
| CNS | 4 | 1 | 0 |
| TR | 1 | 2 | |
| Other | 1 | 0 | 0 |
| SMN | 2 | 3 | 0 |
| Total | 46 | 22 | 3 |
Abbreviations: RER, rapid early responder; SER, slow early responder; M3, bone marrow relapse; EMD, extramedullary relapse; TR, testicular relapse; SMN, second malignant neoplasm.
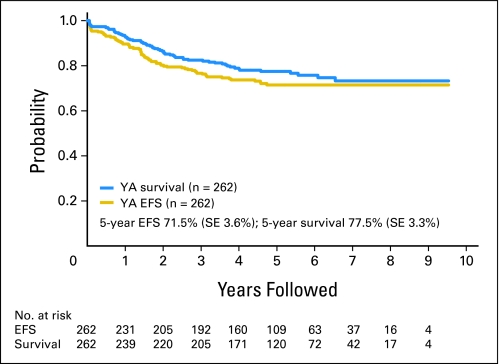
There were six induction deaths and seven deaths in remission. Five patients developed a second malignancy (acute myelogenous leukemia, n = 2; granulocytic sarcoma, n = 1; myelodysplasia, n = 1; large-cell lymphoma, n = 1). Six patients failed to achieve remission, and 47 patients experienced relapse. The 5-year event-free survival and overall survival rates for young adult patients were 71.5% (SE, 3.6%) and 77.5% (SE, 3.3%), respectively (Fig 1). No first events have occurred beyond 5 years. Death after induction failure, relapse, or second malignant neoplasm occurred more frequently in patients 16 to 21 years of age at diagnosis compared with younger patients. In the 16- to 21-year-old subgroup, 80.3% of such patients subsequently died, as compared with 60% for patients 1 to 9 years and 68.5% for patients 10 to 15 years of age.
Fig 1.
Event-free survival (EFS) and overall survival for young adult (YA) patients treated on Children's Cancer Group 1961 (n = 262).
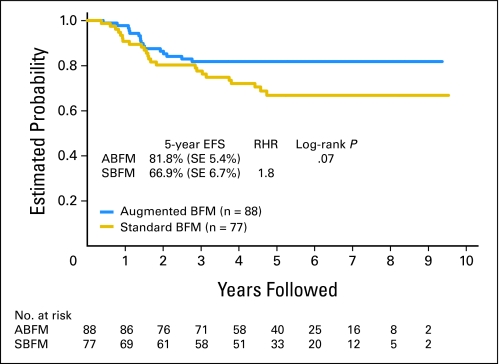
Five-year event-free survival for young adult RER patients was 81.8% (SE, 5.4%) on augmented-intensity arms (n = 88), compared with 66.9% (SE, 6.7%) for patients on standard-intensity arms (n = 76; P = .07; Fig 2). Five-year survival for patients treated with augmented and standard intensity were 83.2% (SE, 6.8%) versus 75.6% (SE, 7.7%) (P = .14). Both deaths occurring beyond 5 years were in patients who received standard-intensity therapy. There was no statistically significant difference in event-free survival for young adult RER patients who were randomly assigned to one or two delayed intensification phases (71.1% v 77.1%; P = .48).
Fig 2.
Event-free survival (EFS) for young adult patients randomly assigned to standard- or augmented-intensity regimens. BFM, Berlin-Frankfurt-Münster regimen; ABFM, augmented-intensity BFM arms; SBFM, standard-intensity BFM arms; RHR, relative hazard rate.
For young adult SER patients, all of whom received augmented postinduction therapy that included two interim maintenance and delayed intensification phases, the 5-year event-free survival rate was 70.7% (SE, 7.3%).
Nine patients underwent allogeneic transplantation in first remission. Eight of these patients had Ph-positive ALL, and one patient had the t(4;11). Five patients remain in continuous remission, two patients died during the early post-transplantation period, and two patients experienced relapse. One of these patients died, and the other remains in remission after a second transplantation.
Prognostic Factors
Sex, race, mediastinal mass, platelet count, hemoglobin, and immunophenotype had no prognostic impact in the young adult subgroup. Patients 16 to 17 and patients 18 to 21 years of age had identical 5-year event-free survival rates of 71.4%.
