Abstract
Objective:
To investigate factors, including cognitive and brain reserve, which may independently predict prevalent and incident dementia of the Alzheimer type (DAT) and to determine whether inclusion of identified factors increases the predictive accuracy of the CSF biomarkers Aβ42, tau, ptau181, tau/Aβ42, and ptau181/Aβ42.
Methods:
Logistic regression identified variables that predicted prevalent DAT when considered together with each CSF biomarker in a cross-sectional sample of 201 participants with normal cognition and 46 with DAT. The area under the receiver operating characteristic curve (AUC) from the resulting model was compared with the AUC generated using the biomarker alone. In a second sample with normal cognition at baseline and longitudinal data available (n = 213), Cox proportional hazards models identified variables that predicted incident DAT together with each biomarker, and the models' concordance probability estimate (CPE), which was compared to the CPE generated using the biomarker alone.
Results:
APOE genotype including an ε4 allele, male gender, and smaller normalized whole brain volumes (nWBV) were cross-sectionally associated with DAT when considered together with every biomarker. In the longitudinal sample (mean follow-up = 3.2 years), 14 participants (6.6%) developed DAT. Older age predicted a faster time to DAT in every model, and greater education predicted a slower time in 4 of 5 models. Inclusion of ancillary variables resulted in better cross-sectional prediction of DAT for all biomarkers (p < 0.0021), and better longitudinal prediction for 4 of 5 biomarkers (p < 0.0022).
Conclusions:
The predictive accuracy of CSF biomarkers is improved by including age, education, and nWBV in analyses.
Disease-modifying therapies likely will be most effective if administered during the preclinical stage of Alzheimer disease (AD), prior to dementia symptom development.1,2 Although several AD biomarkers are under investigation, few studies address their predictive accuracy for dementia of the Alzheimer type (DAT).
Promising CSF biomarkers are based on assays of proteins that are pathologically misfolded, and include amyloid-β42 (Aβ42), the primary component of senile plaques, along with tau and phosphorylated tau (ptau181), the principal components of neurofibrillary tangles.1 These markers may become abnormal, indicating AD-type pathology in cognitively normal individuals a decade or more before the appearance of dementia symptoms.3 This possibility suggests that biomarkers alone may not yield ideal diagnostic accuracy for prevalent DAT and underscores the importance of understanding the temporal relationships between biomarker levels in cognitively normal adults, symptomatic AD (i.e., incident AD), and factors that modify those relationships, to avoid exposing healthy people to potential side-effects of AD medications many years before these drugs are needed.
Factors that are associated with cognitive impairment on their own may confound relationships between biomarkers and clinical symptoms.4 Additionally, cognitive and brain reserve studies5–9 indicate that several factors modify the association between AD pathology and dementia. We sought to identify factors that predict prevalent and incident DAT when considered concomitantly with the CSF biomarkers of Aβ42, tau, and ptau181 and to determine whether including these factors increases the predictive accuracy of biomarker models in identifying prevalent and incident DAT.
METHODS
Participants were volunteers with normal or impaired cognition enrolled in longitudinal, prospective studies of aging and memory at the Washington University Charles F. and Joanne Knight Alzheimer's Disease Research Center. The present study represents a secondary analysis of data from participants who had lumbar puncture (LP) for collection of CSF from June 18, 1998 (when CSF collection was instituted) through October 23, 2008 (figure e-1 on the Neurology® Web site at www.neurology.org). Detailed information regarding recruitment, enrollment, and clinical assessment has been published.10 Briefly, participants are community-dwelling individuals recruited from the greater St. Louis, MO, area. Individuals with a medical or psychiatric illness (e.g., cancer requiring chemotherapy) that could interfere with longitudinal follow-up or adversely impact cognition are excluded. At study entry and all subsequent yearly assessments, each participant is accompanied by a collateral source (CS), usually a family member or close friend. The participant and their CS complete separate semi-structured interviews conducted by experienced clinicians. The participant completes a general physical and neurologic examination, health and medication histories, the Mini Mental-State Exam (MMSE),11 the Geriatric Depression Scale (GDS),12 and psychometric testing. Participants are also asked to undergo brain imaging with MRI.
