Abstract
We evaluated whether insulin resistance in obese people is associated with decreased plasma palmitoleate availability. Palmitoleate content (percentage and absolute concentrations) in FFA and VLDL was measured in obese subjects who were either insulin resistant (IR) or insulin sensitive (IS), based on assessment of multiorgan (skeletal muscle, liver, and adipose tissue) insulin sensitivity by using the hyperinsulinemic-euglycemic clamp procedure in conjunction with infusion of stable isotopically labeled tracers. Plasma palmitoleate concentration and the relative contribution of palmitoleate to total plasma FFA concentration in the IS group (0.018 ± 0.002 mmol/l and 4.4% ± 0.2%, respectively) were not significantly different than values in the IR group (0.023 ± 0.003 mmol/l and 4.4% ± 0.4%, respectively). Plasma VLDL-triglyceride palmitoleate concentration and the proportion of VLDL fatty acids as palmitoleate in the IS group (0.09 ± 0.02 mmol/l and 5.7 ± 0.3%, respectively) were also not significantly different than those in the IR group (0.16 ± 0.04 mmol/l and 5.0% ± 0.4%, respectively). These data demonstrate that decreased palmitoleate in plasma and in VLDL is not associated with insulin resistance in skeletal muscle, liver, or adipose tissue in obese people.
Keywords: free fatty acid, gas chromatography, hyperinsulinemic-euglycemic clamp, stable isotope tracers
An increase in circulating free fatty acids (FFA) impairs insulin action in skeletal muscle and liver and likely contributes to the insulin resistance associated with obesity (1). However, data from studies conducted in rodents have shown that infusing palmitoleic acid (16:1n–7), which is normally produced in adipose tissue and the liver through lipogenesis, followed by desaturation of palmitic acid (16:0) by the stearoyl-CoA desaturase (SCD1) gene, augments skeletal muscle insulin signaling and increases insulin-mediated muscle glucose uptake (2). The importance of circulating palmitoleic acid in regulating insulin sensitivity in humans is unclear because of conflicting data from different studies, which have found that the percentage of palmitoleate in plasma FFA or lipids correlated directly with insulin sensitivity (3) or was greater in insulin-resistant (IR) than insulin-sensitive (IS) subjects (4–8). Moreover, these studies did not measure absolute palmitoleate concentration, which provides a better assessment of palmitoleate availability to peripheral tissues, or palmitoleate within very low-density lipoprotein (VLDL), which may be an important contributor to tissue fatty acid delivery.
The purpose of the present study was to determine whether impaired insulin-mediated skeletal muscle glucose uptake was associated with a decrease in plasma palmitoleic acid availability in obese people. Accordingly, we determined plasma free palmitoleic acid concentration, which is derived primarily from palmitoleate synthesized in adipose tissue, and palmitoleic acid content in VLDL, which is derived primarily from palmitoleate synthesized in the liver. Study subjects were specifically selected to be either IS or IR based on the increase in glucose uptake during a hyperinsulinemic-euglycemic clamp procedure.
MATERIALS AND METHODS
Study participants
A total of 39 overweight and obese (body mass index [BMI], 36.1 ± 0.7 kg/m2; age 42 ± 2 years) men and women who previously underwent a hyperinsulinemic-euglycemic clamp procedure and an evaluation of lipoprotein kinetics (9) participated in this study. Among these 39 subjects, 20 subjects, who comprised the lowest and highest quartiles in skeletal muscle insulin sensitivity, assessed as the increase in glucose disposal during insulin infusion, were used to establish an IR group (n = 10; age, 41 ± 4 years old; 3 men and 7 women; ≤135% increase in glucose disposal) and an IS group (n = 10; age, 44 ± 3 years old; 10 women; ≥261% increase in glucose disposal). All subjects completed a comprehensive medical evaluation, including an oral glucose tolerance test at screening. No subject had any history or evidence of diabetes or other serious chronic diseases or consumed >10 g/day of alcohol. Subjects gave their written informed consent before participating in this study, which was approved by the Human Research Protection Office of Washington University School of Medicine.
Experimental protocol
Body composition.
Percent body fat and intra-abdominal adipose tissue volume were determined by using dual-energy X-ray absorptiometry and magnetic resonance imaging (10).