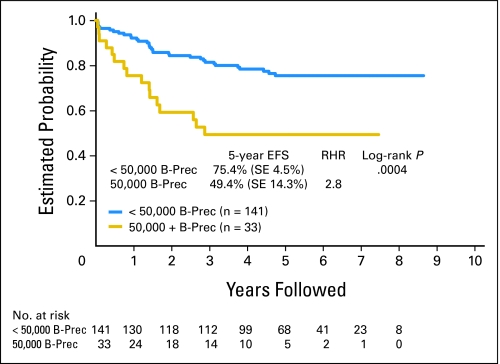
There were no discernible significant prognostic factors in the T-cell group. In the B-precursor group, presenting WBC count was a highly significant prognostic factor. The 5-year event-free survival rate for young adult B-precursor patients with initial WBCs less than 50,000/μL was 75.4% (SE, 4.5%) versus 43.9% (SE, 14.3%) for patients with initial WBCs more than 50,000/μL (P = .0004; Fig 3).
Fig 3.
Prognostic significance of WBCs more than 50,000/μL in young adult patients with B-precursor (B-Prec) acute lymphoblastic leukemia. EFS, event-free survival; RHR, relative hazard rate.
Within the B-precursor group, unfavorable cytogenetics, defined as t(9;22), t(4;11), or hypodiploidy with modal chromosome number less than 44, was a significant prognostic factor (P = .01).
Toxicity
Mortality.
Six patients died during the first 35 days of therapy (induction). Causes of death included bacterial sepsis in three patients (Staphylococcus aureus, n = 2; alpha-hemolytic streptococcus, n = 1), fungal infection in two patients (Aspergillus, n = 1; Candida, n = 1), and sepsis with no organism specified in one patient. Seven patients who achieved remission experienced death as their first event. Causes of death for patients included complications related to bone marrow transplantation performed in first remission in two patients, bacterial sepsis in three patients (E coli, n = 2; alpha-hemolytic streptococcus, n = 1), leukoencephalopathy in one patient, and arrhythmia in one patient. Death as the first event accounted for 11% of events in patients 1 to 9 years of age, 20% of events in patients 10 to 15 years of age, and 18.3% of events in young adult patients.
Other toxicity.
The mean number of hospital days for young adult patients was 32.8, compared with 38.3 days for patients 1 to 9 years of age and 34.2 days for patients 10 to 15 years of age. Table 3 shows a comparison of the incidence of significant toxicities in the young adult patients compared with younger patients. Age at diagnosis does not influence the risk for an L-asparaginase allergy. Avascular necrosis is a major problem in the young adult subgroup.
Table 3.
Incidence of Selected Toxicities for All Patients by Age Group
| Toxicity | Incidence by Patient Age Group (%) |
P | ||
|---|---|---|---|---|
| 1-9 Years | 10-15 Years | 16+ Years | ||
| Hyperglycemia | 0.3 | 2.3 | 4.2 | < .0001 |
| Seizure | 7.5 | 7.8 | 5.3 | .39 |
| Stroke | 0.6 | 2.9 | 1.9 | .003 |
| Encephalopathy | 2.6 | 4.88 | 4.2 | .05 |
| L-asparaginase allergy | 29.4 | 27.9 | 24.4 | .30 |
| Avascular necrosis | 0.96 | 9.8 | 19.9 | < .0001 |
DISCUSSION
Young adult patients entered on the CCG 1961 study had 5-year event-free survival rate of 71.5% (95% CI, 64.4% to 78.6%). In our previous cohort of young adult patients, the 5-year event-free survival rate was 64%.8 Age more than 15 years has historically been a strong adverse prognostic factor on clinical trials for children and adolescents with ALL. This is no longer the case with contemporary trials. The Dana-Farber Cancer Consortium recently reported that a small group of patients 15 to 18 years of age at diagnosis (n = 51) had a 5-year event-free survival rate of 78%, as compared with 85% for those 1 to 10 years of age and 77% for those 10 to 15 years of age.9
Among all patients enrolled onto CCG 1961, RER patients who were randomly assigned to augmented-intensity therapy had a significantly better event-free survival and overall survival (81% and 89%) compared with patients receiving standard-intensity therapy (72% and 83%). There was no benefit to a second delayed intensification phase. Young adult RER patients also had better outcomes with augmented- versus standard-intensity therapy for both event-free (81.8% v 66.9%, P = .07) and overall survival (83.2% v 75.6%, P = .14). Although these differences did not reach conventional levels of statistical significance among the young adult patients, they are quite similar in magnitude to the differences observed in the entire study population. This study was not powered to adequately assess an event-free survival difference among young adult patients by treatment regimen.
Thus we conclude that augmented-intensity therapy improves outcome for young adult RER patients. Event-free survival is similar for the first 2 years for patients receiving standard- or increased-intensity postinduction treatment. However, beyond 2 years, the benefit of increased-intensity postinduction treatment is apparent. This finding was also seen in our prior CCG 1882 study and in the overall RER population in this study.
SER patients had a 5-year EFS rate of 70.7%. The results of the idarubicin versus doxorubicin randomization have not yet been reported.
