Abstract
Satellite cells are myogenic stem cells responsible for the post-natal growth, repair and maintenance of skeletal muscle. This review focuses on the basic biology of the satellite cell with emphasis on its role in muscle repair and parallels between embryonic myogenesis and muscle regeneration. Recent advances have altered the long-standing view of the satellite cell as a committed myogenic stem cell derived directly from the fetal myoblast. The experimental basis for this evolving perspective will be highlighted as will the relationship between the satellite cell and other newly discovered muscle stem cell populations. Finally, advances and prospects for cell-based therapies for muscular dystrophies will be addressed.
Introduction
Skeletal muscle is subject to constant injury resulting from weight bearing, exercise, and trauma, thereby requiring an ever-available, renewable source of cells for muscle repair and regeneration. Since its identification, the satellite cell has been a popular candidate for the adult skeletal muscle "stem cell" [1]. Residing dormant beneath the basal lamina of mature skeletal muscle fibers, this cell is ideally located for timely repair of degenerating muscle fibers. Additionally, these quiescent cells are activated to proliferate upon muscle injury, a necessary step towards generating sufficient numbers of myoblasts for muscle differentiation and myotube formation. However, the identification of multiple stem cell populations resident in skeletal muscle has added further complexity to understanding the process of muscle regeneration. In this mini-review, we will briefly examine the molecular and morphological characteristics of the satellite cell, its role in muscle regeneration, and discuss outstanding questions regarding its origin, developmental potential, and uses in myoblast therapy.
Muscle Regeneration Parallels Myogenesis in the Embryo
Although the developmental origin of satellite cells remains unknown, in vertebrates, the majority of skeletal muscle progenitors arise in the somites. Somites are transient epithelial spheres that pinch off of the paraxial mesoderm lining both sides of the neural tube. Myogenic precursors are first identified in the dermomyotome, an epithelial layer located in the dorsal compartment of the somite. These precursors are characterized by their expression of the paired box transcription factors Pax-3 and Pax-7; in response to signals such as Wnts and Sonic hedgehog from surrounding embryonic structures, the myogenic determination genes Myf-5 and MyoD are activated [3]. Coinciding with the down-regulation of Pax gene expression, muscle precursor cells committed to the skeletal muscle lineage (myoblasts) translocate to the subjacent myotome, where the muscle regulatory factors Myogenin and MRF4 direct differentiation and fusion into multi-nucleated myofibers.Satellite cells are first apparent towards the end of embryogenesis, and function as a primary source for the myogenic cells required for post-natal muscle growth [2].
In adult muscles, dormant, Pax-7-expressing satellite cells reside between the plasmalemma and basal lamina at frequencies that vary with age, muscle fiber type, and species [4]. The activation of satellite cells in vivo can be induced by muscle fiber injury brought on by acute injury [5-7], exercise [8-10], and denervation [11]. Upon injury, satellite cells are stimulated to re-enter the cell cycle to generate a pool of proliferating myogenic precursors analogous to the embryonic myoblasts, while the inflammatory response mounted by the immune system clears affected myofibers [2]. Recently, certain Wnt-family members were found to be up-regulated in muscle following injury, suggesting a parallel to myogenic signaling pathways in the embryo [12]. Additionally, up-regulation of Myf-5 and MyoD occurs at the injury site in proliferating satellite cells indicating cell commitment [13-17]. Pax-7 expression declines with the up-regulation of MRF-4 and Myogenin, and differentiated myocytes fuse to new and existing fibers as part of the repair process. One of the hallmarks of regenerating myofibers is the centrally located position of the myonuclei; upon maturing, muscle fiber nuclei are located along the cell periphery [4]. Notably, repeated cycles of injury and regeneration do not appear to deplete satellite cell numbers, suggesting that these cells have the ability to self-renew [2].