Standard protocol approvals, registrations, and patient consents.
Study protocols were approved by the Washington University Medical Center Human Subjects Committee, and written informed consent was obtained from all participants.
Clinical assessment for dementia.
A Clinical Dementia Rating (CDR)13–15 is generated for each participant by experienced clinicians who use the information obtained from the participant and CS interviews to determine the presence of dementia. Clinician trainees review teaching and reliability videotapes of participants until 80% agreement or better is achieved with the gold standard videotapes. Impairment in each of 6 domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care) is defined as decline due only to cognitive change. The global CDR is derived from ratings of each domain in accordance with a standard scoring algorithm: CDR 0 = normal cognition and CDR 0.5 = very mild, CDR 1 = mild, CDR 2 = moderate, and CDR 3 = severe dementia. The reliability of the CDR has been established.15–17
For participants who receive a CDR of 0.5 or above, indicating abnormal cognition, a clinical diagnosis is assigned. A diagnosis of DAT is based on evidence that the participant has experienced the gradual onset and progression of memory and other cognitive problems that represents a change from a previous higher level of functioning, and that interferes with usual activities at home and in the community. The validity of this diagnosis has been demonstrated by subsequent progressive cognitive and functional decline18 and by the neuropathologic diagnosis of AD in 92% of those coming to autopsy.19 Clinicians are unaware of the CSF results for each participant.
Collection and analysis of CSF.
Following an overnight fast, 20–30 mL of CSF was obtained at 8:00 am from participants by a trained neurologist using a 22-gauge Sprotte spinal needle. To avoid possible gradient effects, samples were gently inverted and centrifuged at low speed. Tubes were frozen at −84°C20 after aliquotion into polypropylene tubes. ELISA (Innotest; Innogenetics, Ghent, Belgium) was used to analyze CSF samples for Aβ42, tau, and ptau181. Analysis of CSF is conducted by 2 trained scientists with over 25 years of experience, who are blind to the results of the participant's clinical assessment.
Inclusion criteria.
Patients were 50 years or older at the time of first LP. Only participants with data available for all study variables at the time of data analysis were included so that predictive models could be compared for the same individuals.
Statistical analyses.
Analyses were conducted using SAS version 9.1 (SAS Institute, Inc, Cary, NC).
Cross-sectional analyses.
These analyses used data from participants with a diagnosis of normal cognition or DAT at the closest clinical assessment within 1 year before or after the LP. Logistic regression was used to identify candidate variables (see below) that were independently related to DAT diagnosis when considered together with each CSF biomarker (Aβ42, tau, ptau181, and the ratios of tau/Aβ42 and ptau181/Aβ42). The biomarker was entered into the model first, and the stepwise selection procedure then identified additional demographic, brain reserve, cognitive reserve, and other variables linked to cognitive impairment that improved model fit. A significance level of 0.05 was used for entering and exiting effects. A receiver operating characteristic curve (ROC) and the area under the curve (AUC) were calculated from the resulting model. Higher AUC values indicate better predictive accuracy, reaching a maximum at 1, which signifies perfect prediction. A second logistic regression was performed including the biomarker as the sole predictor of a DAT diagnosis, which generated an AUC based on the biomarker alone. The AUCs from both models were then compared and tested21 to determine whether the expanded model resulted in increased predictive accuracy.
We also compared the AUCs generated from the 5 models assessing the biomarkers as sole predictors with each other, and the AUCs generated from the 5 stepwise models with each other. Leave-one-out estimation was used to cross-validate the ROCs and their AUCs.
Longitudinal analyses.
Data from participants with normal cognition (CDR 0) at the closest clinical assessment within 1 year prior to or 1 month after their first LP and with at least one subsequent clinical assessment were used. A concordance probability estimate (CPE),22 reflecting the predictive accuracy of the Cox proportional hazards model, was calculated for models using each of the 5 biomarker variables as the sole predictor of time to a diagnosis of DAT. Additional Cox proportional hazards models were used to determine which of the candidate variables were independent predictors of time to DAT when considered together with each of the biomarker variables. In these analyses, the biomarker was entered into the model first, and stepwise selection was used to identify additional variables that improved model fit. A significance level of 0.05 was used for entering and exiting effects. The CPE resulting from the expanded stepwise model was then compared to the CPE yielded using the biomarker as the sole predictor, to determine whether addition of the variables identified in the stepwise procedure resulted in increased predictive accuracy. In all models, data from participants who died, did not develop dementia, or did not return for follow-up assessment were censored at the date of their most recent clinical assessment. The CPEs generated from the 5 models assessing the biomarkers as sole predictors were compared with each other, as were the CPEs generated from the 5 stepwise models.