Young adult patients had a higher incidence of induction death and death in remission compared with younger patients. These patients also had a higher incidence of avascular necrosis and corticosteroid-induced hyperglycemia.
Young adult patients with B-precursor ALL and a presenting WBC count more than 50,000/μL had a worse prognosis compared with patients with WBCs less than 50,000/μL.
In reports published to date, young adult patients treated by pediatric oncologists have a better outcome compared with similar patients treated by medical oncologists.10–13 The event-free survival advantage is substantial, with a 20% to 30% absolute improvement observed in most comparisons. Whether the difference in outcome is due to physician compliance, patient compliance, protocol differences, or other differences is not easily ascertained. More 16- and 17-year-old patients are treated in pediatric centers, whereas more 18- to 21-year-old patients are treated in adult centers. There are no demographic differences between the 16- and 17-year-old patients compared with the 18- to 21-year-old patients, but there could be psychosocial factors that differ between the two populations. Pediatric and adult protocols differ significantly in the drugs used. Compared with adult protocols, pediatric protocols feature significantly more nonmyelosuppressive therapy (vincristine, corticosteroids, L-asparaginase) elements and more intensive early CNS-directed therapy.
The Medical Research Council (MRC) and the Eastern Cooperative Oncology Group (ECOG) conducted a joint study for adult patients 15 to 59 years of age with ALL between 1993 and 2006. Patients enrolled onto this trial received two phases of induction therapy and then underwent an allogeneic stem-cell transplantation if a matched sibling donor was available. Other patients were randomlyassigned to receive continuation chemotherapy or autologous stem-cell transplantation. The overall survival among 234 Ph-negative patients younger than 20 years of age was only 43%.14 Adult patients in the MRC/ECOG study considered standard risk (age < 35 years; no t(9; 22); B precursor and WBCs < 100,000/μL, T cell and WBCs < 30,000/μL) showed a significant 5-year survival benefit if they had a matched sibling donor available. Five-year survival was 62% for patients with a donor (n = 239) compared with 52% for patients without a donor (n = 323; P = .02).
We determined outcome for patients 16 to 21 years of age enrolled onto CCG 1961 meeting the MRC/ECOG criteria for “standard” risk. Five-year event-free and overall survival rates were 72.6% and 80.3%, respectively, markedly better than that observed in the adult trial. One could argue that the outcome for patients 22 to 30 years of age might be worse than for patients 16 to 21 years of age. However, a report from Ribera et al15 showed that for patients treated on a pediatric-type treatment regimen, there was no difference in outcome for patients 15 to 18 years of age (n = 35) and those 19 to 30 years of age (n = 46). This result needs to be confirmed in trials with larger patient numbers. There was also no difference in outcome in our study for patients 16 to 17 years of age versus those 18 to 21 years of age, although the numbers in the older age group were small.
Our results establish that young adult patients with ALL who do not have the t(9;22) or other high-risk features such as induction failure or hypodiploidy with less than 44 chromosomes have an event-free survival rate greater than 70% with chemotherapy alone and therefore do not meet criteria generally used to select patients for allogeneic stem-cell transplantation in first remission. This study was not designed to test the hypothesis that routine use of allogeneic stem-cell transplantation in first remission might improve outcome for young adult patients with ALL. Because of concerns regarding early mortality and both early and late morbidity, we generally consider patients candidates for allogeneic stem-cell transplantation in first remission only if they have an expected event-free survival rate of less than 50%.
Whether adult centers can attain outcomes similar to those obtained in pediatric centers is being explored in a current study conducted by the major adult cooperative groups in the United States. This trial uses one of the established arms of the current Children's Oncology Group high-risk, B-precursor ALL trial used for young adult patients.
We believe that young adult patients with ALL between the ages of 16 and 21 years should either be enrolled onto a clinical trial that is studying a pediatric-type treatment regimen or should be referred to a pediatric center for treatment. When a pediatric-type treatment regimen is used, routine use of stem-cell transplantation in first remission for this patient population seems unwarranted.
