Abstract
Background:
Therapeutic hypothermia (TH) is becoming standard of care in newborns with hypoxic-ischemic encephalopathy (HIE). The prognostic value of the EEG and the incidence of seizures during TH are uncertain.
Objective:
To describe evolution of EEG background and incidence of seizures during TH, and to identify EEG patterns predictive for MRI brain injury.
Methods:
A total of 41 newborns with HIE underwent TH. Continuous video-EEG was performed during hypothermia and rewarming. EEG background and seizures were reported in a standardized manner. Newborns underwent MRI after rewarming. Sensitivity and specificity of EEG background for moderate to severe MRI brain injury was assessed at 6-hour intervals during TH and rewarming.
Results:
EEG background improved in 49%, remained the same in 38%, and worsened in 13%. A normal EEG had a specificity of 100% upon initiation of monitoring and 93% at later time points. Burst suppression and extremely low voltage patterns held the greatest prognostic value only after 24 hours of monitoring, with a specificity of 81% at the beginning of cooling and 100% at later time points. A discontinuous pattern was not associated with adverse outcome in most patients (73%). Electrographic seizures occurred in 34% (14/41), and 10% (4/41) developed status epilepticus. Seizures had a clinical correlate in 57% (8/14) and were subclinical in 43% (6/14).
Conclusions:
Continuous video-EEG monitoring in newborns with HIE undergoing TH provides prognostic information about early MRI outcome and accurately identifies electrographic seizures, nearly half of which are subclinical.
Hypoxic-ischemic encephalopathy (HIE) after perinatal asphyxia is an important cause of mortality and neurologic morbidity.1,2 Randomized clinical trials have demonstrated that moderate hypothermia is associated with a reduction in death and neurologic impairment at 18 to 22 months of age.3,4 As a result, many centers now offer therapeutic hypothermia (TH) as a neuroprotective strategy for neonatal HIE.
Reliable early predictors of neurologic outcome in newborns after acute hypoxic-ischemic insult are important for counseling families and for making thoughtful treatment decisions. Clinical assessment can help with prognosis,5–7 although sedating medications and clinical changes with TH8 are complicating factors. Brain MRI is highly predictive of neurologic outcome,9,10 including in the setting of hypothermia,11 although it does not provide functional assessment and its sensitivity for hypoxic-ischemic injury in the first 48 hours of life is limited. A single EEG recording during the first week of life in noncooled infants with HIE,12–14 and in infants with HIE treated with TH,15 may help predict neurologic outcome. However, continuous video-EEG monitoring is the gold standard for evaluating brain function and for recording electrographic seizures in neonates with HIE16,17 and it has not yet been reported in neonates with HIE undergoing TH.
Our aim was to describe the evolution of the EEG background during TH and rewarming and to determine the incidence of electrographic seizures in newborns with HIE treated with moderate hypothermia. We also sought to determine the prognostic value of EEG background during TH using brain MRI as an early outcome measure.
METHODS
Consecutive newborns with HIE who underwent TH with whole-body cooling at University of California, San Francisco (UCSF), between November 2007 and July 2009 were included in this cohort study. Our TH clinical protocol is based on published trials.3,4 Selection for TH included 1) ≥36 weeks gestational age at birth, 2) any of the following: cord or first gas pH <7.0, cord or first gas base deficit >12, 10-minute Apgar score <5, or prolonged resuscitation, and 3) moderate to severe encephalopathy within 6 hours of birth. Exclusion criteria included suspected or known congenital malformation and inborn errors of metabolism. TH was initiated as soon as possible after birth at UCSF or at the time of referral from the outside hospital and consisted of whole-body moderate hypothermia (Cincinnati Sub-Zero Blanketrol III) (target temperature 33.5°C) for 72 hours followed by rewarming over approximately 6 hours. All patients received morphine infusion throughout TH to prevent discomfort and shivering (10–25 μg/kg/h, with boluses as needed). Clinical and electrographic seizures were treated with antiepileptic drugs (AEDs) including lorazepam, phenobarbital, fosphenytoin, and levetiracetam according to institutional guidelines. Clinical data were extracted from medical records.
Standard protocol approvals, registrations, and patient consents.