Fiber- and Age-related Variation in Satellite Cell Frequency
Satellite cells were initially identified in frog leg muscles by electron microscopy [1], and subsequently have been identified in all higher vertebrates. In humans and mice, these quiescent [18], non-fibrillar, mononuclear cells are most plentiful at birth (estimated at 32% of sublaminar nuclei) [19]. The frequency declines post-natally, stabilizing to between 1 to 5% of skeletal muscle nuclei in adult mice [2]. Satellite cell frequency varies in different muscles, likely as a function of variation in fiber type composition (i.e. slow oxidative, fast oxidative, or fast glycolytic fibers). For example, the mouse soleus muscle, which is predominantly made up of slow oxidative fibers, has a higher number of satellite cells than the extensor digitorum longus (EDL) muscle, which primarily contains fast glycolytic fibers. Additionally, the absolute numbers of satellite cells increases in the soleus but not the EDL between 1 and 12 months of age, although the proportion of satellite cells decreases in both muscle types with increasing age [20]. In humans, the proportion of satellite cells in skeletal muscles also decreases with age, which may explain the decreased efficiency of muscle regeneration in older subjects [21]. Satellite cells from aged muscle also display reduced proliferative and fusion capacity, as well as a tendency to accumulate fat, all of which likely contribute to deteriorating regeneration capability [22,23]. That endurance training can offset the decline in satellite cell number with age suggests that poorer regeneration is not simply a result of limited replicative potential of older satellite cells [24].
Molecular Basis of Satellite Cell Activation and Differentiation
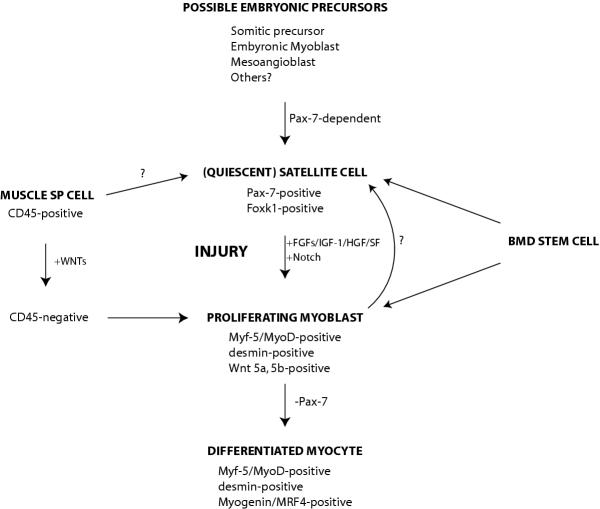
Several signals and growth factors have been implicated in promotion of satellite cell activation and proliferation (Figure 1). For example, the Notch signaling pathway, which is activated upon muscle injury, regulates satellite cell transition from quiescence to proliferation in single fiber cultures, thereby expanding the myoblast population in injured muscle [25]. Basic fibroblast growth factor (bFGF) stimulates satellite cell proliferation while inhibiting differentiation [2]. bFGF also promotes muscle regeneration in mdx mice [26], which undergo repeated cycles of degeneration and regeneration resulting from a mutation in the dystrophin gene; in humans, deficiency of dystrophin causes Duchenne muscular dystrophy [27,28]. In addition to expressing all known FGF receptors [29,30], satellite cells also express the tyrosine kinase receptor c-met [16,31]. The c-met ligand, hepatocyte growth factor/scatter factor (HGF/SF), is also a known activator of satellite cells [29,32].
Figure 1.
Model for the development, activation, and maintenance of the satellite cell. Upon skeletal muscle injury, quiescent satellite cells expressing Pax-7 and Foxk1 are activated to proliferate, up-regulating the myogenic determination factors, MyoD and Myf-5 [13-17], the myoblast marker desmin [16,79], and Wnts 5a and 5b [12]. Satellite cell activation is regulated by the Notch signaling pathway [25], and proliferation is stimulated by a number of growth factors, including basic FGF, insulin-like growth factor-1, and HGF/SF [26,32,80]. Transition from proliferation to differentiation, which is accompanied by the down-regulation of Pax-7 [57] and up-regulation of Myogenin and MRF-4 [13,14,16], is dependent on both MyoD [36] and the Foxk1 pathway [33,34]. Candidate satellite cell progenitors, which must activate Pax-7 for satellite cell development [57], include embryonic myoblast precursors, fetal myoblasts, and vessel-associated mesoangioblasts, the latter of which exhibits strong myogenic potential [60-62]. Additionally, bone marrow-derived (BMD) stem cells can contribute directly to quiescent satellite cells and regenerating muscle fibers following injury [54,65,68], and muscle SP cells have been used with some success in myoblast transplantation experiments into dystrophic muscle [53,54]. Importantly, members of the Wnt family of secreted glycoproteins can convert SP cells favoring the hematopoietic fate into highly myogenic cells [12].
