Abstract
Objectives:
To investigate whether multiple sclerosis (MS) and non-MS white matter brain lesions can be distinguished by their appearance on 7 T T2*-weighted MRI.
Methods:
This was an observational study of 28 patients with MS and 17 patients with cerebral white matter lesions who did not have MS. Subjects were imaged using 7 T T2*-weighted imaging. White matter lesions were identified and analyzed for volume, location, and perivenous appearance.
Results:
Out of 901 lesions identified in patients with MS, 80% were perivenous. In comparison, 19% of 428 lesions identified in patients without MS had a perivenous appearance. Seven-Tesla T2*-weighted MRI reliably distinguished all patients with clinically definite MS (>40% lesions appeared perivenous) from those without clinical MS (<40% lesions appeared perivenous). Perivenous lesion appearance was more predictive of MS (odds ratio [OR] 14, p < 0.001) than subcortical or periventricular lesion location (OR 4.5, p < 0.001, and OR 2.4, p = 0.009). Perivenous lesion appearance was observed with a similar frequency in patients with clinically isolated syndrome of demyelination and in early (gadolinium-enhancing) MS lesions.
Conclusion:
Perivenous lesion location on 7 T T2*-weighted imaging is predictive of the presence of demyelination. Optimization of this imaging technique at lower magnetic resonance field strengths would offer benefit for the diagnosis of MS.
The diagnosis of multiple sclerosis (MS) rests on the identification of multifocal demyelinating lesions which are disseminated in their time of onset.1 Demyelinating lesions are readily detected as discrete hyperintense lesions on T2-weighted MRI.2 However, focal T2 signal change is not specific to MS, occurring in several other conditions.3 In particular, small hyperintense lesions are commonly seen in the cerebral white matter (WM) in patients of advanced age or with vascular risk factors.4,5 Histologic studies have revealed these incidental WM hyperintensities to reflect foci of ischemia.6 This overlap in the imaging features of demyelination and other conditions such as ischemia can lead to diagnostic uncertainty or invasive testing.
We have shown, using ultra-high-field (7 T) imaging with high T2* weighting, that most MS lesions are centered on small parenchymal veins.7 Here we compare the 7 T T2*-weighted MRI appearance of brain lesions between patients with MS and patients with asymptomatic white matter lesions to assess whether a perivenous lesion appearance differentiates between these states.
METHODS
Subjects.
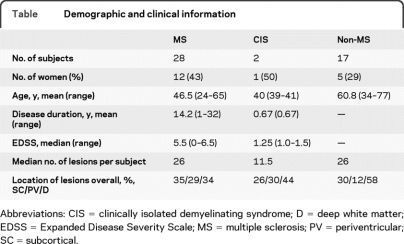
Twenty-eight patients with clinically definite MS according to internationally recognized criteria were recruited from neurology outpatient clinics at Nottingham's University Hospital between August 2007 and August 2009.1 Disease classification was as follows: 12 relapsing-remitting, 9 primary progressive, 7 secondary progressive. Two patients with demyelinating clinically isolated syndromes (CIS) were also recruited. In addition, 17 subjects who were found incidentally on clinical MRI scanning to have WM brain lesions were included in the study (referred to as the non-MS group). Five of these subjects were healthy volunteers who were incidentally found to have WM brain lesions. The remaining 12 were patients with multiple vascular risk factors and a clinical MRI scan which revealed incidental WM lesions. All lesions in the non-MS group had been reviewed by a neuroradiologist and none of these subjects were thought to have imaging or clinical features of CNS demyelination. Patient characteristics are shown in the table. Subject numbers were determined by power calculations from preliminary data on 10 patients with MS and 5 patients without MS.
Table.
Demographic and clinical information
Abbreviations: CIS = clinically isolated demyelinating syndrome; D = deep white matter; EDSS = Expanded Disease Severity Scale; MS = multiple sclerosis; PV = periventricular; SC = subcortical.
Standard protocol approvals, registrations, and patient consents.
All subjects gave informed consent and the study received ethical approval from the local research ethics committee.
