The crystallization of the C. perfringens delta-toxin is reported.
Keywords: Clostridium perfringens, delta-toxin, haemolysins
Abstract
Clostridium perfringens is a Gram-positive anaerobic bacterium that is responsible for a wide range of diseases in humans and both wild and domesticated animals, including birds. C. perfringens is notable for its ability to produce a plethora of toxins, e.g. phospholipases C (alpha-toxin), pore-forming toxins (epsilon-toxin, beta-toxin and enterotoxin) and binary toxins (iota-toxin). Based on alpha-, beta-, epsilon- and iota-toxin production, the bacterium is classified into five different toxinotypes (A–E). Delta-toxin, which is a 32.6 kDa protein with 290 amino acids, is one of three haemolysins released by type C and possibly by type B strains of C. perfringens. This toxin is immunogenic and lytic to erythrocytes from the even-toed ungulates sheep, goats and pigs, and is cytotoxic to other cell types such as rabbit macrophages, human monocytes and blood platelets from goats, rabbits, guinea pigs and humans. The recombinant delta-toxin has been cloned, expressed, purified and crystallized in two different crystal forms by the hanging-drop vapour-diffusion method. Of these two different crystal forms, only the form II crystal diffracted to atomic resolution (d min = 2.4 Å), while the form I crystal diffracted to only 15 Å resolution. The form II crystals belonged to space group P21212, with one molecule in the crystallographic asymmetric unit and unit-cell parameters a = 49.66, b = 58.48, c = 112.93 Å.
1. Introduction
Clostridium perfringens is an anaerobic Gram-positive, rod-shaped spore-forming bacterium which causes a wide range of diseases in both humans and animals through its ability to release numerous toxins and enzymes (Popoff & Bouvet, 2009 ▶). Individual strains of C. perfringens produce only subsets of various toxins and the bacterium is classified into five different toxinotypes (A–E) based on its ability to produce alpha-, beta-, epsilon- and iota-toxins (Petit et al., 1999 ▶). Delta-toxin is one of the three haemolysins produced by C. perfringens type C and possibly type B strains. This toxin has been shown to be immunogenic and lyses the erythrocytes of even-toed ungulates but not those of humans, rabbits and horses (Alouf & Jolivet-Reynaud, 1981 ▶). However, the toxin is cytotoxic to other cell types from other taxonomic groups, such as rabbit macrophages, human monocytes and blood platelets from goats, rabbits, guinea pigs and humans (Manich et al., 2008 ▶; Jolivet-Reynaud et al., 1982 ▶, 1988 ▶, 1989 ▶; Cavaillon et al., 1986 ▶). The restricted cytotoxicity spectrum of delta-toxin is associated with its ability to bind specifically to the ganglioside GM2 (Jolivet-Reynaud et al., 1982 ▶, 1989 ▶; Cavaillon et al., 1986 ▶; Manich et al., 2008 ▶; Alouf & Jolivet-Reynaud, 1981 ▶; Jolivet-Reynaud & Alouf, 1983 ▶). Studies utilizing iodinated toxin show that it can also bind to GM2 extracted from sensitive cell membranes (Jolivet-Reynaud & Alouf, 1983 ▶) and liposomes containing GM2 (Jolivet-Reynaud et al., 1989 ▶) and can lyse selective malignant cells that are expressing GM2 (Jolivet-Reynaud et al., 1993 ▶). It has been revealed that the toxin is effective in reducing the growth of tumour cells expressing GM2 in vivo in mouse (Jolivet-Reynaud et al., 1993 ▶). As reported recently, characterization of delta-toxin at the amino-acid level reveals that the secreted protein is basic (pI 9.1), with 290 amino acids and a molecular mass of 32.6 kDa (Alouf & Jolivet-Reynaud, 1981 ▶; Manich et al., 2008 ▶). It has 44 and 42% sequence identity to beta-toxin and necrotic enteritis toxin B (NetB), respectively, of C. perfringens and also has approximately 30% sequence identity to the α- and γ-haemolysins and leukocidin F of Staphylococcus aureus. The cloning, expression and purification of the delta-toxin-encoding gene (del) into plasmid pET28a (Novagen) in Escherichia coli has been reported (Manich et al., 2008 ▶) and the purified toxin produced in E. coli has similar biophysical properties to the toxin produced from C. perfringens. Moreover, the purified recombinant toxin is fully active on target cells and retains its binding capacity to GM2 (Manich et al., 2008 ▶). It has also been shown that the recombinant delta-toxin forms hexamers and heptamers that insert into lipid bilayers and form channels, suggesting that delta-toxin is a β-pore-forming toxin (Manich et al., 2008 ▶). However, the three-dimensional structure of C. perfringens delta-toxin has not yet been solved and its cytolytic mechanism and function at the molecular level are still uncharacterized. Here, we report the crystallization and preliminary X-ray diffraction characterization of delta-toxin crystals (form II) that we obtained from the purified recombinant protein.