Targeted deletion of the gene encoding the Forkhead/winged helix transcription factor Foxk1 [previously known as myocyte nuclear factor (MNF)], which is expressed in quiescent satellite cells, causes a severely runted phenotype, and cardiotoxin-induced muscle regeneration is delayed and accompanied by prominent accumulation of adipose cells, suggesting a defect in skeletal muscle commitment [33]. Interestingly, the myopathy associated with the Foxk1 mutant is rescued when bred into a p21-null background. p21 is up-regulated in Foxk1-null muscles, and while mice lacking this cyclin-dependent kinase inhibitor show a defect in satellite cell differentiation, double mutants exhibit normal muscle growth and regeneration, suggesting that p21 is a downstream target of Foxk1 [34,35].
The muscle determination gene MyoD is also required for normal muscle regeneration [36]. Regenerating muscles in MyoD-null animals accumulate high numbers of mononuclear cells and have few differentiated myotubes; this phenotype is exacerbated in an mdx background, with MyoD-/-; mdx muscles exhibiting severely reduced cross-sectional area and mass. MyoD-null animals exhibit increased numbers of satellite cells, suggesting that the cells fail to progress through the differentiation program and instead participate in self-renewal [36]. The abnormal proliferation observed with MyoD-null adult myoblasts and failure to up-regulate the muscle differentiation factors MRF-4 or Myogenin under differentiation conditions support this hypothesis [37,38]. In addition, MyoD-null satellite cells express increased levels of Myf-5 [37,38]. In embryos lacking MyoD, myogenesis is dependent on Myf-5 and vice versa: while single mutant embryos have normal muscles at birth, MyoD-/-; Myf-5-/- double mutant embryos fail to develop myoblasts or myotubes [39-41]. Given the defects in muscle regeneration observed in adult MyoD mutants, it is evident that the functional redundancy between MyoD and Myf-5 that ultimately rescues embryonic muscle development is not sufficient to rescue myogenesis in injured muscle.
Muscle Stem Cell Plasticity
Interestingly, while traditionally thought to be committed to the skeletal muscle fate, it is now evident that muscle stem cells, including satellite cells, are multipotent. For example, bone morphogenetic protein (BMP) treatment activates osteogenic markers while down-regulating MyoD in C2C12 myoblasts, an immortalized cell line derived from mouse limb muscle [42,43]. Additionally, treatment with thiazolidinediones and fatty acids converts C2C12 cells to the adipogenic cell fate [44]. Primary myoblast cultures from adult muscles respond similarly to C2C12 cells in the presence of strong osteogenic and adipogenic inducers; interestingly, satellite cells derived from intact single fiber cultures (and thought to be more representative of true myogenic stem cells) spontaneously form adipocytes and osteocytes when cultured on Matrigel, a soluble basement membrane matrix lacking strong osteogenic or adipogenic signals [45]. The finding that undifferentiated cells in adult myoblast cultures co-express MyoD, Runx2, and PPARγ, key regulators for myogenesis, osteogenesis, and adipogenesis, respectively, supports the hypothesis that satellite cells have a multipotential predisposition [46].