Candidate variables available for stepwise selection.
With one exception, the same candidate variables were used in the cross-sectional and longitudinal analyses. These included age at LP, gender, race, APOE genotype, and the CS rating of the participant's general physical health (excellent, good, mild, or moderate impairment). Candidate variables used as proxies for cognitive reserve (i.e., the efficient use of brain networks or the ability to recruit alternate brain networks or cognitive strategies)7 were years of education23–29 and occupational attainment23,30 as reflected in the occupation ranking of the Hollingshead Index of Social Position.31 Normalized whole brain volume (nWBV)32 (measured using the methods of Buckner et al.33) and clinical history of stroke were taken as proxies of brain reserve, which reflects the numbers and health of neurons in the cortex.7 Other candidate variables represented factors known or suggested to cause cognitive impairment themselves, which may confound the association between AD pathology and cognition. These included depressive symptoms34 (scores on the GDS12 and a clinical diagnosis of depression or bereavement), a concomitant medical condition that may interfere with cognition (e.g., vitamin B12 deficiency, alcoholism, sleep apnea), or use of a medication that may interfere with cognition (e.g., benzodiazepines/sedatives, anticholinergics, opiates). In preliminary analyses, the tau and the ptau181/Aβ42 variables yielded very small, and very large, odds and hazards ratios, respectively. Therefore, tau values were divided by 10, and ptau181/Aβ42 ratios were multiplied by 10, before use in the statistical analyses. These linear transformations resulted in exactly the same p values throughout the models, but easier interpretation of odds and hazards ratios for these variables.
Because 32.9% of participants who would have otherwise met criteria for the longitudinal analyses did not have structural imaging data available at the time of analysis, nWBV was not included in the primary longitudinal analyses. In exploratory analyses, however, we repeated the Cox proportional hazards models using stepwise selection with the subsample for which nWBV was available.
RESULTS
Table 1 shows the demographic characteristics for the cross-sectional (n = 247) and longitudinal (n = 213) samples. Some participants (n = 161) were represented in both samples.
Table 1.
Demographics for cross-sectional and longitudinal samples at baseline
Abbreviations: DAT = dementia of the Alzheimer type; LP = lumbar puncture.
Cross-sectional identification of DAT.
Models testing each biomarker alone.
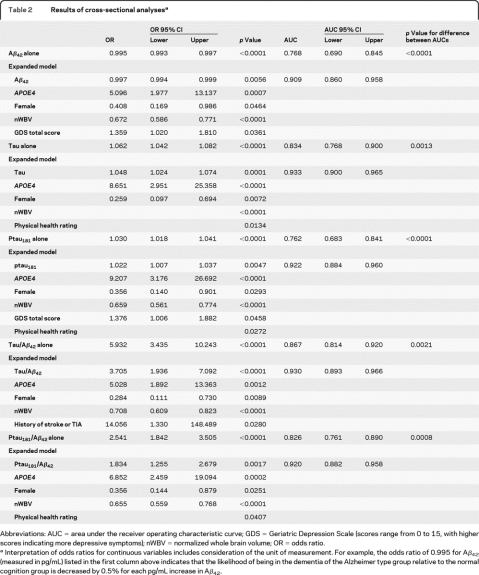
Forty-six (18%; 34 = CDR 0.5, 11 = CDR 1, 1 = CDR 2) participants in the cross-sectional sample had DAT. Lower values of CSF Aβ42, and higher values of each of the other biomarker variables, were associated with having DAT vs normal cognition (table 2). AUC values for models based on the individual biomarkers ranged from 0.762 to 0.867 (table e-1). The highest AUCs were generated by CSF tau and tau/Aβ42, and the lowest by CSF Aβ42 and ptau181 (table e-2).