The Committee on Human Research at UCSF approved the retrospective review of the clinical, EEG, and imaging data for this study.
Video-EEG monitoring.
Video-EEG was initiated as soon as possible after admission to the nursery. As part of our clinical protocol, infants are continuously monitored with a NicoletOne video-EEG system throughout TH and rewarming. A trained technician applied surface electrodes according to the international 10–20 system, modified for neonates. EEG recordings were interpreted by a pediatric neurophysiologist (J.S.) for clinical use in the acute setting. Using stored files, full video-EEG recordings were scored in a standardized fashion by a pediatric neurophysiologist (M.R.C.) blinded to all clinical factors except patient age. Predominant background pattern and occurrence of electrographic seizures and status epilepticus (SE), with or without clinical manifestations, were reported in 6-hour intervals with the initiation of recording as time “0.” Clinical seizures without EEG correlates were not considered. For the analysis, we used the initial 6-hour interval (beginning of cooling), the 24- to 30-hour interval (midcooling), the last interval prior to rewarming (end of cooling), and the first interval after rewarming (postcooling). A seizure was defined as a repetitive, evolving, and stereotyped pattern, with a definite beginning and end, a minimum duration of 10 seconds, and a minimal amplitude of 2 μV.18,19 SE was defined as continuous seizure activity for at least 30 minutes or recurrent seizures for over 50% of 1–3 hours of recording time.20 EEG background was classified into 5 patterns as previously described21,22: 1) normal pattern for gestational age, including recordings with transient periods of discontinuous activity occupying less than 50% of the recording, with presence of distinct state changes; 2) excessively discontinuous, with persistence of discontinuous activity occupying more than 50% of the recording and consisting of bursts of normal activity separated by abnormally long, interburst intervals of more than 6 seconds duration, and amplitude <25 and >5 μV, with poor state changes; 3) depressed and undifferentiated, with persistently low-voltage background activity with amplitude between 5 and 15 μV and without normal features; 4) burst suppression (BS), invariant and unreactive pattern of bursts of paroxysmal activity with mixed features but no age-appropriate activity lasting less than 10 seconds alternating with periods of marked voltage attenuation with amplitude ≤5 μV; 5) extremely low voltage, invariant and unreactive pattern, with amplitude <5 μV or with no discernible cerebral activity.
Brain MRI.
Infants were imaged shortly after rewarming (median of 5 days of life) using a specialized neonatal head coil on a 1.5-Tesla Signa EchoSpeed system (GE Medical Systems). Imaging sequences included T1- and T2-weighted MRI and diffusion-weighted imaging. A pediatric neuroradiologist (A.J.B.), blinded to the clinical history, evaluated all images. Injury was scored using a system strongly predictive of neurodevelopmental outcome following neonatal HIE.23 We defined normal to mild MRI injury as basal ganglia/thalamus score <2 and watershed score <3 and moderate to severe MRI injury as basal ganglia/thalamus score ≥2 (involving both the thalamus and the lentiform nucleus) or watershed pattern ≥3 (involving both watershed cortex and white matter). A similar classification was highly predictive for neurologic disability at 18 months of age in newborns with HIE treated with hypothermia.11
Statistical analysis.
All analyses were performed with STATA software (Stata 10.1, Stata Corporation, College Station, TX). χ2 and Fisher exact tests were used to compare dichotomous variables, and t test was used for continuous variables. Wilcoxon rank sum test (Mann-Whitney U) was used to compare nonparametric data. Receiver operating characteristic (ROC) curves were used to assess the prognostic value of EEG background patterns at each time interval. We considered significant a p value < 0.05.
RESULTS
Patient population.
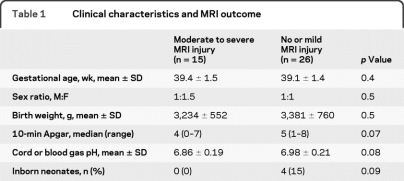
During the study period, 49 newborns were treated with hypothermia and 46 had continuous video-EEG available for review. Of these, 41 were evaluated with MRI. Five infants who were deceased following redirection of care and were not studied with MRI were excluded. Four of them had an initial extremely low voltage cerebral activity, and one had BS pattern, both persisting for more than 24 hours. Their clinical severity was similar to those with persistent BS or extremely low voltage activity and moderate to severe MRI injury. Clinical characteristics of newborns with respect to brain injury are presented in table 1. Mean hour of life to reach target temperature was 5.2 ± 1.9.
