Crystallographic data of organic solvent-tolerant lipase from Bacillus sp. strain 42 was collected at 2.0 Å with unit-cell parameters a =117.41, b = 80.85, c = 99.44 Å, β=96.40°. The protein–solvent interactions will be studied since lipase 42 was stable in water-miscible solvent.
Keywords: lipases, Bacillus sp. strain 42, organic solvent tolerance
Abstract
An organic solvent-tolerant lipase from Bacillus sp. strain 42 was crystallized using the capillary-tube method. The purpose of studying this enzyme was in order to better understand its folding and to characterize its properties in organic solvents. By initially solving its structure in the native state, further studies on protein–solvent interactions could be performed. X-ray data were collected at 2.0 Å resolution using an in-house diffractometer. The estimated crystal dimensions were 0.09 × 0.19 × 0.08 mm. The crystal belonged to the monoclinic space group C2, with unit-cell parameters a = 117.41, b = 80.85, c = 99.44 Å, β = 96.40°.
1. Introduction
Lipases are ubiquitous enzymes that catalyze the hydrolysis of long-chain triglycerides at water–oil interfaces to form glycerol and free fatty acids (Thomson et al., 1999 ▶; Carrasco-López et al., 2009 ▶). Their properties, such as their stability in solvents, substrate specificity, regioselectivity and high enantioselectivity, indicate that lipases are potential biocatalysts for industrial use (Reetz & Jaeger, 1998 ▶). Although many solvent-tolerant enzymes are currently available for industrial use, their interactions (especially in solvents) are not yet understood. An organic solvent-tolerant lipase (called L42) which is thermophilic and that can tolerate various solvents has been isolated from Bacillus sp. strain 42 (Eltaweel et al., 2005 ▶). Based on previous studies, L42 is stable in water-miscible solvents such as acetone, ethanol, methanol, DMSO and DMF, but its stability decreases drastically in water-immiscible solvents at a concentration of 25%(v/v). DMSO at a concentration of 30–45%(v/v) enhances lipase activity, but this enhancement drops abruptly at a concentration of 60%(v/v) (Hamid, Eltaweel et al., 2009 ▶; Hamid, Rahman et al., 2009 ▶). In fluorescence studies, tertiary-structure collapse was found at a methanol concentration of 60%(v/v) and at a DMSO concentration of 75%(v/v). The same result was found in a circular-dichroism (CD) analysis, in which more than 60%(v/v) methanol disrupted the secondary structure of L42. Expansion of α-helices and loss of β-sheets were suggested from the CD analysis (Manavalan & Johnson, 1983 ▶). L42 could possibly serve as an industrial biocatalyst for use with organic solvents (biodiesel production) as it functions well in hydrophilic solvents.
2. Experimental procedures
2.1. Protein overproduction and purification
Recombinant L42 was overproduced in a pET-51b vector system under the regulation of the strong T7 promoter in Escherichia coli BL21 (DE3) pLysS. The recombinant culture was grown in LB broth supplemented with 50 µg ml−1 ampicillin and 25 µg ml−1 chloramphenicol in a rotary shaker (150 rev min−1) at 310 K. After 3.5 h, L42 overproduction was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at an OD600 of ∼0.5. The cell culture was harvested by centrifugation, resuspended in washing buffer (100 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA pH 8.0) and subjected to sonication for 2 min (Branson 250 sonifier; output 2 and duty cycle 35). The clear lysate was collected after centrifugation (10 000g for 15 min) and loaded onto Strep-tag II High Performance matrix (GE Healthcare, USA) in an XK16/20 column (GE Healthcare, USA) pre-equilibrated with five column volumes (CVs) of washing buffer. The unbound protein was removed with seven CVs of washing buffer and eluted with two CVs of elution buffer (100 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 mM CaCl2, 2.5 mM d-desthiobiotin). The pooled sample was desalted using a HiTrap desalting column (GE Healthcare, USA) into 100 mM Tris–HCl pH 8.0 and subjected to ion-exchange chromatography (Tosoh SuperQ-650S, Japan) to increase its purity. The eluted protein (in 100 mM Tris–HCl pH 8.0, 200 mM NaCl) was desalted again into 100 mM Tris–HCl pH 8.0 supplemented with 1 mM CaCl2 to ensure its stability using a HiTrap desalting column (GE Healthcare, USA) and concentrated to 10 mg ml−1 using Amicon ultracentrifugation (Millipore-15, 10 kDa cutoff). The homogeneity of the protein was assessed using SDS–PAGE (Laemmli, 1970 ▶) and native PAGE.