Insulin sensitivity measurement.
Hepatic tissue, skeletal muscle, and adipose tissue insulin sensitivity levels were measured by using a two-stage hyperinsulinemic-euglycemic clamp procedure in conjunction with stable isotopically labeled glucose and palmitate tracer infusions, as described previously (9). Subjects were admitted to the Clinical Research Unit on the evening before the clamp procedure, consumed a standard meal at 1900 h, and then fasted until completion of the clamp procedure the next day. At 0600 h, the following morning, a primed, continuous infusion of [6,6-2H2]glucose and a continuous infusion of [2,2-2H2]palmitate were started and continued for 9.5 h. After 3.5 h of tracer infusion (basal period), a two-stage euglycemic-hyperinsulinemic clamp procedure was started and continued for 6 h. Insulin was infused at a rate of 20 mU ∙ m−2 body surface area (BSA) ∙ min−1 during stage 1 (3.5–5.5 h) and at a rate of 50 mU ∙ m−2 BSA ∙ min−1 during stage 2 (5.5–9.5 h) of the clamp procedure. Euglycemia (100 mg/dl) was maintained by infusing 20% dextrose enriched to 2.5% with [6,6-2H2]glucose at various rates. Glucose and palmitate tracer-to-tracee ratios were determined by using gas chromatography-mass spectrometry (10, 11). Adipose tissue and skeletal muscle insulin sensitivity levels were assessed as the relative decrease in palmitate rate of appearance (Ra) in plasma during stage 1 (low-dose insulin) and the relative increase in glucose rate of disappearance (Rd) from plasma during stage 2 (high-dose insulin) of the clamp procedure (9). Hepatic insulin sensitivity was calculated as the hepatic insulin sensitivity index (HISI) (9).
Adipose tissue, plasma, and VLDL palmitoleate measurement.
To measure palmitoleate content in adipose tissue, subcutaneous abdominal adipose tissue biopsies were performed and tissue was obtained by aspiration through a 4-mm liposuction cannula at 1 h after the basal stage of the hyperinsulinemic-euglycemic clamp procedure was started. Immediately after being collected, adipose tissue samples were rinsed in ice-cold saline, frozen in liquid nitrogen, and stored at −80°C until final analyses were performed.
Blood samples obtained during a study conducted to measure hepatic lipoprotein kinetics, performed ∼1 week apart from the clamp procedure, were used to assess circulating palmitoleate content in plasma FFA and VLDL. Subjects were admitted to the Clinical Research Unit in the evening before the study and consumed a standard meal. The following morning, after subjects fasted overnight, 17 blood samples were obtained over 12 h (0600–1800 h) of continued fasting to determine free palmitoleate concentrations, plasma FFA composition, and palmitoleate in VLDL lipid by using gas chromatography (11). To isolate VLDL particles, approximately 1.5 ml of plasma was transferred into OptiSeal polyallomer tubes (Beckman Instruments, Palo Alto, CA) overlaid with an NaCl-EDTA solution (1.006 g/ml) and centrifuged at 100,000 g for 16 h at 10°C in an Optima LE-80K preparative ultracentrifuge equipped with a type 50.4 Ti rotor (Beckman Instruments, Palo Alto, CA). The top layer containing VLDL was removed by tube slicing (CentriTube slicer; Beckman Instruments, Palo Alto, CA). Values from all samples for each subject were averaged to provide a robust measure of fatty acid composition in plasma and VLDL. Plasma-free palmitoleate (in mmol/l) was calculated as percent palmitoleate in plasma × FFA concentration. Palmitoleate in VLDL (in mmol/l) was calculated as the percentage of VLDL fatty acids as palmitoleate × VLDL-triglyceride (TG) concentration (in mmol/l) × 3 (because each TG contains 3 fatty acids). An estimate of adipose tissue palmitoleate content (in moles) was calculated as the percentage of palmitoleate × moles of fatty acids in adipose tissue TG, the latter calculated on the basis of total body fat mass and the average molecular mass of TG (861 g/mol).
SCD1 gene expression and activity.