Image acquisition.
Subjects were imaged using a 7 T Achieva scanner (Philips Medical Systems, Best, the Netherlands), equipped with whole body gradients, 16-channel SENSE RF receive coil, and head only volume transmit coil. A 3-dimensional gradient echo sequence was acquired using 200 transverse slices acquired in 4 stacks, each stack overlapping by 10 slices (192 × 164 × 85 mm field of view, 0.5 mm isotropic voxels, echo time = 20 msec, repetition time = 150 msec, and flip angle 14°). A parallel imaging SENSE factor of 2 (right–left direction) and an EPI factor of 3 were employed; acquisition time was 8.8 minutes.
In addition, standard clinical MRI, performed on a 1.5 T or 3 T scanner within 2 years of recruitment to this study, were available for 22 of the patients (8 MS and 14 non-MS).
Image analysis.
All 7 T images were converted to Analyze format and image stacks were merged using in-house software.8 All analysis was performed with the primary observer (E.C.T.) blinded to disease status.
White matter lesions in patients with MS and controls were outlined on 7 T T2* magnitude images using a manually defined edge threshold technique. Each lesion was outlined on each axial slice on which it appeared so that lesion volumes could be calculated. Lesions were classified as periventricular (if within one pixel from the ventricle), subcortical (if within one pixel of the cortex), or deep WM.
The 7 T T2*-weighted magnitude images were viewed in orthogonal planes using MRIcro.9 For each lesion, the presence or absence of a central vein was noted. Veins were counted if they 1) could be visualized in at least 2 perpendicular planes, 2) appeared linear in at least one plane, and 3) were completely surrounded by hyperintense signal in at least one plane (to avoid the inclusion of adjacent rather than central veins). Lesions were classed as perivenous is they contained one or more central veins.
Where available, clinical T2-weighted MRI were analyzed for whether they met Fazekas criteria (more than 3 lesions plus 2 of the following: one lesion >6 mm diameter, one infratentorial lesion, one lesion abutting the body of the lateral ventricle).10
Statistics and reproducibility.
χ2 was used to compare the frequencies of perivenous lesions between patients with MS and patients without MS. Regression analysis (logit with standard error adjusted for clusters in patients) was used to determine how strongly the perivenous appearance of lesions predicted disease status (MS vs non-MS) when controlling for potential confounders. The dependent variable was disease status (MS or non-MS); independent variables were lesion volume, lesion location, the presence of a central vein, age, and gender. Lesion location variable was assigned 3 categories (subcortical, periventricular, and deep). As a robustness check, in a second regression, the subcortical and periventricular lesions were combined and compared to deep white matter locations in order to mimic more closely what is done clinically. Further analysis using the same method was performed in a subgroup of patients aged ≥50 years (9 MS and 14 non-MS).
It was recognized that there was potential for unblinding of the primary observer to disease status (MS or control) on the basis of lesion distribution. To ensure that lesion classification had not been influenced by unblinding, 20 lesions, selected at random, were cropped such that images of the lesion excluded the remainder of the brain. Using cropped images, lesions were analyzed by a second observer for the presence or absence of a central vein (N.E.; blinded to the original results). Results revealed almost perfect agreement (Cohen kappa coefficient = 0.91, p < 0.001).
For patients with high lesion loads, our method of analyzing every lesion was recognized to be time-consuming. In order to estimate the smallest number of lesions that could be analyzed while maintaining an acceptable accuracy for predicting MS/non-MS, we applied hypergeometric distribution theory. Hypergeometric distribution describes the distribution of probabilities of various outcomes when n items (in our case lesions) are sampled out of a total number of N items, without replacement (each lesion can only be analyzed once). Using this model, we investigated how often sampling 10 lesions (in any patient who had >10 lesions) would provide the correct outcome where ≤40% perivenous lesions was taken to imply non-MS and >40% perivenous lesions was taken to indicate MS.