Table 1.
Clinical characteristics and MRI outcome
Video-EEG monitoring.
EEG monitoring was initiated at a mean of 10.2 ± 2.9 hours of life. All infants reached target temperature before or within 1 hour after EEG initiation. Mean duration of monitoring was 90.9 ± 28.2 hours. Nine infants received AEDs prior to the onset of EEG monitoring, and one of them subsequently developed subclinical seizures. In 2 newborns, monitoring was discontinued prior to rewarming.
EEG background pattern.
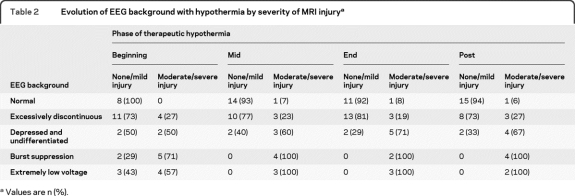
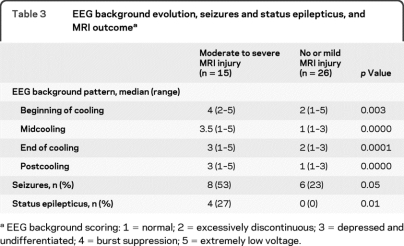
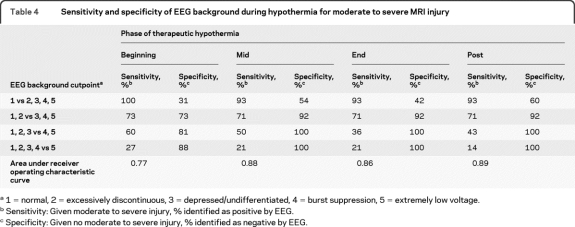
From the beginning of cooling to rewarming, the background improved in 19 newborns (49%), remained the same in 15 (38%), and worsened in 5 (13%). EEG background pattern and the burden of moderate to severe MRI injury at each time interval are presented in table 2. The background pattern for newborns with moderate to severe injury was significantly worse than for those with no or mild injury for all monitoring time points (table 3). The sensitivity and specificity of EEG background at specified time intervals was greatest at midcooling and beyond (table 4).
Table 2.
Evolution of EEG background with hypothermia by severity of MRI injurya
Values are n (%).
Table 3.
EEG background evolution, seizures and status epilepticus, and MRI outcomea
EEG background scoring: 1 = normal; 2 = excessively discontinuous; 3 = depressed and undifferentiated; 4 = burst suppression; 5 = extremely low voltage.
Table 4.
Sensitivity and specificity of EEG background during hypothermia for moderate to severe MRI injury
1 = normal, 2 = excessively discontinuous, 3 = depressed/undifferentiated, 4 = burst suppression, 5 = extremely low voltage.
Sensitivity: Given moderate to severe injury, % identified as positive by EEG.
Specificity: Given no moderate to severe injury, % identified as negative by EEG.
Beginning of cooling.
None of the newborns with a normal background at the beginning of cooling had moderate to severe injury. Of the 15 newborns with an excessively discontinuous pattern, 4 (27%) had moderate to severe injury, 5 had mild injury, and 6 were normal. In contrast, of the 14 newborns with BS or extremely low voltage patterns, 9 (64%) had moderate to severe injury. Of these, 4 had maximal MRI injury scores. Interestingly, 5 newborns with BS or extremely low voltage patterns at the beginning of cooling had a normal MRI or only mild injury. In all 5 cases, however, the EEG improved by 12–18 hours of recording, and in 3 of them the background normalized by midcooling.
Midcooling.
One newborn whose EEG had normalized by this interval from a discontinuous background at the beginning of cooling had moderate to severe MRI injury; none of the remaining 14 newborns with a normal EEG during midcooling had moderate to severe injury. Of the 13 infants with a discontinuous EEG, again about one-quarter (23%) had moderate to severe injury, accounting for 2 infants whose background was unchanged from the beginning of cooling and an additional infant whose initial background was depressed and undifferentiated. By this time period, all newborns (7/7, 100%) with BS or extremely low voltage patterns had moderate to severe injury, with 3 having maximal MRI injury scores.