The product-to-precursor ratio of palmitoleate to palmitate (C16:1:C16:0) in VLDL-TG was used as an index of hepatic SCD1 activity, because SCD1 is the enzyme that mediates the desaturation of palmitate (C16:0) into palmitoleate (C16:1) (12). The adipose tissue SCD1 gene expression was determined by using quantitative real-time PCR. Total RNA was isolated from adipose tissues by using TRIzol (Invitrogen). RNA was quantified by using spectrophotometry (NanoDrop 1000, Thermo Scientific, Wilmington, DE), and cDNA was synthesized by using a TaqMan reverse transcription kit (Applied BioSystems). cDNA samples were then amplified by using SYBR Green PCR Master Mix (Applied BioSystems) on an ABI 7500 real-time PCR system (Applied BioSystems). Results were analyzed by comparing the cycle threshold (Ct) value of each sample after normalization to the housekeeping 36B4 gene. The changes, Δ, in Ct (ΔCt) were used to calculate the relative levels of each mRNA compared with that of the control gene from the various samples, using the formula.
The forward and reverse primers used for SCD1 transcription were 5′-ACACTTGGGAGCCCTGTATG-3′ and 3′-GCAGCCGAGCTTTGTAAGA-5′, respectively; and the forward and reverse primers used for 36B4 transcription were 5′-GTGATGTGCAGCTGATCAAGACT-3′ and 3′-GATGACCAGCCCAAAGGAGA-5′, respectively.
Statistical analyses
All data sets were tested for normality according to the Shapiro-Wilk test. For normally distributed variables, comparisons between groups were performed by using parametric procedures. Levene's test was used to assess the equality of group variances on each dependent variable, and Student's t-test for unpaired samples was used to determine the significance of differences between groups. When variables were not normally distributed and could not be normalized by using standard mathematical transformations, datasets were ranked for all statistical analyses. Pearson correlation analysis was used to assess the relationships between variables for all available subjects (n = 39). Results are presented as means ± standard errors of the mean (SEM). A P value of ≤0.05 was considered statistically significant. Analyses were performed by using SPSS version 17.0 software (SPSS Inc., Chicago, IL).
RESULTS
The metabolic characteristics of the study subjects are shown in Table 1. There was a wide range in insulin sensitivity, measured as the percent increase in muscle glucose uptake during the hyperinsulinemic-euglycemic clamp procedure, from 46% to 475%. Plasma palmitoleate and palmitoleate in VLDL ranged from 2.3% to 7% and from 2.9% to 7.5%, respectively. By study design, skeletal muscle insulin-mediated glucose uptake was 3-fold greater in the IS than in the IR group. Mean values for the HISI and insulin-mediated suppression of palmitate Ra (a measure of adipose tissue insulin sensitivity) were also significantly greater in the IS that in the IR group. However, both free palmitoleate and palmitoleate in VLDL, measured as either a proportion of total fatty acids or as absolute concentrations, were not significantly different between the two groups. In fact, there was a trend toward lower plasma palmitoleate availability as either free palmitoleate or within VLDL in the IS group. We detected a weak negative correlation between palmitoleate availability as either free palmitoleate or palmitoleate within VLDL and skeletal muscle insulin sensitivity. In addition, BMI was positively correlated with both plasma (r = 0.418, P = 0.008) and adipose tissue (r = 0.470, P = 0.003) palmitoleate content.
TABLE 1.