2.2. Crystallization of L42
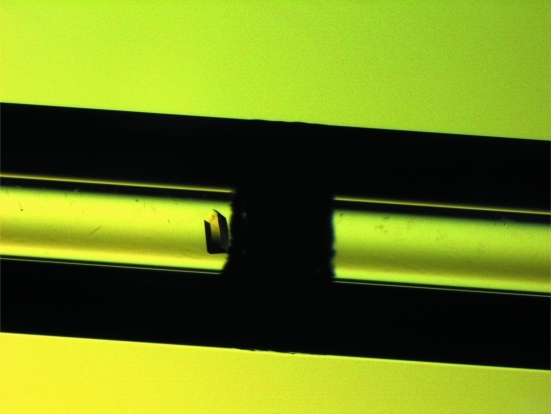
A preliminary screen was initially conducted by vapour diffusion using the Crystal Screen and Crystal Screen 2 reagent kits (Hampton Research, USA) and was optimized using the counter-diffusion method. L42 was crystallized in 2.0 M NaCl, 0.1 M NaH2PO4, 0.1 M KH2PO4 and 0.1 M MES pH 6.5 using the counter-diffusion method. The purified protein (10 mg ml−1) was mixed with the formulation (90:10) and inserted into the capillary (0.3 mm diameter) with one end sealed with clay and the other end attached to a piece of Gel-Tube from the kit (Confocal Science Inc., Japan). The capillary was incubated at 293 K with the Gel-Tube soaked with 1 ml mother liquor (in a test tube with a screw cap). The crystal grew within 3 d. The estimated dimensions of the crystal were 0.09 × 0.19 × 0.08 mm (Fig. 1 ▶).
Figure 1.
An L42 crystal observed under a stereomicroscope. The inner diameter of the capillary is 0.3 mm.
2.3. X-ray data collection and processing
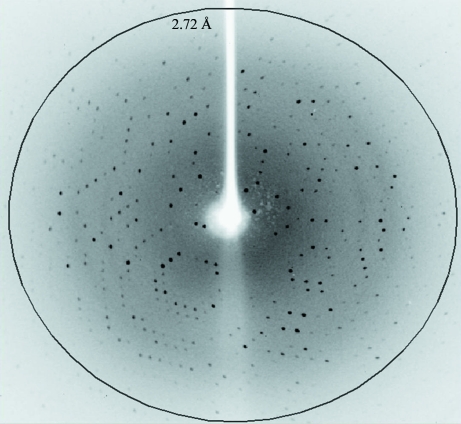
The L42 crystal was mounted in 30% glycerol in mother liquor as a cryoprotectant using cryoloops (0.3 mm; Hampton Research, USA) and flash-cooled in a 100 K nitrogen stream. Data were collected to ∼2.0 Å resolution using an in-house X-ray diffractometer (Bruker X8 PROTEUM, λ = 1.54 Å) with a PLATINUM 135 CCD detector (0.5° per image) at a distance of 60 mm and with an exposure time of 120 s (Fig. 2 ▶). The data were indexed with the PROTEUM software, integrated with SAINT and scaled with SADABS (Bruker). After the statistical data had been obtained, the space group was determined using XPREP (Bruker).
Figure 2.
A diffraction pattern from an L42 crystal.
3. Results and discussion
Recombinant L42 was purified in two steps using affinity and ion-exchange chromatography. The molecular mass of this protein is approximately 43 kDa. The L42 crystals belonged to the monoclinic space group C2, with unit-cell parameters a = 117.41, b = 80.85, c = 99.44 Å, β = 96.40°. From our calculations, the Matthews coefficient V M (the volume per unit of protein molecular mass) was 2.73 Å3 Da−1 (Matthews, 1968 ▶), with an estimated solvent content of 55% and two molecules per asymmetric unit. Additional information from our data analysis is listed in Table 1 ▶. Comparing L42 with other lipases with solved structures, we found sequence identities of 97% to BTL from Geobacillus thermocatenulatus and 98% and 94% to P1 and L1 from G. stearothermophilus, respectively (Tyndall et al., 2002 ▶; Choi et al., 2005 ▶; Carrasco-López et al., 2009 ▶). BTL has the highest optimum temperature for enzymatic activity (348 K); L42 has an optimum temperature of 343 K, followed by L1 (333 K) and P1 (328 K). Of the aforementioned lipases with solved crystal structures, no mention has been made of their incidence in organic solvents. L42 is stable in polar organic solvents [for instance, 45%(v/v) DMSO or 25%(v/v) acetone, ethanol, methanol, heptanol or octanol; Hamid, Eltaweel et al., 2009 ▶; Hamid, Rahman et al., 2009 ▶]. It has also been reported that lipases from Bacillus sp. J33, Cryptococcus sp. S-2 and Burkholderia are stable in 40–60%(v/v) DMSO or 30–50%(v/v) methanol (Nawani & Kaur, 2006 ▶; Nawani et al., 2006 ▶; Kamini et al., 2000 ▶; Ishimoto et al., 2001 ▶), that Bacillus thermoleovorans CCR11 is stable in 70%(v/v) acetone, methanol, ethanol or propanol (Castro-Ochoa et al., 2005 ▶) and that Pseudomonas aeruginosa LST-03 retains its activity in 25%(v/v) DMSO or methanol (Ogino et al., 1999 ▶, 2000 ▶). To date, L42 is the only solvent-tolerant lipase that has been crystallized. The structure of L42 will be elucidated by the molecular-replacement method using the CCP4 software suite (Collaborative Computational Project, Number 4, 1994 ▶; Dodson et al., 1997 ▶) with the crystal structure of lipase P1 (PDB code 1ji3; Tyndall et al., 2002 ▶) as a template. Further investigations are currently in progress in order to obtain insights into protein–solvent interactions.