RESULTS
Using 7 T T2*-weighted imaging, 901 WM lesions were detected in the brains of 28 patients with MS (range 4–83 lesions per patient, mean 33 lesions; mean lesion volume 0.095 mL) and 428 WM lesions were detected in the brains of 17 subjects without MS (range 1–76 lesions per subject; mean 25 lesions; mean lesion volume 0.094 mL). Examples of MS and non-MS WM lesions are shown in figure 1. White matter lesions observed in patients with MS were significantly more likely to have perivenous location than lesions observed in the non-MS group (80% vs 19%; p < 0.001). The proportion of perivenous lesions in individual patients with MS (mean 80%, range 53%–100%) was consistently much higher than in individual subjects without MS (mean 16%, range 0%–34%; figure 2A). Perivenous lesion appearance was equally common in patients with CIS, relapsing-remitting MS, primary progressive MS, and secondary progressive MS (figure 2B).
Figure 1. Multiple sclerosis (MS) and non-MS lesion appearances on 7 T T2*-weighted imaging.
(A) A white matter lesion from a patient with MS is shown in 3 orthogonal planes, highlighted by red crosshairs. Inset are enlarged versions of the lesion as it appears in each plane. The lesion can be seen to have a perivenous appearance in each plane. (B) A white matter lesion in a patient without MS is shown for comparison. Even on the enlarged images, no central vessel is evident within this lesion.
Figure 2. Comparison of the proportion of perivenous lesions seen (A) in patients with multiple sclerosis (MS) vs subjects without MS and (B) in subjects with different clinical phenotypes of demyelinating disease.
(A) This scatterplot shows the proportion of perivenous lesions which were observed in each subject. Lack of overlap between the patients with MS and subjects without MS supports the discriminatory value of this imaging marker. (B) This scatterplot shows the proportion of perivenous lesions which were observed in subjects with different clinical phenotypes. CIS = clinically isolated syndrome; PP = primary progressive MS; RR = relapsing-remitting MS; SP = secondary progressive MS.
In patients for whom recent clinical images were available, 7 of the 8 patients with MS (88%) were found to meet Fazekas criteria for predicting the presence of demyelination. However, 4 of the 14 patients without MS (29%) also met Fazekas criteria.
Regression analysis confirmed that perivenous lesion appearance on 7 T T2*-weighted imaging was highly predictive of the presence of MS after taking into account other potential contributing factors (OR 14.0, confidence interval 9.6–20.3, p < 0.001). Even in a subgroup analysis of patients aged over 50 years, perivenous lesion appearance still strongly predicted the presence of MS (OR 13.7). While lesion location was also predictive of MS, the magnitude of this effect was considerably lower than for perivenous appearance (periventricular, OR 2.4, p = 0.009; subcortical, OR 4.5, p < 0.001 compared to deep white matter) (figure 3). When subcortical and periventricular locations were combined and compared with deep WM, the OR was 3.4 (presence of central vein OR 13.3; p < 0.001).
Figure 3. Proportion of perivenous lesions seen in multiple sclerosis (MS) and non-MS groups according to lesion location.
Lesions in all 3 locations were more likely to be perivenous in patients with MS. However, even in subjects without MS, a high proportion of periventricular lesions contained at least one vein.
In order to determine whether the perivenous appearance was seen in very early demyelinating lesions, we performed T1-weighted, gadolinium-enhanced images in one patient with active relapsing MS. These images demonstrated 10 enhancing lesions, of which 8 were perivenous. Follow-up imaging 3 months later confirmed persistence of the perivenous location of these lesions on T2* imaging. In 2 patients with CIS, 23 lesions were identified, of which 19 (83%) were perivenous.
Hypergeometric distribution methodology suggested that if only 10 lesions per patient were sampled (in patients with >10 lesions), the diagnosis of MS/non-MS could be correctly predicted with 90% certainty in 44 out of 45 patients tested.