The plasticity of muscle stem cells has also been demonstrated using ex vivo approaches. Muscle stem cells isolated via serial preplating enrich for a population of cells which, in addition to contributing to regenerating myofibers when injected directly into dystrophic muscle, are detected in differentiated vascular and nerve cells [47,48]. Furthermore, these cells, which express the myoblast markers desmin and MyoD, are sufficient to completely heal skull defects in vivo when engineered to express BMP [49]. These muscle-derived stem cells are also capable of reconstituting bone marrow in lethally irradiated mice [50].
Another muscle-based stem cell with hematopoietic potential is the muscle side population (SP) cell, which can be isolated based on its specific exclusion of the vital dye Hoechst 33342 [51]. Initially sorted from bone marrow derived (BMD) stem cells by FACS analysis and observed to possess the majority of hematopoietic stem cell activity in bone marrow [52], SP cells have since been identified in a variety of tissues, including skeletal muscle, brain, heart, spleen, kidney and lung, although they are notably absent in peripheral blood [53]. It is important to note that the relationship between these different SP populations, and whether or not they derive from a common precursor, remains to be determined. Muscle SP cells reconstitute bone marrow in lethally irradiated mdx mice, although less efficiently than BMD SP cells. Interestingly, donor-derived nuclei also appear in regenerating muscle fibers after bone marrow reconstitution, indicating a contribution by the hematopoietic system in muscle repair [51,54]. The heterogeneity of muscle stem cells is underscored by the observation that SP cells within normal, uninjured skeletal muscle can be distinguished as positive for the hematopoietic marker CD45 (and poorly myogenic) or CD45-negative (a population that readily differentiates along the myogenic pathway) [55]. The CD45-positive subpopulation of cells has also been shown to contribute to neo-vascularization in regenerating muscle, whereas the CD45-negative population does not [56]. Interestingly, Wnts 5a, 5b, 7a and 7b, which are up-regulated in myoblasts and myofibers of regenerating muscle, convert the normally resistant CD45-positive muscle SP fraction to the myogenic program; this property to induce a switch in fate could contribute to the recruitment of much-needed progenitors upon injury [12].
The Developmental Origin of Satellite Cells
A recent study of the Pax-7-null mouse revealed that this paired box transcription factor is essential for satellite cell formation. In addition to exhibiting severe muscle deficiency at birth and premature lethality, Pax-7 mutants are completely devoid of satellite cells [57]. However, while this observation demonstrates the requirement for Pax-7 in satellite cell formation, it remains to be seen whether the satellite cell arises from a pre-determined myoblast in the dermomyotome, a fetal myoblast, or from a non-somitic progenitor. Satellite cells may originate from specified Pax-7-positive cells prior to the activation of Myf-5 and MyoD, and thus represent a true precursor to the myogenic lineage. Alternatively, satellite cells may arise from determined myoblasts which, instead of differentiating, continue to proliferate until withdrawing from the cell cycle and taking up residence beneath the basal lamina of myofibers. While relatively little is known about the cis regulation of the Pax-7 gene, the extensive characterization of Myf-5 and MyoD regulatory elements [3,58] can be used to determine if satellite cells originate from a Myf-5 or MyoD-positive population by in vivo cell tracing. Interestingly, while Pax-7-null animals lack satellite cells, the muscle SP population remains intact, although exhibiting increased hematopoietic potential; Pax-7 may direct specification of pluripotent SP cells to satellite cells [57,59].
The observation that various non-muscle stem cells can participate in skeletal muscle regeneration has expanded the candidate pool for the satellite cell precursor. For example, myogenic potential has been demonstrated in vivo by mesoangioblasts, which are vessel-associated stem cells [60-62], neural stem cells [63], and, as mentioned previously, bone marrow cells [64,65].