Table 2.
Results of cross-sectional analysesa
Abbreviations: AUC = area under the receiver operating characteristic curve; GDS = Geriatric Depression Scale (scores range from 0 to 15, with higher scores indicating more depressive symptoms); nWBV = normalized whole brain volume; OR = odds ratio.
Interpretation of odds ratios for continuous variables includes consideration of the unit of measurement. For example, the odds ratio of 0.995 for Aβ42 (measured in pg/mL) listed in the first column above indicates that the likelihood of being in the dementia of the Alzheimer type group relative to the normal cognition group is decreased by 0.5% for each pg/mL increase in Aβ42.
Expanded models.
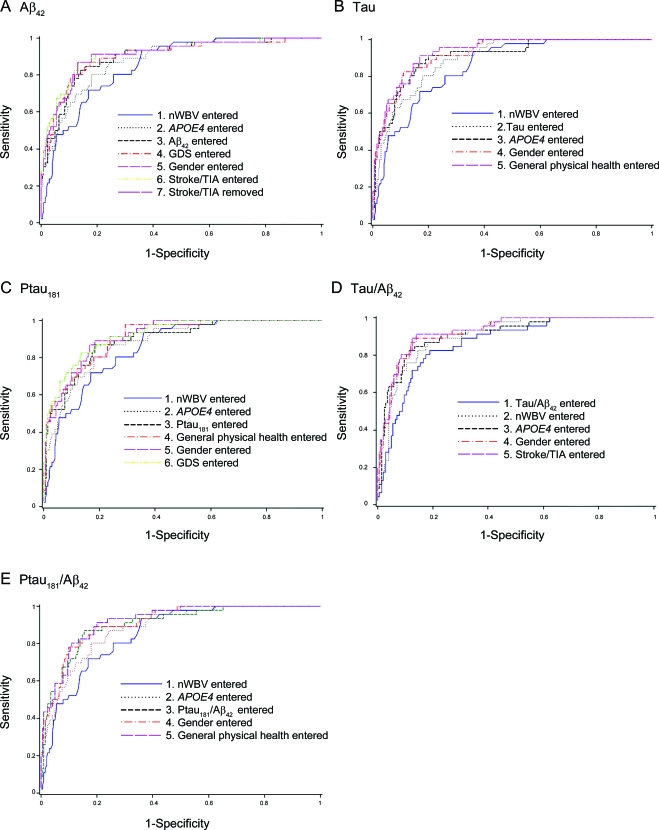
An APOE genotype containing an ε4 allele (APOE4), gender, and nWBV were independent predictors of DAT when considered with each of the CSF biomarkers in the expanded models, such that APOE4 genotype increased, but female gender and larger nWBVs decreased, the likelihood of DAT (table 2). Worse physical health rating also helped to predict DAT in the models testing tau, ptau181, and ptau181/Aβ42, and higher GDS scores were associated with DAT in the Aβ42 and ptau181 models (table 2). A history of stroke or TIA was an additional independent predictor of DAT in the tau/Aβ42 stepwise model (table 2). Figure 1 shows the increase in the AUC as each variable is added to the expanded model for each biomarker. Each of the expanded models yielded an AUC that was higher than that obtained for the model based on the CSF biomarker alone (table e-1). There was no difference across the AUCs generated by the 5 expanded models (p = 0.3531).
Figure 1. Discriminating persons with dementia of the Alzheimer type from those with normal cognition.
(A) Aβ42, (B) Tau, (C) Ptau181, (D) Tau/Aβ42, (E) Ptau181/Aβ42. Increase in area under the receiver operating characteristic curve as each variable is added to the expanded model for each biomarker. GDS = Geriatric Depression Scale; nWBV = normalized whole brain volume.
Longitudinal prediction of future DAT.
Models testing each biomarker alone.