DISCUSSION
Conventional T2-weighted MRI continues to play a valuable role in diagnosing MS in patients with appropriate clinical features. However, diagnostic uncertainty arises when T2 hyperintense brain lesions cannot be confidently classified as being demyelinating. Definitive diagnosis may be achieved by further paraclinical testing (lumbar puncture or electrophysiologic evoked potentials), but in many instances a period of uncertainty ensues. Apart from diagnostic delay being unsettling for both patient and physician, growing evidence suggests that emerging treatments are most beneficial when used early in the disease course.11
Imaging criteria (Barkhof/Tintore) are useful for predicting the likelihood of clinically definite MS developing in a patient presenting with a CIS. However, these criteria have not been tested in a study that compares patients with MS and patients with ischemia or in patients who lack a typical CIS history. The Fazekas criteria, on the other hand, were devised specifically to help distinguish the imaging appearances of MS from ischemia.10 Retrospective evaluation has suggested Fazekas criteria to be highly specific (96%) and moderately sensitive (81%) to the presence of MS.12 However, these features are not pathognomonic and some patients with cerebral ischemia meet all Fazekas criteria.13
Small venules in the center of lesions are visualized using T2*-weighted MRI because of the paramagnetic effect of deoxyhemoglobin. Here we show that using 7 T T2*-weighted MRI we can reliably distinguish all patients with clinically definite MS (>40% lesions appeared perivenous) from those without clinical MS (<40% lesions appeared perivenous). Perivenous lesions were equally common in patients with CIS and all phenotypes of MS. In combination, these features suggest that perivenous lesion appearance may be a useful imaging marker of demyelination. In comparison, where clinical images were available (n = 22), Fazekas criteria had a sensitivity of 88% in detecting MS but a specificity of only 71%.
Our trial is not without limitations. The MS and non-MS groups were relatively small and had significantly different ages. Age was controlled for in our analyses but nevertheless, further work with more close age matching may be desirable. Furthermore, in order to predict disease status, analysis of all white matter lesions was required. This approach was time-consuming in patients with a heavy lesion load. However, in our sample, using hypergeometric distribution methodology it was possible to predict the diagnosis of MS/non-MS with at least 90% accuracy by sampling only 10 lesions in all but one of our patients. This suggests that in patients with high lesion loads, only a proportion of the lesions will need to be analyzed to make a confident diagnosis. Further work will be required to validate how few lesions could be analyzed to maintain a high diagnostic confidence once this technique is translated to a clinical scanner. Finally, the lack of availability of ultra-high-field scanners for clinical use means that our results are not immediately applicable to clinical practice. Work is in progress in our department to translate this technique to clinically available MRI scanners operating at lower field strengths with promising results to date. In previous work, we found 3 T T2*-weighted imaging to identify 89% of 3 T fluid-attenuated inversion recovery lesions (vs 94% at 7 T). Of the visible lesions, 3 T T2*-weighted imaging identified veins in 45% (vs 87% at 7 T).14 Our more recent work has shown that the sensitivity to veins can be improved considerably at 3 T, by altering voxel size and shape, and echo time.15 Using susceptibility-weighted imaging at 3 T and restricting analysis to large lesions (>3 mm diameter), Lummel et al16 were able to visualize central vessels in almost all MS lesions but they also observed vessels in most large non-MS white matter lesions. More work at lower field strengths will therefore be necessary, first to determine the optimum sequence parameters and secondly to establish a protocol for image analysis which balances accuracy with efficiency for distinguishing between MS and non-MS lesions.
This study was aimed at distinguishing patients with demyelination from those with incidental asymptomatic WM lesions (which were mostly expected to be ischemic). Further work will be required to investigate differences between lesions seen in MS and other specific conditions such as migraine. Work is in progress to determine whether this imaging marker is predictive of the development of clinically definite MS in patients with CIS by following patients longitudinally.
ACKNOWLEDGMENT
The authors thank Dr. Su-Yin Lim and Professor Cris Constantinescu (Department of Clinical Neurology, University of Nottingham, UK) who recruited volunteers with clinically isolated syndromes. In addition, the authors thank Dr. Timothy Jaspan (Department of Neuroradiology, Nottingham University Hospital, UK) for guidance in image analysis and comments on the manuscript.