End of cooling.
Moderate to severe MRI injury was present in 1 of the 12 infants (8%) whose background was normal and 3 of the 16 infants (19%) whose background was excessively discontinuous, compared with all 5 (100%) whose background showed BS or extremely low voltage.
Postcooling.
During this interval, the same newborn with a normal background (6%) and the same 3 newborns with a discontinuous background (27%) had moderate to severe injury. All 6 infants with BS or extremely low voltage patterns after rewarming had moderate to severe injury.
Electrographic seizures and SE.
Electrographic seizures were identified in 14 newborns (34%), 4 of whom had SE. Among the 14 newborns with seizures, 13 (93%) had seizure onset within the first 18 hours of recording, 8 in the first 6 hours. One infant had seizure onset during rewarming. Recurrent seizures were recorded during midcooling in 2 patients and during rewarming in 3. Six of 14 patients (43%) never showed a clinical correlate during seizures, including 3 with subclinical SE. All 4 infants with SE had BS or extremely low voltage patterns at the beginning of cooling and never recovered better than depressed and undifferentiated at last time point. Isolated or recurrent seizures were more frequent in patients with moderate to severe MRI injury compared with those with no or mild injury (53% vs 23%, p = 0.05), and SE was only seen in newborns with moderate to severe injury (p = 0.01).
Nonsurviving infants.
Among the 5 nonsurviving infants, 4 had persistent BS or extremely low voltage backgrounds throughout the duration of monitoring and maximal MRI injury, and goals of care were redirected to comfort measures. One infant had an excessively discontinuous background at the initiation of monitoring, depressed and undifferentiated by midcooling, with less severe MRI injury. Goals of care were redirected due to persistent multisystem failure.
DISCUSSION
This study demonstrates that continuous video-EEG recording is an important diagnostic and prognostic tool in newborns with HIE undergoing TH. In our cohort, EEG background was associated with early MRI findings throughout the treatment period. A normal EEG was associated with no or mild MRI brain injury at all time points, although a normal background at the beginning of cooling was even more predictive of a favorable MRI outcome (100% specific) than at later time points (93% specific), as the EEG background of one newborn with moderate to severe MRI injury improved from excessively discontinuous to normal over the first 24 hours of monitoring. In contrast, the prognostic value of a BS pattern or extremely low voltage background for moderate to severe injury increased from the beginning of cooling (81% specific) to midcooling and thereafter (100% specific), reflecting 5 newborns with these concerning patterns at the onset of monitoring who rapidly improved by midcooling and were spared from moderate to severe MRI injury. The greatest prognostic value of EEG background in this population for predicting moderate to severe MRI brain injury was not achieved until midcooling, highlighting the importance of continuous monitoring or sequential EEGs in this population.
Our findings are substantiated by prior studies in noncooled infants with HIE, which demonstrated that a normal EEG within the first 2–7 days of life is associated with favorable developmental outcome and a severely abnormal EEG (BS or extremely low voltage) on the second day of life or thereafter is associated with poor outcome.12–14,24 The few studies that reported EEG background in this population within the first 24 hours of life17,25,26 showed a relatively poor specificity for adverse developmental outcome following a severely abnormal background during the first 12 hours of life because of EEG normalization by 12 to 24 hours of life in some infants with normal outcome. Similarly, in our cohort, a BS or extremely low voltage EEG was not highly predictive for moderate to severe MRI injury until the second day of life, around the time of midcooling. This finding is supported by a prior study evaluating EEG during hypothermia in neonatal HIE by a single sample recorded sometimes in the first 48 hours of life, which found that a background of <5 μV was associated with death or major neurologic disability.15 Similarly, a recent study evaluating the prognostic value of amplitude-integrated EEG in newborns with HIE exposed to normothermia compared to those treated with TH showed that a severely abnormal background pattern in the hypothermia-treated group was not specific for abnormal developmental outcome until 48 hours of life.27
Our findings differ from prior studies of noncooled newborns with HIE in 2 main ways. First, prior studies have shown that a discontinuous EEG in the first several days of life is often associated with poor outcome,28–31 whereas the majority of newborns in our cohort (73%) with an excessively discontinuous pattern after rewarming had no or only mild MRI injury. While it is difficult to directly compare our study to prior studies given the differences in methodology and the wide range of definitions of discontinuous background in the literature, the outcome of newborns with an excessively discontinuous background appears to be different in newborns treated with hypothermia. Interestingly, clinical encephalopathy on the fourth day of life following TH has also been shown to be less predictive of outcome in cooled infants compared to noncooled infants.8 Second, in comparison to the single study of continuous EEG monitoring in noncooled newborns with HIE which showed improvement of the background pattern over the first 3 days of life in all newborns,17 the background worsened over the course of monitoring in 13% of newborns in our cohort. Whether the EEG differences seen in our cohort of infants with HIE and cooling are attributed to hypothermia itself or the evolution of injury in this population is uncertain.