Characteristics of the study subjects
| All subjects | Insulin resistant group | Insulin sensitive group | |
|---|---|---|---|
| N of subjects (female:male) | 39 (27:12) | 10 (7:3) | 10 (10:0) |
| Age (years) | 42 ± 2 (20-63) | 41 ± 4 | 44 ± 3 |
| Body mass index (kg/m ) | 36.1 ± 0.7 (29.0-45.0) | 36.7 ± 1.6 | 32.9 ± 0.9 |
| Fat mass (%) | 40 ± 1 (28-52) | 39 ± 2 | 43 ± 1 |
| Intra-abdominal fat (cm ) | 1,604 ± 154 (413-4321) | 1,910 ± 353* | 883 ± 121* |
| Insulin-mediated increase in glucose Rd (%) | 210 ± 15 (46-475) | 106 ± 9† | 327 ± 20† |
| Insulin-mediated suppression of FFA Ra (%) | 71 ± 1 (50-87) | 63 ± 3† | 78 ± 2† |
| Hepatic Insulin Sensitivity Index (100/[µmol · min] × mU/l) | 0.11 ± 0.02 (0.03-0.53) | 0.05 ± 0.01† | 0.13 ± 0.02† |
| Plasma FFA (mmol/l) | 0.51 ± 0.04 (0.51-0.02) | 0.51 ± 0.04 | 0.41 ± 0.04 |
| Proportion of plasma FFA as palmitoleate (%) | 4.2 ± 0.2 (2.3-7.0) | 4.4 ± 0.4 | 4.4 ± 0.2 |
| Plasma palmitoleate (mmol/l) | 0.020 ± 0.001 (0.006-0.043) | 0.023 ± 0.003 | 0.018 ± 0.002 |
| Plasma VLDL-TG (mmol/l) | 0.8 ± 0.1 (0.2-2.6) | 1.2 ± 0.3* | 0.5 ± 0.1* |
| Proportion of VLDL-lipid as palmitoleate (%) | 5.1 ± 0.2 (2.9-7.5) | 5.0 ± 0.4 | 5.7 ± 0.3 |
| Plasma VLDL-TG palmitoleate (mmol/l) | 0.12 ± 0.01 (0.02-0.35) | 0.16 ± 0.04 | 0.09 ± 0.02 |
Adipose tissue and skeletal muscle insulin sensitivity levels were calculated as the relative decrease in FFA rate of appearance during stage 1 and the relative increase in glucose rate of disappearance during stage 2 of the clamp procedure, respectively. The hepatic insulin sensitivity index (HISI) was calculated as the reciprocal of the hepatic insulin resistance index (glucose Ra [µmol/min] × insulin [mU/l]). Data are presented as mean ± SEM (range).
*, P < 0.05 and †, P < 0.01 indicate values significantly different than the corresponding insulin resistant group value.
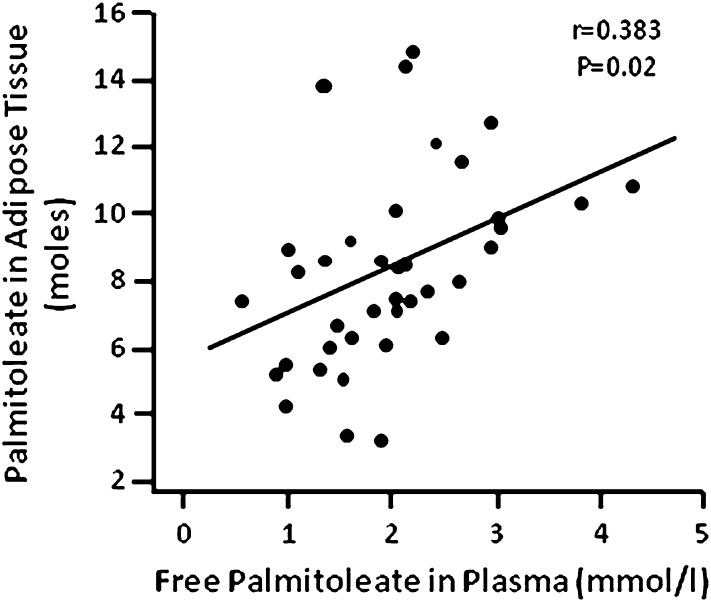
Palmitoleate in adipose tissue TG ranged from 3.3% to 11.4% of total fatty acids in TG. Plasma-free palmitoleate correlated directly with adipose tissue palmitoleate content (r = 0.383, P = 0.016) (Fig. 1) and with adipose tissue SCD1 gene expression (r = 0.437, P = 0.008). Adipose tissue palmitoleate content correlated directly with SCD1 gene expression (r = 0.397, P = 0.017), and VLDL palmitoleate concentration correlated directly with hepatic SCD1 activity, estimated by using the C16:1:C16:0 ratio in VLDL (r = 0.999, P = 0.001). In addition, plasma-free palmitoleate correlated directly with total plasma FFA concentration (r = 0.813, P = 0.0001).