Bone marrow cells have long been known to have myogenic potential [66,67]. Direct injection of β-galactosidase-positive bone marrow cells into cardiotoxin-injured muscle gives rise to labeled myofibers, although at a lower frequency than injected satellite cells [64]. Interestingly, bone marrow cells contribute directly to regenerating myofibers in lethally irradiated mdx bone marrow transplant recipients [68]. Surprisingly, in the absence of myogenic induction, a subset of bone marrow cells in mdx mice are positive for both early and late myogenic markers including Pax3, MyoD, and myosin heavy chain, suggesting that muscle commitment and differentiation are underway [69]. Also intriguing is the finding that GFP-labeled BMD cells take up residence beneath the basal lamina of skeletal muscle fibers in irradiated transplant recipients following injury (in this case, an exercise model), with subsequent injury provoking an increased contribution of BMD cells to regenerated muscle fibers [65]. This suggests that satellite cells are maintained in regenerating fibers through self-renewal as well as replenishment from the bone marrow. It remains to be seen what proportion of satellite cells arise anew with each round of injury, and whether multiple rounds of injury results in a complete turnover of host satellite cells with donor bone marrow cells.
Advances in Muscle Stem Cell Therapy
The use of muscle stem cells for therapeutic purposes holds much promise for treatment of diseases affecting skeletal muscle, including muscular dystrophy [70]. Dystrophic muscles that receive myoblast transplants exhibit some dystrophin-positive myofibers, and persistence of donor fibers in regenerated muscles is observed [71-73]. However, certain roadblocks hinder the efficacy of this therapy, including the limited migration of donor cells into dystrophic muscle and problems with poor donor cell survival and inefficient myogenic contribution. Advances have been made in identifying chemotactic factors and cell surface molecules that enhance the migration of transplanted cells [74-76]. In addition, careful selection of donor cells has been shown to enhance efficiency of rescue and cell survival in transplant hosts. In particular, Huard and colleagues have found that their serial preplated muscle stem cell cultures display enhanced proliferative capabilities and readily contribute to regenerating muscle while failing to trigger a strong immune response [47,77]. Furthermore, CD45-positive muscle SP cells also contribute to regenerating muscle with high efficiency [54,59].
The use of bone marrow transplants for treating muscular dystrophy has been contemplated as an alternative therapy to myoblast injection and, as mentioned previously, BMD cells do contribute to regenerating muscle. In fact, bone marrow derived nuclei have been identified in muscle biopsies from a 15-year-old patient who received a bone marrow transplant at age 1, and was diagnosed with a mild case of Duchenne muscular dystrophy at age 12 [78]. While this demonstrates the longevity of transplanted cells in muscle, it remains to be seen whether the contribution of these cells to regenerating muscle is responsible for the mild form of the patient's disease. Intriguingly, intra-arterial injection of wild-type mesoangioblasts into mice suffering from limb girdle muscle dystrophy results in complete functional recovery of all affected muscles [62]. This presents a promising solution to difficulties encountered with myoblast transplantation therapy, and makes all muscles accessible for treatment. This is especially important for the treatment of essential muscles such as the diaphragm, impairment of which results in severe respiratory problems.
Contributor Information
Jennifer CJ Chen, Email: jennifer.chen@uconn.edu.
David J Goldhamer, Email: david.goldhamer@uconn.edu.