Footnotes
Supported by Grant No. CA 98543 from the Children's Oncology Group.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002812.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: James Nachman, Enzon Pharmaceuticals; Paul S. Gaynon, Sanofi Aventis (C) Stock Ownership: None Honoraria: James Nachman, Enzon Pharmaceuticals; Paul S. Gaynon, Enzon Research Funding: Paul S. Gaynon, Genzyme Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: James B. Nachman, Paul S. Gaynon, David R. Freyer, Peter G. Steinherz, Nita L. Seibel
Administrative support: Stephen P. Hunger
Provision of study materials or patients: Nyla A. Heerema, Paul S. Gaynon, Leonard A. Mattano Jr, David R. Freyer, Peter G. Steinherz, Nita L. Seibel
Collection and assembly of data: James B. Nachman, Paul S. Gaynon, Leonard A. Mattano Jr, Harland Sather, David R. Freyer, Peter G. Steinherz, Nita L. Seibel
Data analysis and interpretation: James B. Nachman, Mei K. La, Stephen P. Hunger, Paul S. Gaynon, Caroline Hastings, Leonard A. Mattano Jr, Harland Sather, Meenakshi Devidas, David R. Freyer, Peter G. Steinherz, Nita L. Seibel
Manuscript writing: James B. Nachman, Stephen P. Hunger, Paul S. Gaynon, Leonard A. Mattano Jr, Meenakshi Devidas, David R. Freyer, Peter G. Steinherz, Nita L. Seibel
Final approval of manuscript: James B. Nachman, Stephen P. Hunger, Nyla A. Heerema, Paul S. Gaynon, Caroline Hastings, Leonard A. Mattano Jr, Harland Sather, David R. Freyer, Peter G. Steinherz,Nita L. Seibel
REFERENCES
- 1.Plasschaert L, Kamps W, Vellenga E, et al. Prognosis in childhood and adult acute lymphoblastic leukemia: A question of maturation? Cancer Treat Rev. 2004;30:37–51. doi: 10.1016/S0305-7372(03)00140-3. [DOI] [PubMed] [Google Scholar]
- 2.Nachman J. Clinical Characteristics, biologic features and outcome for young adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2005;130:166–173. doi: 10.1111/j.1365-2141.2005.05544.x. [DOI] [PubMed] [Google Scholar]
- 3.Möricke A, Zimmermann M, Reiter A, et al. Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia. Klin Padiatr. 2005;217:310–320. doi: 10.1055/s-2005-872515. [DOI] [PubMed] [Google Scholar]
- 4.Santana V, Dodge R, Crist W, et al. Presenting features and treatment outcome of adolescents with acute lymphoblastic leukemia. Leukemia. 1990;4:87–90. [PubMed] [Google Scholar]
- 5.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 6.Seibel N, Steinherz P, Sather H, et al. Early post induction intensification therapy Improves survival for children and adolescents with high risk acute lymphoblastic leukemia: A report from the Children's Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattano L, Sather H, La M, et al. Modified dexamethasone reduces the incidence of treatment related osteonecrosis in children and adolescents with higher risk acute lymphoblastic leukemia: A report of CCG – 1961. Blood. 2003;102:221a. abstr. [Google Scholar]
- 8.Nachman J, Sather H, Buckley J, et al. Young adults 16-21 years of age at diagnosis entered on Children's Cancer Group acute lymphoblastic leukemia and acute myeloblastic leukemia protocols. Cancer. 1993;71:3377–3385. doi: 10.1002/1097-0142(19930515)71:10+<3377::aid-cncr2820711741>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Barry E, DeAngelo D, Neuberg D, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium protocols. J Clin Oncol. 2007;25:813–819. doi: 10.1200/JCO.2006.08.6397. [DOI] [PubMed] [Google Scholar]
- 10.Stock W, Sather H, Dodge RK, et al. Outcome of adolescents and young adults with ALL: A Comparison of Children's Cancer Group (CCG) and Cancer and Leukaemia Group B (CALGB) regimens. Blood. 2008;111:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bont J, Holt B, Dekker A, et al. Significant difference in outcome for adolescent with acute lymphoblastic leukemia treated on pediatric vs. adult protocols in the Netherlands. Leukemia. 2004;18:2032–2035. doi: 10.1038/sj.leu.2403538. [DOI] [PubMed] [Google Scholar]
- 12.Boissel N, Auclerc MF, Lheritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Ramanujachar R, Richards S, Hann I, et al. Adolescents with acute lymphoblastic leukaemia: Outcome on UK National Paediatric (ALL97) and Adult (UKALLXII/E2993) Trials. Pediatric Blood Cancer. 2007;48:254–261. doi: 10.1002/pbc.20749. [DOI] [PubMed] [Google Scholar]
- 14.Goldstone AH, Richards S, Lazarus H, et al. In adults with standard- risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/ maintenance chemotherapy in all patients: Final results of the International ALL Trial (MRN UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 15.Ribera JM, Oriol A, Sanz MA, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Espanol de Tratamiento en Hematolgia Pediatric Based Protocol ALL-96. J Clin Oncol. 2008;26:1843–1849. doi: 10.1200/JCO.2007.13.7265. [DOI] [PubMed] [Google Scholar]