Table 1. Preliminary crystallographic data for L42.
Values in parentheses are for the highest resolution shell.
| Crystal data | |
| Space group | C2 |
| Unit-cell parameters | |
| a (Å) | 117.41 |
| b (Å) | 80.85 |
| c (Å) | 99.44 |
| β (°) | 96.40 |
| Data processing | |
| Temperature (K) | 100 |
| Wavelength (Å) | 1.5418 |
| Resolution (Å) | 56.84–2.00 (2.10–2.00) |
| No. of diffraction images | 1046 |
| Oscillation angle per frame (°) | 0.5 |
| Unique data | 78680 |
| Multiplicity | 2.02 (1.38) |
| Data completeness (%) | 95.9 (92) |
| Mean I/σ(I) | 8.59 (2.77) |
| Matthews coefficient (Å3 Da−1) | 2.73 |
| Molecules per asymmetric unit | 2 |
| Solvent content (%) | 55 |
| Rmerge (%) | 10.3 (38.1) |
Acknowledgments
The authors wish to thank Dr Naoki Shibata of Hyogo University, Japan for his assistance and helpful discussions while completing this paper. This project was fully sponsored by the Ministry of Science, Technology and Innovation of Malaysia (09-05-MGI-GMB001).
References
- Carrasco-López, C., Godoy, C., de Las Rivas, B., Fernandez-Lorente, G., Palomo, J. M., Guisan, J. M., Fernandez-Lafuente, R., Martinez-Ripoll, M. & Hermoso, J. A. (2009). J. Biol. Chem. 284, 4365–4372. [DOI] [PubMed]
- Castro-Ochoa, L. D., Rodríguez-Gomez, C., Valerío-Alfaro, G. & Ros, R. O. (2005). Enzyme Microb. Technol. 37, 648–654.
- Choi, W.-C., Kim, M. H., Ro, H.-S., Ryu, S. R., Oh, T.-K. & Lee, J.-K. (2005). FEBS Lett. 579, 3461–3466. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Dodson, E. J., Winn, M. & Ralph, A. (1997). Methods Enzymol. 277, 620–633. [DOI] [PubMed]
- Eltaweel, M. A., Rahman, R. N. Z. R. A., Salleh, A. B. & Basri, M. (2005). Ann. Microbiol. 55, 187–192.
- Hamid, T. H. T. A., Eltaweel, M. A., Rahman, R. N. Z. R. A., Basri, M. & Salleh, A. B. (2009). Ann. Microbiol. 59, 111–118.
- Hamid, T. H. T. A., Rahman, R. N. Z. A., Salleh, A. B. & Basri, M. (2009). Biotechnol. Biotech. Eq. 23, 1524–1530.
- Ishimoto, R., Sugimoto, M. & Kawai, F. (2001). FEMS Microbiol. Lett. 195, 231–235. [DOI] [PubMed]
- Kamini, N. R., Fujii, T., Kurosu, T. & Iefuji, H. (2000). Process Biochem. 36, 317–324.
- Laemmli, U. K. (1970). Nature (London), 227, 680–685. [DOI] [PubMed]
- Manavalan, P. & Johnson, W. C. Jr (1983). Nature (London), 305, 831–832.
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Nawani, N. & Kaur, J. (2006). Mol. Cell. Biochem. 206, 91–96. [DOI] [PubMed]
- Nawani, N., Singh, R. & Kaur, J. (2006). Electron. J. Biotechnol. 9, 559–565.
- Ogino, H., Miyamoto, K., Yasuda, M., Ishimi, K. & Ishikawa, H. (1999). Biochem. Eng. J. 4, 1–6.
- Ogino, H., Nakagawa, S., Shinya, K., Muto, T., Fujimura, N., Yasuda, M. & Ishikawa, H. (2000). J. Biosci. Bioeng. 89, 451–457. [DOI] [PubMed]
- Reetz, M. T. & Jaeger, K.-E. (1998). Chem. Phys. Lipids, 93, 3–14. [DOI] [PubMed]
- Thomson, C. A., Delaquis, P. J. & Mazza, G. (1999). Crit. Rev. Food Sci. Nutr. 39, 165–187. [DOI] [PubMed]
- Tyndall, J. D., Sinchaikul, S., Fothergill-Gilmore, L. A., Taylor, P. & Walkinshaw, M. D. (2002). J. Mol. Biol. 323, 859–869. [DOI] [PubMed]