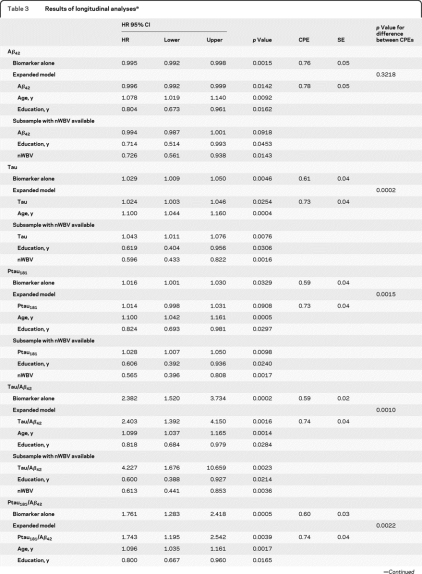
Fourteen participants (6.6%) with normal cognition at baseline developed DAT over a mean follow-up period of 3.2 ± 1.6 years. Each biomarker was associated with time to DAT, with lower values of CSF Aβ42 and higher values of the other biomarkers associated with more rapid time to DAT development (table 3). The model testing Aβ42 generated a higher CPE than each of the remaining biomarkers, the CPEs for which did not differ from each other (tables 3 and e-3).
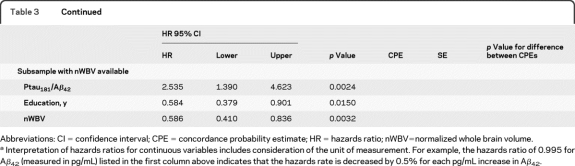
Table 3.
Results of longitudinal analysesa
Abbreviations: CI = confidence interval; CPE = concordance probability estimate; HR = hazards ratio; nWBV=normalized whole brain volume.
Interpretation of hazards ratios for continuous variables includes consideration of the unit of measurement. For example, the hazards ratio of 0.995 for Aβ42 (measured in pg/mL) listed in the first column above indicates that the hazards rate is decreased by 0.5% for each pg/mL increase in Aβ42.
Expanded models.
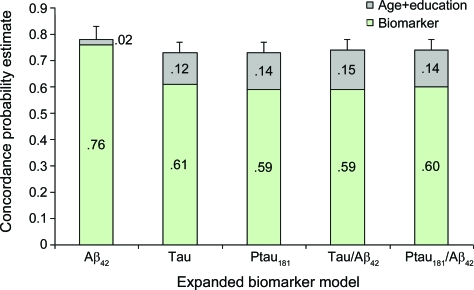
Considered together with the biomarker variable, older age was independently related to a faster time to DAT diagnosis in all predictive models and more years of education predicted a slower time to DAT in 4 of the 5 models (table 3). With the exception of CSF Aβ42, the CPEs resulting from the expanded models were significantly higher than the CPE generated by testing the biomarker alone (tables 3 and e-4). Although the small increase in the CPE for Aβ42 was not significant, the enhanced models for the other biomarkers increased the CPEs for their respective models to a level that did not differ (p = 0.2740) from that of the expanded Aβ42 model, nor from that of each of the other expanded biomarker models (figure 2).
Figure 2. Predicting time to incident dementia of the Alzheimer type from cognitive normality at baseline.
Increase in concordance probability estimate with the addition of age and education in the expanded biomarker models.
Exploratory analysis for the subsample with nWBV.
Seven (4.9%) of the 143 participants with baseline nWBV measurement developed DAT over the follow-up period. In the analyses including nWBV among the candidate variables for stepwise selection, education and nWBV were independently associated with time to DAT when considered together with each biomarker (table 3). Unlike the analyses on the entire longitudinal sample, age no longer met entry criteria for any of the models (table 3).
DISCUSSION
We found that the identification by CSF biomarkers of individuals with prevalent symptomatic AD and the predictive power of the biomarkers for the future onset of symptomatic AD among individuals with normal cognition can be improved by including variables reflecting attributes of the individual in predictive equations. Although the predictive value of the biomarkers differed from each other when considered alone, addition of these ancillary variables led to predictive values that were similar across all biomarker models. This was true for both cross-sectional and longitudinal prediction of DAT.
The accurate cross-sectional identification of DAT was improved by nWBV, gender, and APOE ε4 when considered together with all CSF biomarkers studied. Larger nWBVs were associated with a lower likelihood of DAT, consistent with the idea that volume may function as a marker of brain reserve,32 enabling one to cope better with AD-related pathology prior to overt symptoms. Smaller brain volumes may also reflect neuronal death. These two explanations are not incompatible.