Electrographic seizures were present in 34% of newborns during TH, and continuous video-EEG revealed that almost 50% of newborns with seizures, including 3 with SE, had seizures without clinical correlate. Although experimental studies showed a potent effect of hypothermia in controlling seizures,32,33 a high incidence of seizures has been reported in children during TH.34 This discrepancy may be related to the earlier and deeper cooling used in animal models.32,33 Most studies rely on clinical evaluation for seizure diagnosis and classification of seizure severity in newborns.35,36 However, it is known that the majority of seizures, especially in critically ill infants, do not have a clinical correlate and will not be recognized without continuous EEG.16,37,38 Moreover, it is often impossible to accurately differentiate between seizure-related and nonseizure movements in infants using clinical evaluation alone.39 While isolated or recurrent seizures were recorded in more than 50% of infants with moderate to severe brain injury, not all were associated with moderate to severe brain injury. In contrast, all newborns with SE had severely abnormal MRI. These results are in keeping with a recent work suggesting that a significantly worse outcome occurs in newborns with SE compared to newborns with recurrent seizures.20
There are several limitations to our study. First, due to the referral pattern at our institution, newborns did not start monitoring at the exact same time in the first day of life. However, in most of our patients, monitoring was initiated within the first 12 hours of life. Second, an excessively discontinuous background by our system encompassed a broad range of interburst intervals. Therefore, it is not surprising that a discontinuous pattern was a relatively poor predictor of MRI brain injury. Third, we used MRI as a short-term outcome measure and do not yet know the long-term outcome in this cohort. It has been reported that hypothermia does not affect the prognostic value of MRI in newborns with HIE.11 However, long-term developmental follow-up of this cohort is needed to confirm our results. Finally, as many patients with poor EEG backgrounds and moderate to severe brain injury were treated with AEDs, we were unable to assess whether depressed background activity was an effect of medication or due to underlying brain injury.
EEG monitoring in newborns is noninvasive, provides data from the entire cortex, and can be easily performed at the bedside. Our findings underscore the importance of continuous EEG monitoring in this population to assist with seizure management and discussions regarding prognosis and goals of care. Even in the setting of hypothermia, EEG remains a strong predictive tool, and its routine use alongside clinical evaluation and MRI is warranted. Establishing consensus on neonatal EEG nomenclature and classification will help future studies on the prognostic value of EEG during TH. Particularly, further analysis and revalidation of the excessively discontinuous pattern in neonates being treated with HIE is warranted. Given emerging data suggesting that seizures may be associated with increased brain injury following neonatal HIE,35,36,40 accurate seizure detection is becoming an important issue in the context of neuroprotection. Future studies that evaluate whether rapid and effective treatment of seizures will improve neurologic outcome will rely on continuous EEG monitoring.
ACKNOWLEDGMENT
The authors thank the neonatal research nurses of the Pediatric Clinical Research Center at UCSF for their work on this study.
Footnotes
- AED
- antiepileptic drug
- BS
- burst suppression
- HIE
- hypoxic-ischemic encephalopathy
- ROC
- receiver operating characteristic
- SE
- status epilepticus
- TH
- therapeutic hypothermia
- UCSF
- University of California, San Francisco
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. H.C. Glass, Dr. S.L. Bonifacio, and Dr. K.B. Nash.