Fig. 1.
Relationship between plasma-free palmitoleate and adipose tissue palmitoleate content.
DISCUSSION
The findings from the present study demonstrate it is unlikely that decreased palmitoleate availability is an important factor in the pathogenesis of liver, skeletal muscle, and adipose tissue insulin resistance in obese human subjects. We found that free palmitoleate and palmitoleate in VLDL, which are the two major sources of circulating palmitoleate available for insulin-responsive organs, are not different in obese IS and obese IR subjects, matched by age, BMI, and percent body fat. In fact, there was a weak inverse relationship between circulating palmitoleate and skeletal muscle insulin sensitivity, presumably because plasma palmitoleate tracks closely with total plasma FFA concentration, which is often associated with insulin resistance.
Data from studies conducted in isolated myocytes and in a rodent model have found palmitoleate improves insulin signaling and increases insulin-mediated skeletal muscle glucose uptake (2, 13). These data led to the hypothesis that palmitoleate provides a link between adipose tissue and distant organs that is involved in regulating systemic metabolic function. We found that adipose tissue palmitoleate content correlated directly with SCD1 gene expression and that plasma free palmitoleate was directly correlated with adipose tissue palmitoleate content. These findings are consistent with the idea that plasma free palmitoleate is derived predominantly from adipose tissue fatty acid metabolism. Palmitoleate can also be delivered to peripheral tissues by lipoprotein lipase-mediated hydrolysis of circulating VLDL-TG, which is produced by the liver. This potential source of palmitoleate is 5-fold greater than free palmitoleate in plasma. Even though the relative amount of palmitoleate in VLDL tended to be higher in the IS subjects, the absolute concentration of VLDL palmitoleate tended to be higher in the IR subjects because total VLDL-TG concentration was greater. Therefore, both adipose tissue and liver are important sources of systemic palmitoleate availability.
Although few studies have evaluated the relationship between plasma palmitoleate and insulin sensitivity in humans, the results from most of these studies indicate that palmitoleate is not associated with improved insulin sensitivity, as follows: (i) the percent plasma FFA as palmitoleate was greater in obese than in lean children and was positively correlated with measures of adiposity (8); (ii) serum palmitoleate composition in phospholipids was directly correlated with IR (assessed by the homeostasis model assessment of insulin resistance) in subjects with type 2 diabetes (5); and (iii) palmitoleate in plasma cholesterol esters was inversely associated with peripheral insulin sensitivity in elderly adults (6). In contrast, data from a study conducted in overweight subjects indicated that the percentage of plasma FFA as palmitoleate was directly associated with whole-body insulin sensitivity, measured as the glucose infusion rate required to maintain euglycemia during a hyperinsulinemic clamp procedure (3). However, all these studies measured the percent palmitoleate in FFA or other lipids but did not evaluate absolute plasma palmitoleate concentrations. If palmitoleate acts as a circulating hormone, the absolute plasma concentration is more important than relative composition in regulating metabolic function. In our study, we measured both the percent contribution and absolute concentration of palmitoleate within plasma FFA and VLDL, which provides a more robust assessment of total palmitoleate availability than previous studies.
We evaluated whether decreased availability of the two major circulating sources of palmitoleate, present in plasma FFA and in VLDL, was associated with insulin resistance. Accordingly, we selected two distinct groups of obese subjects, identified from among a larger cohort of subjects who were either IR or IS based on the increase in insulin-mediated muscle glucose uptake determined by using the hyperinsulinemic-euglycemic clamp procedure in conjunction with isotope tracer infusion. In addition, these subjects were also found to be either insulin resistant or sensitive in the liver (ability of insulin to suppress glucose production) and adipose tissue (ability of insulin to suppress lipolysis). Subjects with type 2 diabetes were purposely excluded to avoid the confounding effect of diabetes drug therapy on insulin action. An important strength of our study is that we measured average palmitoleate content obtained from multiple blood samples collected over a 12-h period, rather than a single basal sample as measured in previous studies. Although our study was designed to maximize the ability to detect an effect of palmitoleate by evaluating its availability from both free palmitoleate in plasma and palmitoleate in VLDL, we did not find a trend toward higher free palmitoleate in plasma or in VLDL in the IS group compared with the IR group. However, our study does not preclude the possibility that plasma palmitoleate availability may be related to insulin sensitivity in lean subjects or that palmitoleate availability within muscle and liver tissue might be different in IS and IR subjects. An additional limitation of our study is the small number of men within our study cohort, which precluded us from evaluating possible sex differences in the relationship between palmitoleate and glucose homeostasis.