The cross-sectional association of gender with DAT may be due to the greater tendency for men to enroll in our longitudinal studies when they are experiencing dementia symptoms compared to women. Therefore, the cross-sectional effect of gender may differ in other samples. We found no effect of gender on prediction of incident DAT.
We found that, after controlling for relationships between APOE ε4 and the biomarker levels themselves, APOE ε4 adds additional predictive power. This suggests that APOE genotype may be linked to additional pathologic processes other than those reflected in CSF biomarker values studied or investigated here. We previously found that APOE ε4, together with age, is related to CSF AB42 levels among cognitively normal individuals.35 That study also suggested that other, as yet unknown, factors are related to abnormal biomarker levels, as some individuals with low CSF Aβ42 did not have an APOE ε4 allele, and APOE ε4 was unrelated to CSF tau and ptau levels.
Age did not enhance the cross-sectional predictive ability of any CSF biomarker model, despite its well-known status as an AD risk factor. However, brain volume decreases with normal aging,36 and the Pearson product-moment correlation between age and nWBV was −0.80 (p < 0.0001) in this sample. Since variation of the two factors is shared (i.e., they are highly correlated), once one factor is present in the model, the other adds little additional predictive value and does not meet the criteria for stepwise selection.
Interestingly, education did not independently predict DAT when considered together with CSF biomarkers in the cross-sectional sample, although it was previously found to interact with fibrillar brain Aβ to predict dementia symptoms.27 Thus, the ability of particular ancillary variables to improve prediction using biomarkers may differ depending on the type of biomarker used (e.g., CSF measures vs amyloid imaging).
Longitudinally, only education and age helped to predict incident DAT in the primary analyses. However, our follow-up period and number of incident DAT cases were modest. Studies examining a longer follow-up period may reveal additional important ancillary variables. In the exploratory analyses conducted on the smaller subsample with nWBV available, education continued to contribute to predictive accuracy but nWBV replaced age as an important longitudinal predictor. Again, this is probably due to the close correlation between age and nWBV.
In our previous work, Aβ42 values below 500 pg/mL were generally considered to be abnormal.37,38 Among participants in the main longitudinal analyses, and over a modest follow-up period, 64.3% (9/14) of those who developed DAT had Aβ42 values below 500 pg/mL at baseline, whereas only 31.7% (63/199) of those who did not develop DAT had Aβ42 values below this level. Similarly, tau/Aβ42 ratios of greater than 1.0 (roughly indicating both abnormal tau and abnormal Aβ42 levels) were found for 50% (7/14) of those who developed DAT vs 12.6% (25/199) of those who did not. Stated differently, our results suggest that cognitively normal individuals who later develop DAT are likely to have “abnormal” biomarker levels at baseline.
Considered alone, tau-based CSF assays were superior to Aβ42 in identifying prevalent DAT, whereas Aβ42 better predicted incident DAT, consistent with a model of AD development whereby the major pathologic effects of Aβ are exerted in preclinical AD, and of tau in symptomatic AD.3
Limitations include the use of a convenience sample, the few participants with nWBV available in the longitudinal sample, as well as the relatively short follow-up period (a mean of 3.2 years). Given these limitations, our results suggest that factors shown in previous research to mediate, or confound, associations between AD pathology and dementia can be used to improve the predictive accuracy of CSF biomarkers. These results provide a starting point toward the development of AD risk models which incorporate CSF biomarker values together with individual patient attributes. These risk models can be used in the clinical setting to accurately predict time to development of AD symptoms, helping physicians to make informed treatment decisions and patients to plan for the future.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants, investigators, and staff of the AD Research Center Clinical (participant assessments) and Genetics Cores (genotyping) and the investigators and staff of the Adult Children Study's Biomarker Core for CSF analytes. They also thank Dr. Mithat Gönen of Memorial Sloan-Kettering Cancer Center for providing statistical software and Halley Hindman for help in data collection.