DISCLOSURE
Dr. Nash reports no disclosures. Dr. Bonifacio has received fellowship support from the NIH. Dr. Glass has received research support from the NIH. Dr. Sullivan has received research support from Pfizer Inc. and the NIH. Dr. Barkovich serves on the editorial boards of the American Journal of Neuroradiology, Neuroradiology, Brain and Development, and Neuropediatrics; receives royalties from the publication of Pediatric Neuroimaging, 4th ed. (Lippincott Williams & Wilkins, 2005), Pediatric Neuroradiology (Amirsys, 2008), and Neuroradiology, 2nd ed. (Amirsys, 2009); and receives research support from the NIH. Dr. Ferriero serves on a scientific advisory board for the NIH/NINDS; serves as an Associate Editor for Annals of Neurology and Pediatric Research; receives royalties from the publication of Principle and Practices of Pediatric Neurology (Elsevier, 2005); and receives research support from the NIH. Dr. Cilio receives research support from the European Commission.
REFERENCES
- 1. Pierrat V, Haouari N, Liska A, Thomas D, Subtil D, Truffert P. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch Dis Child Fetal Neonatal Ed 2005;90:F257–F261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pin TW, Eldridge B, Galea MP. A review of developmental outcomes of term infants with post-asphyxia neonatal encephalopathy. Eur J Paediatr Neurol 2009;13:224–234 [DOI] [PubMed] [Google Scholar]
- 3. Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–1584 [DOI] [PubMed] [Google Scholar]
- 4. Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–1358 [DOI] [PubMed] [Google Scholar]
- 5. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol 1976;33:696–705 [DOI] [PubMed] [Google Scholar]
- 6. Mercuri E, Guzzetta A, Haataja L, et al. Neonatal neurological examination in infants with hypoxic ischaemic encephalopathy: correlation with MRI findings. Neuropediatrics 1999;30:83–89 [DOI] [PubMed] [Google Scholar]
- 7. Miller SP, Latal B, Clark H, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol 2004;190:93–99 [DOI] [PubMed] [Google Scholar]
- 8. Gunn AJ, Wyatt JS, Whitelaw A, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr 2008;152:55–58 [DOI] [PubMed] [Google Scholar]
- 9. Barnett A, Mercuri E, Rutherford M, et al. Neurological and perceptual-motor outcome at 5–6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics 2002;33:242–248 [DOI] [PubMed] [Google Scholar]
- 10. Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr 2005;146:453–460 [DOI] [PubMed] [Google Scholar]
- 11. Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monod N, Pajot N, Guidasci S. The neonatal EEG: statistical studies and prognostic value in full-term and pre-term babies. Electroencephalogr Clin Neurophysiol 1972;32:529–544 [DOI] [PubMed] [Google Scholar]
- 13. Watanabe K, Miyazaki S, Hara K, Hakamada S. Behavioral state cycles, background EEGs and prognosis of newborns with perinatal hypoxia. Electroencephalogr Clin Neurophysiol 1980;49:618–625 [DOI] [PubMed] [Google Scholar]
- 14. Holmes G, Rowe J, Hafford J, Schmidt R, Testa M, Zimmerman A. Prognostic value of the electroencephalogram in neonatal asphyxia. Electroencephalogr Clin Neurophysiol 1982;53:60–72 [DOI] [PubMed] [Google Scholar]
- 15. Mariani E, Scelsa B, Pogliani L, Introvini P, Lista G. Prognostic value of electroencephalograms in asphyxiated newborns treated with hypothermia. Pediatr Neurol 2008;39:317–324 [DOI] [PubMed] [Google Scholar]
- 16. Murray DM, Ryan CA, Boylan GB, Fitzgerald AP, Connolly S. Prediction of seizures in asphyxiated neonates: correlation with continuous video-electroencephalographic monitoring. Pediatrics 2006;118:41–46 [DOI] [PubMed] [Google Scholar]
- 17. Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcome at 2 years. Pediatrics 2009;124:e459–e467 [DOI] [PubMed] [Google Scholar]