2. Materials and methods
2.1. Protein isolation, purification and concentration
C. perfringens delta-toxin was cloned, expressed and purified as described in Manich et al. (2008 ▶). Briefly, the DNA sequence coding for the delta-toxin gene was PCR-amplified from C. perfringens strain CP24-03 and cloned into the expression vector pET28a (Novagen) downstream of the six-histidine motif. The fusion protein was expressed in E. coli BL21 CodonPlus DE3 RIL (Stratagene) at 299 K and purified by affinity chromatography with a cobalt column (Talon, Qiagen). The delta-toxin was eluted with 100 mM imidazole. Interestingly, dialyzing the eluted sample against an imidazole-free buffer resulted in complete precipitation of the protein. The precipitated protein could be redissolved by slow addition of imidazole and the toxin only remained stable and soluble in the presence of imidazole. It has previously been proposed that in the absence of imidazole the six histidine residues of His-tagged proteins can form salt bridges or cross-links, leading to insolubility problems (Hamilton et al., 2003 ▶). Shi and coworkers reported a chaperone-like activity for imidazole, showing that imidazole could catalyze the refolding of a chemically denatured protein (Shi et al., 2007 ▶). Nonetheless, the effect of imidazole on the solubility of the delta-toxin could also simply be a consequence of its buffering properties maintaining the toxin at an appropriate pH.
Concentration of the delta-toxin using standard methods (either a Centricon or an Amicon Ultra concentrator) resulted in very low recovery (<20%) of the protein. However, attempts using stirred cells in the presence of nitrogen gas yielded a recovery of over 75%, but resulted in some precipitation. The precipitated protein was redissolved by adding 5%(v/v) glycerol and the dropwise addition of NaCl and imidazole to a final concentrations of 50 and 100 mM, respectively. The final protein concentration used for all crystallization work was 6.6 mg ml−1 in 20 mM Tris–HCl pH 8.0, 5%(v/v) glycerol, 50 mM NaCl and 100 mM imidazole. Throughout the entire concentration procedure, loss of protein did not exceed 10% of the starting amount.
Electronspray mass spectrometry (ESI-MS) of the soluble toxin sample revealed a single mass species of mass 33 279 ± 20 Da, which is consistent with the theoretical mass of the 6×His-tagged protein (33.2 kDa), and circular-dichroism spectroscopy gave a spectrum that was characteristic of mainly β-sheet-containing protein (data not shown).
2.2. Crystallization
Initial crystallization trials were carried out at both 277 and 293 K using Crystal Screen and Crystal Screen 2 from Hampton Research, Wizard I and II from Emerald BioSystems and Stura FootPrint Screens, MacroSol and PACT premier from Molecular Dimensions. A number of conditions resulted in initial success. However, only two of these initial conditions could be optimized to produce crystals that were suitable for X-ray diffraction analysis. All trials used the hanging-drop vapour-diffusion technique.
2.2.1. Crystal form I
The optimal conditions for reproducible form I crystals were found to be 2 µl 6.6 mg ml−1 protein mixed with 1 µl well buffer composed of 100 mM MES–NaOH pH 6.2 and 1–1.4 M ammonium sulfate and equilibrated at 293 K against 150 µl well buffer. Tetrahedral bipyramidal crystals of maximum dimensions 0.05 × 0.02 × 0.04 mm grew after 3–4 weeks (Fig. 1 ▶ a). The crystals were cryoprotected in crystallization buffer supplemented with 25–28%(v/v) glycerol and vitrified in liquid nitrogen.
Figure 1.
(a) Crystals of form I. (b) Crystals of form II.
2.2.2. Crystal form II
Form II crystals were also grown using 2 µl 6.6 mg ml−1 protein mixed with 1 µl of a well buffer composed of 100 mM MES–NaOH pH 6.0, 25–30%(v/v) PEG 550 and 25 mM ZnSO4 and equilibrated at 293 K against 150 µl well buffer. Crystals appeared after 6–10 d and had a rectangular needle morphology with maximum dimensions of 0.4 × 0.05 × 0.02 mm (Fig. 1 ▶ b). The crystals were cryoprotected in the well buffer supplemented with 10%(v/v) ethylene glycol and vitrified in liquid nitrogen.
2.3. Data collection and processing
Both form I and form II crystals were tested for X-ray diffraction on station ID29 at the ESRF in Grenoble, France. The form I crystal produced diffraction that was limited to only 15 Å resolution and were not investigated further. However, form II crystals diffracted to a resolution of 2.4 Å and data were collected. Analysis of the data indicated an orthorhombic space group, with unit-cell parameters a = 49.66, b = 58.48, c = 112.93 Å. Reflections h00 = 2n + 1, k00 = 2n + 1 are absent, suggesting that the space group is P21212. The Matthews coefficient indicates that the asymmetric unit contains one molecule, with a V M of 2.47 Å3 Da−1 and an estimated solvent content of 50%.
Data sets were processed with the MOSFLM program (Leslie, 1992 ▶). Scaling and merging of the data were performed using the program SCALA (Evans, 1993 ▶). All subsequent data manipulations were performed with the CCP4 software suite (Collaborative Computational Project, Number 4, 1994 ▶).