Editorial, page 496
Supplemental data at www.neurology.org
- AD
- Alzheimer disease
- AUC
- area under the receiver operating characteristic curve
- CDR
- Clinical Dementia Rating
- CPE
- concordance probability estimate
- CS
- collateral source
- DAT
- dementia of the Alzheimer type
- GDS
- Geriatric Depression Scale
- LP
- lumbar puncture
- MMSE
- Mini Mental-State Examination
- nWBV
- normalized whole brain volume
- ROC
- receiver operating characteristic
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Catherine M. Roe.
DISCLOSURE
Dr. Roe receives research and salary support from the NIH/NIA and from the Charles and Joanne Knight Alzheimer Research Initiative of the Knight Alzheimer's Disease Research Center. Dr. Fagan serves on the speakers' bureau for the Alzheimer's Association. Dr. Williams serves on a scientific advisory board for Centene; serves on a speakers' bureau for the Alzheimer's Association; and receives research support from Eli Lilly and Company, Bristol-Myers Squibb, and the NIH. Dr. Ghoshal receives research support from Elan Corporation/Janssen, Eli Lilly and Company, Wyeth/Pfizer Inc, Novartis, Bristol-Myers Squibb, and the NIH (NIA/NINDS). Ms. Aeschleman reports no disclosures. Dr. Grant receives research and salary support from the NIH/NIA. Dr. Marcus has a patent pending re: a software system to select and perform automated medical imaging analysis; serves as a consultant for Avid Radiopharmaceuticals, Inc.; and receives research support from the US Department of Defense and the NIH. Dr. Mintun is currently employed as Chief Medical Officer for Avid Radiopharmaceuticals, Inc. (all work on this project was done while faculty at Washington University); has served as a consultant for Avid Radiopharmaceuticals, Inc.; and receives research support from the NIH. Dr. Holtzman serves on scientific advisory boards for Satori Pharmaceuticals and EnVivo Pharmaceuticals; serves as an Associate Editor of Annals of Neurology, the Journal of Neuroscience, Neurobiology of Disease, and Experimental Neurology; may accrue revenue on pending patents re: Methods for Measuring the Metabolism of Neurally Derived Biomolecules in Vivo; Use of Anti-AB Antibody to Treat Traumatic Brain Injury; Methods to Treat Alzheimer's Disease or Other Amyloid Beta Accumulation Associated Disorders; Humanized Antibodies That Sequester abeta Peptide; Diagnostic for Early Stage Alzheimer's Disease; and Predictive Diagnostic for Alzheimer's Disease; serves as a consultant to Merck Serono, Eli Lilly and Company, Takeda Pharmaceutical Company Limited, Abbott, Comentis, Inc., Eisai Inc., and AstraZeneca; is cofounder of and receives board of directors compensation from C2N Diagnostics LLC; receives research support from AstraZeneca, Pfizer Inc., Eli Lilly and Company, Elan Corporation, Forest Laboratories, Inc., the NIH, Cure Alzheimer's Fund, and Fidelity Foundation; has received compensation from Washington University from license revenue received for licensing of patent applications to C2N Diagnostics LLC; and may receive future royalty payments for Washington University licensing patents to C2N Diagnostics, LLC and Eli Lilly and Company. Dr. Morris serves on scientific advisory boards for AstraZeneca, Bristol-Myers Squibb, Genentech, Inc., Merck Serono, Novartis, Pfizer Inc, Schering-Plough Corp., Eli Lilly and Company, Wyeth, and Elan Corporation; serves on the editorial advisory board of Alzheimer's Disease and Associated Disorders; receives royalties from publishing Mild Cognitive Impairment and Early Alzheimer's Disease (John Wiley and Sons, 2008), Dementia (Clinical Publishing, 2007), Handbook of Dementing Illnesses, 2nd edition (Taylor & Francis, 2006) and for an editorial in Lancet Neurology (Elsevier, 2008); and receives research support from Elan Corporation, Wyeth, Eli Lilly and Company, Novartis, Pfizer Inc, Avid Radiopharmaceuticals, the NIH, and from the Dana Foundation.