- 18. Clancy RR, Legido A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia 1987;28:537–541 [DOI] [PubMed] [Google Scholar]
- 19. Scher MS, Hamid MY, Steppe DA, Beggarly ME, Painter MJ. Ictal and interictal electrographic seizure durations in preterm and term neonates. Epilepsia 1993;34:284–288 [DOI] [PubMed] [Google Scholar]
- 20. Pisani F, Cerminara C, Fusco C, Sisti L. Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology 2007;69:2177–2185 [DOI] [PubMed] [Google Scholar]
- 21. Holmes GL, Lombroso CT. Prognostic value of background patterns in the neonatal EEG. J Clin Neurophysiol 1993;10:323–352 [DOI] [PubMed] [Google Scholar]
- 22. Lamblin MD, André M, Challamel MJ, et al. Électroencéphalographie du nouveau-né prématuré et à terme: aspects maturatifs et glossaire. Neurophysiol Clin 1999;29:123–219 [DOI] [PubMed] [Google Scholar]
- 23. Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 1998;19:143–149 [PMC free article] [PubMed] [Google Scholar]
- 24. Selton D, Andre M. Prognosis of hypoxic-ischaemic encephalopathy in full-term newborns–value of neonatal electroencephalography. Neuropediatrics 1997;28:276–280 [DOI] [PubMed] [Google Scholar]
- 25. Pezzani C, Radvanyi-Bouvet MF, Relier JP, Monod N. Neonatal electroencephalography during the first twenty-four hours of life in full-term newborn infants. Neuropediatrics 1986;17:11–18 [DOI] [PubMed] [Google Scholar]
- 26. Pressler RM, Boylan GB, Morton M, Binnie CD, Rennie JM. Early serial EEG in hypoxic ischaemic encephalopathy. Clin Neurophysiol 2001;112:31–37 [DOI] [PubMed] [Google Scholar]
- 27. Thoresen M, Hellström-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 2010;126:131–139 [DOI] [PubMed] [Google Scholar]
- 28. Biagioni E, Mercuri E, Rutherford M, et al. Combined use of electroencephalogram and magnetic resonance imaging in full-term neonates with acute encephalopathy. Pediatrics 2001;107:461–468 [DOI] [PubMed] [Google Scholar]
- 29. Wertheim D, Mercuri E, Faundez JC, Rutherford M, Acolet D, Dubowitz L. Prognostic value of continuous electroencephalographic recording in full term infants with hypoxic ischaemic encephalopathy. Arch Dis Child 1994;71:F97–F102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Lieshout HB, Jacobs JW, Rotteveel JJ, Geven W, v't Hof M. The prognostic value of the EEG in asphyxiated newborns. Acta Neurol Scand 1995;91:203–207 [DOI] [PubMed] [Google Scholar]
- 31. Menache CC, Bourgeois BF, Volpe JJ. Prognostic value of neonatal discontinuous EEG. Pediatr Neurol 2002;27:93–101 [DOI] [PubMed] [Google Scholar]
- 32. Liu Z, Gatt A, Mikati M, Holmes GL. Effect of temperature on kainic acid seizures. Brain Res 1993;631:51–58 [DOI] [PubMed] [Google Scholar]
- 33. Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis 2006;23:689–696 [DOI] [PubMed] [Google Scholar]
- 34. Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology 2009;72:1931–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr 2009;155:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology 2002;58:542–548 [DOI] [PubMed] [Google Scholar]
- 37. Murray DM, Boylan GB, Ryan CA, Connolly S. Early continuous video-EEG in acute near-total intrauterine asphyxia. Pediatr Neurol 2006;35:52–56 [DOI] [PubMed] [Google Scholar]
- 38. Clancy RR, Legido D, Lewis D. Occult neonatal seizures. Epilepsia 1988;29:256–261 [DOI] [PubMed] [Google Scholar]
- 39. Malone A, Ryan CA, Fitzgerald A, Burgoyne L, Connolly S, Boylan GB. Interobserver agreement in neonatal seizure identification. Epilepsia 2009;50:2097–2101 [DOI] [PubMed] [Google Scholar]
- 40. Bjorkman ST, Miller SM, Rose SE, Burke C, Colditz PB. Seizures are associated with brain injury severity in a neonatal model of hypoxia-ischemia. Neuroscience 2010;166:157–167 [DOI] [PubMed] [Google Scholar]