Data statistics for the form II crystal are listed in Table 1 ▶.
Table 1. Data-processing statistics.
Values in parentheses are for the highest resolution shell.
| Crystal form | II |
| No. of crystals used | 1 |
| Beamline | ID29 |
| Wavelength (Å) | 1.0332 |
| Detector | ADSC Quantum 315r |
| Crystal-to-detector distance (mm) | 336.8 |
| Rotation angle per image (°) | 1.0 |
| Total rotation range (°) | 250–430 |
| Exposure time per image (s) | 0.5 |
| Resolution range (Å) | 100.0–2.4 (2.53–2.4) |
| Space group | P21212 |
| Unit-cell parameters (Å) | a = 49.66, b = 58.48, c = 112.93 |
| Mosaicity (°) | 0.4 |
| Total no. of measured observations | 140319 (20111) |
| No. of unique reflections | 13444 (1898) |
| Multiplicity | 10.4 (10.6) |
| Rmerge† | 0.148 (0.691) |
| Rp.i.m.‡ | 0.047 (0.218) |
| Mean I/σ(I) | 115.8 (3.5) |
| Completeness (%) | 99.9 (99.8) |
| Overall B factor from Wilson plot (Å2) | 20.29 |
R
merge = 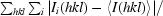
 .
.
R
p.i.m. = 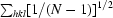
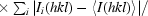
 , where N is the data redundancy, I
i(hkl) is the observed intensity and 〈I(hkl)〉 is the mean intensity from multiple observations of symmetry-related reflections.
, where N is the data redundancy, I
i(hkl) is the observed intensity and 〈I(hkl)〉 is the mean intensity from multiple observations of symmetry-related reflections.
3. Conclusions
The recombinant delta-toxin of C. perfringens was purified and crystallized using the hanging-drop vapour-diffusion method. A native data set to 2.4 Å resolution was collected from a form II crystal at the ESRF, France. Preliminary analysis of the X-ray diffraction data revealed that the form II crystal belonged to the primitive orthorhombic space group P21212, with unit-cell parameters a = 49.66, b = 58.48, c = 112.93 Å and one molecule in the crystallographic asymmetric unit.
We are presently working to solve the phase problem by the molecular-replacement method using the available related protein structures from the haemolysin group of S. aureus. We anticipate that these molecular-replacement phases supplemented with phase information from heavy-atom derivatives or single anomalous dispersion will lead to structure solution of the delta-toxin of C. perfringens.
Acknowledgments
The authors wish to thank Dr Robert Sarra for his assistance with ESI-MS and CD spectroscopy, and the beamline staff at ID29, ESRF, Grenoble for assistance with data collection.
References
- Alouf, J. E. & Jolivet-Reynaud, C. (1981). Infect. Immun. 31, 536–546. [DOI] [PMC free article] [PubMed]
- Cavaillon, J. M., Jolivet-Reynaud, C., Fitting, C., David, B. & Alouf, J. E. (1986). J. Leukoc. Biol. 40, 65–72. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Evans, P. R. (1993). Proceedings of the CCP4 Study Weekend. Data Collection and Processing, edited by L. Sawyer, N. Isaacs & S. Bailey, pp. 114–122. Warrington: Daresbury Laboratory.
- Hamilton, S., Odili, J., Pacifico, M. D., Wilson, G. D. & Kupsch, J. M. (2003). Hybrid. Hybridomics, 22, 347–355. [DOI] [PubMed]
- Jolivet-Reynaud, C. & Alouf, J. E. (1983). J. Biol. Chem. 258, 1871–1877. [PubMed]
- Jolivet-Reynaud, C., Cavaillon, J.-M. & Alouf, J. E. (1982). Infect. Immun. 38, 860–864. [DOI] [PMC free article] [PubMed]
- Jolivet-Reynaud, C., Estrada, J., West, L. A., Alouf, J. E. & Chedid, L. (1993). Anticancer Drugs, 4, 65–75. [DOI] [PubMed]
- Jolivet-Reynaud, C., Hauttecoeur, B. & Alouf, J. E. (1989). Toxicon, 27, 1113–1126. [DOI] [PubMed]
- Jolivet-Reynaud, C., Launay, J.-M. & Alouf, J. E. (1988). Arch. Biochem. Biophys. 262, 59–66. [DOI] [PubMed]
- Leslie, A. G. W. (1992). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr. 26
- Manich, M., Knapp, O., Gibert, M., Maier, E., Jolivet-Reynaud, C., Geny, B., Benz, R. & Popoff, M. R. (2008). PLoS One, 3, e3764. [DOI] [PMC free article] [PubMed]
- Petit, L., Gibert, M. & Popoff, M. R. (1999). Trends Microbiol. 7, 104–110. [DOI] [PubMed]
- Popoff, M. R. & Bouvet, P. (2009). Future Microbiol. 4, 1021–1064. [DOI] [PubMed]
- Shi, R., Pan, Q., Guan, Y., Hua, Z., Huang, Y., Zhao, M. & Li, Y. (2007). Arch. Biochem. Biophys. 459, 122–128. [DOI] [PubMed]