REFERENCES
- 1. Carrillo MC, Blackwell A, Hampel H, et al. Early risk assessment for Alzheimer's disease. Alzheimers Dement 2009;5:182–196 [DOI] [PubMed] [Google Scholar]
- 2. Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006;5:228–234 [DOI] [PubMed] [Google Scholar]
- 3. Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid AB42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol 2009;65:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roe CM, Mintun MA, Ghoshal N, et al. Alzheimer's disease identification using amyloid imaging and reserve variables: proof of concept. Neurology 2010;75:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 1988;23:138–144 [DOI] [PubMed] [Google Scholar]
- 6. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460 [PubMed] [Google Scholar]
- 7. Stern Y. Cognitive reserve. Neuropsychologia 2009;47:2015–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mortimer JA. Brain reserve and the clinical expression of Alzheimer's disease. Geriatrics 1997;52:S50–S53 [PubMed] [Google Scholar]
- 9. Valenzuela MJ. Brain reserve and the prevention of dementia. Curr Opin Psychiatry 2008;21:296–302 [DOI] [PubMed] [Google Scholar]
- 10. Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 1998;55:326–335 [DOI] [PubMed] [Google Scholar]
- 11. Folstein MF, Folstein SE, McHugh PR. Mini-mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 12. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986:165–173 [Google Scholar]
- 13. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–572 [DOI] [PubMed] [Google Scholar]
- 14. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 15. Williams MM, Roe CM, Morris JC. Stability of the Clinical Dementia Rating: 1979–2007. Arch Neurol 2009;66:773–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burke WJ, Miller JP, Rubin EH, et al. Reliability of the Washington University Clinical Dementia Rating. Arch Neurol 1988;45:31–32 [DOI] [PubMed] [Google Scholar]
- 17. Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer's Disease Cooperative study experience. Neurology 1997;48:1508–1510 [DOI] [PubMed] [Google Scholar]
- 18. Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer's disease. Arch Neurol 2001;58:397–405 [DOI] [PubMed] [Google Scholar]
- 19. Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathological outcomes in original versus revised MCI and in PreMCI. Neurology 2006;67:467–473 [DOI] [PubMed] [Google Scholar]
- 20. Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol 2006;59:512–519 [DOI] [PubMed] [Google Scholar]
- 21. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845 [PubMed] [Google Scholar]
- 22. Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika 2005;92:965–970 [Google Scholar]
- 23. Stern Y, Gurland B, Tatemichi TK, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–1010 [PubMed] [Google Scholar]
- 24. Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann Neurol 1992;32:371–375 [DOI] [PubMed] [Google Scholar]
- 25. Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education, and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol 2003;25:671–679 [DOI] [PubMed] [Google Scholar]
- 26. Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia; Support for the cognitive reserve hypothesis. Neurology 2007;68:223–228 [DOI] [PubMed] [Google Scholar]
- 27. Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: education effect varies with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol 2008;65:1467–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Ann Neurol 2008;63:112–118 [DOI] [PubMed] [Google Scholar]
- 29. Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 2003;60:1909–1915 [DOI] [PubMed] [Google Scholar]
- 30. Stern Y, Alexander GE, Prohovnik I, et al. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer's disease pathology. Neurology 1995;45:55–60 [DOI] [PubMed] [Google Scholar]
- 31. Hollingshead AB. Hollingshead two factor index of social position (1957). In: Miller DC, ed. Handbook of Research Design and Social Measurement, 5th ed Newbury Park, CA: Sage Publications; 1991:351–359 [Google Scholar]
- 32. Mori E, Hirono N, Yamashita H, et al. Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer's disease. Am J Psychiatry 1997;154:18–24 [DOI] [PubMed] [Google Scholar]
- 33. Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage 2004;23:724–738 [DOI] [PubMed] [Google Scholar]
- 34. Vicioso BA. Dementia: when is it not Alzheimer disease? Am J Med Sci 2002;324:84–95 [DOI] [PubMed] [Google Scholar]
- 35. Morris JC, Roe CM, Xiong C, et al. APOE predicts AB but not tau Alzheimer's pathology in cognitively normal aging. Ann Neurol 2010;67:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 2005;64:1032–1039 [DOI] [PubMed] [Google Scholar]
- 37. Fagan AM, Roe CM, Xiong C, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 2007;64:343–349 [DOI] [PubMed] [Google Scholar]
- 38. Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and ptau181 increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med 2009;1:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.