In summary, our data do not support the notion that palmitoleate is an important physiological regulator of insulin sensitivity in humans and demonstrate that insulin resistance in obese people is not associated with a decrease in circulating palmitoleate. Nonetheless, these results do not eliminate the possibility that palmitoleate administration can improve insulin sensitivity in humans, as observed in rodent models (2).
Acknowledgments
The authors thank Freida Custodio, Jennifer Shew, and Terri Pietka for technical assistance, the staff of the Clinical Research Unit for help in performing the studies, and the study subjects for their participation.
Footnotes
Abbreviations:
- BMI
- body mass index
- BSA
- body surface area
- Ct
- threshold crossing
- HISI
- hepatic insulin sensitivity index
- IR
- insulin resistant
- IS
- insulin sensitive
- Ra
- rate of appearance
- Rd
- rate of disappearance
- TG
- triglyceride
This study was supported by National Institutes of Health Grants DK-37948, UL1 RR-024992 (Clinical and Translational Science Award), DK 56341 (Nutrition and Obesity Research Center), and RR-00954 (Biomedical Mass Spectrometry Resource). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1.Boden G. 2002. Interaction between free fatty acids and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 5: 545–549. [DOI] [PubMed] [Google Scholar]
- 2.Cao H., Gerhold K., Mayers J. R., Wiest M. M., Watkins S. M., Hotamisligil G. S. 2008. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 134: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefan N., Kantartzis K., Celebi N., Staiger H., Machann J., Schick F., Cegan A., Elcnerova M., Schleicher E., Fritsche A., et al. 2010. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care. 33: 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusunoki M., Tsutsumi K., Nakayama M., Kurokawa T., Nakamura T., Ogawa H., Fukuzawa Y., Morishita M., Koide T., Miyata T. 2007. Relationship between serum concentrations of saturated fatty acids and unsaturated fatty acids and the homeostasis model insulin resistance index in Japanese patients with type 2 diabetes mellitus. J. Med. Invest. 54: 243–247. [DOI] [PubMed] [Google Scholar]
- 5.Pelikanova T., Kazdova L., Chvojkova S., Base J. 2001. Serum phospholipid fatty acid composition and insulin action in type 2 diabetic patients. Metabolism. 50: 1472–1478. [DOI] [PubMed] [Google Scholar]
- 6.Vessby B., Tengblad S., Lithell H. 1994. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia. 37: 1044–1050. [DOI] [PubMed] [Google Scholar]
- 7.Ho R. C., Davy K. P., Hickey M. S., Summers S. A., Melby C. L. 2002. Behavioral, metabolic, and molecular correlates of lower insulin sensitivity in Mexican-Americans. Am. J. Physiol. Endocrinol. Metab. 283: E799–E808. [DOI] [PubMed] [Google Scholar]
- 8.Okada T., Furuhashi N., Kuromori Y., Miyashita M., Iwata F., Harada K. 2005. Plasma palmitoleic acid content and obesity in children. Am. J. Clin. Nutr. 82: 747–750. [DOI] [PubMed] [Google Scholar]
- 9.Fabbrini E., Magkos F., Mohammed B. S., Pietka T., Abumrad N. A., Patterson B. W., Okunade A., Klein S. 2009. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA. 106: 15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korenblat K. M., Fabbrini E., Mohammed B. S., Klein S. 2008. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 134: 1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabbrini E., Mohammed B. S., Magkos F., Korenblat K. M., Patterson B. W., Klein S. 2008. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 134: 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peter A., Cegan A., Wagner S., Lehmann R., Stefan N., Konigsrainer A., Konigsrainer I., Haring H. U., Schleicher E. 2009. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin. Chem. 55: 2113–2120. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos N., Watson M., Sakamoto K., Hundal H. S. 2006. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem. J. 399: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]