Footnotes
- CIS
- clinically isolated syndrome
- MS
- multiple sclerosis
- OR
- odds ratio
- WM
- white matter
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Trudy Owens.
DISCLOSURE
Dr. Tallantyre receives fellowship support from the MRC/MS Society UK; and has received funding for travel from Bayer Schering Pharma and Novartis. Dr. Dixon reports no disclosures. Dr. Donaldson received funding travel from Novartis. Dr. Owens reports no disclosures. Dr. Morgan receives research support from the NIH. Prof. Morris serves as Associate Editor for Journal of Magnetic Resonance Imaging and Editor for MAGMA; and receives research support from the Medical Research Council UK and the MS Society. Dr. Evangelou has served on scientific advisory boards for Bayer Schering Pharma and Merck Serono; has received funding for travel from Bayer Schering Pharma, Merck Serono, and Novartis; and receives research support from Multiple Sclerosis of Great Britain and Northern Ireland.
REFERENCES
- 1. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58:840–846 [DOI] [PubMed] [Google Scholar]
- 2. Newcombe J, Hawkins CP, Henderson CL, et al. Histopathology of multiple sclerosis lesions detected by magnetic resonance imaging in unfixed postmortem central nervous system tissue. Brain 1991;114:1013–1023 [DOI] [PubMed] [Google Scholar]
- 3. Ormerod IE, Miller DH, McDonald WI, et al. The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions: a quantitative study. Brain 1987;110:1579–1616 [DOI] [PubMed] [Google Scholar]
- 4. Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology 1997;16:149–162 [DOI] [PubMed] [Google Scholar]
- 5. Shintani S, Shiigai T, Arinami T. Silent lacunar infarction on magnetic resonance imaging (MRI): risk factors. J Neurol Sci 1998;160:82–86 [DOI] [PubMed] [Google Scholar]
- 6. Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683–1689 [DOI] [PubMed] [Google Scholar]
- 7. Tallantyre EC, Brookes MJ, Dixon JE, Morgan PS, Evangelou N, Morris PG. Demonstrating the perivascular distribution of MS lesions in vivo with 7-Tesla MRI. Neurology 2008;70:2076–2078 [DOI] [PubMed] [Google Scholar]
- 8. Morgan PS. Image analysis and related computer issues. Available at: http://www.nottingham.ac.uk/∼njzwww/paul/software. Accessed August 5, 2009 [Google Scholar]
- 9. Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 2000;12:191–200 [DOI] [PubMed] [Google Scholar]
- 10. Fazekas F, Offenbacher H, Fuchs S, et al. Criteria for an increased specificity of MRI interpretation in elderly subjects with suspected multiple sclerosis. Neurology 1988;38:1822–1825 [DOI] [PubMed] [Google Scholar]
- 11. Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med 2008;359:1786–1801 [DOI] [PubMed] [Google Scholar]
- 12. Offenbacher H, Fazekas F, Schmidt R, et al. Assessment of MRI criteria for a diagnosis of MS. Neurology 1993;43:905–909 [DOI] [PubMed] [Google Scholar]
- 13. Miller DH, McDonald WI, Smith KJ. The diagnosis of multiple sclerosis. In:Compston A. ed. McAlpine's Multiple Sclerosis. London: Churchill Livingstone Elsevier; 2005:372 [Google Scholar]
- 14. Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol 2009;44:491–494 [DOI] [PubMed] [Google Scholar]
- 15. Dixon JE, Tallantyre EC, Morgan PS, Evangelou N, Morris PG. Optimisation of 3 T and 7 T T2*-weighted MRI for the detection of small parenchymal veins in MS lesions. Int Soc Magn Reson Med 2010;18:4322 [Google Scholar]
- 16. Lummel N, Boeckh-Behrens T, Schoepf V, Burke M, Bruckmann H, Linn J. Presence of a central vein within white matter lesions on susceptibility weighted imaging: a specific finding for multiple sclerosis? Neuroradiology. doi: 10.1007/s00234-010-0736-z. Epub 2010 Jun 29. [DOI] [PubMed] [Google Scholar]