References
- Mauro A. Satellite cells of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. The satellite cell and muscle regeneration. In: Engel A G and Franzini-Armstrong C, editor. Myology. Second. Vol. 1. New York, McGraw-Hill, Inc.; 1994. pp. 97–118. [Google Scholar]
- Pownall ME, Gustafsson MK, Emerson C. P., Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol. 2002;18:747–783. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. I. A fine structural study. Anat Rec. 1977;188:181–199. doi: 10.1002/ar.1091880205. [DOI] [PubMed] [Google Scholar]
- Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat Rec. 1977;188:201–217. doi: 10.1002/ar.1091880206. [DOI] [PubMed] [Google Scholar]
- Maltin CA, Harris JB, Cullen MJ. Regeneration of mammalian skeletal muscle following the injection of the snake-venom toxin, taipoxin. Cell Tissue Res. 1983;232:565–577. doi: 10.1007/BF00216429. [DOI] [PubMed] [Google Scholar]
- Darr KC, Schultz E. Exercise-induced satellite cell activation in growing and mature skeletal muscle. J Appl Physiol. 1987;63:1816–1821. doi: 10.1152/jappl.1987.63.5.1816. [DOI] [PubMed] [Google Scholar]
- Appell HJ, Forsberg S, Hollmann W. Satellite cell activation in human skeletal muscle after training: evidence for muscle fiber neoformation. Int J Sports Med. 1988;9:297–299. doi: 10.1055/s-2007-1025026. [DOI] [PubMed] [Google Scholar]
- Snow MH. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat Rec. 1990;227:437–446. doi: 10.1002/ar.1092270407. [DOI] [PubMed] [Google Scholar]
- Snow MH. A quantitative ultrastructural analysis of satellite cells in denervated fast and slow muscles of the mouse. Anat Rec. 1983;207:593–604. doi: 10.1002/ar.1092070407. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Eftimie R, Brenner HR, Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Apone L, Morasso MI, Beers R, Brenner HR, Eftimie R. The MyoD family of myogenic factors is regulated by electrical activity: isolation and characterization of a mouse Myf-5 cDNA. Nucleic Acids Research. 1992;20:539–544. doi: 10.1093/nar/20.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Developmental Biology. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- Cardasis CA, Cooper GW. An analysis of nuclear numbers in individual muscle fibers during differentiation and growth: a satellite cell-muscle fiber growth unit. J Exp Zool. 1975;191:347–358. doi: 10.1002/jez.1401910305. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Schultz E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve. 1983;6:574–580. doi: 10.1002/mus.880060807. [DOI] [PubMed] [Google Scholar]
- Renault V, Rolland E, Thornell LE, Mouly V, Butler-Browne G. Distribution of satellite cells in the human vastus lateralis muscle during aging. Exp Gerontol. 2002;37:1513–1514. doi: 10.1016/S0531-5565(02)00095-5. [DOI] [PubMed] [Google Scholar]
- Peterson CA. Cell culture systems as tools for studying age-related changes in skeletal muscle. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:142–144. doi: 10.1093/gerona/50a.special_issue.142. [DOI] [PubMed] [Google Scholar]
- Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev. 2002;123:649–661. doi: 10.1016/S0047-6374(01)00411-0. [DOI] [PubMed] [Google Scholar]
- Charifi N, Kadi F, Feasson L, Denis C. Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve. 2003;28:87–92. doi: 10.1002/mus.10394. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Sebille A. Basic fibroblast growth factor promotes in vivo muscle regeneration in murine muscular dystrophy. Neurosci Lett. 1995;202:121–124. doi: 10.1016/0304-3940(95)12223-0. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Brown R. H., Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Sheehan SM, Allen RE. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J Cell Physiol. 1999;181:499–506. doi: 10.1002/(SICI)1097-4652(199912)181:3<499::AID-JCP14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem. 2000;48:1079–1096. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Garry DJ, Meeson A, Elterman J, Zhao Y, Yang P, Bassel-Duby R, Williams RS. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc Natl Acad Sci U S A. 2000;97:5416–5421. doi: 10.1073/pnas.100501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ, Jiang N, Garry DJ. Absence of p21CIP rescues myogenic progenitor cell proliferative and regenerative capacity in Foxk1 null mice. J Biol Chem. 2003;278:4015–4020. doi: 10.1074/jbc.M209200200. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Meeson AP, Jiang N, Graham S, Hutcheson K, DiMaio MJ, Garry DJ. p21 is Essential for Normal Myogenic Progenitor Cell Function in Regenerating Skeletal Muscle. Am J Physiol Cell Physiol. 2003. [DOI] [PubMed]
- Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes & Development. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA. Reduced Differentiation Potential of Primary MyoD-/- Myogenic Cells Derived from Adult Skeletal Muscle. J Cell Biol. 1999;144:631–643. doi: 10.1083/jcb.144.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol. 1999;210:440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, Braun T, Hinuma S, Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Braun T, Rudnicki MA, Arnold HH, Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992;71:369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage [published erratum appears in J Cell Biol 1995 Feb;128(4):following 713] Journal of Cell Biology. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Akiyama S, Namiki M, Komaki M, Yamaguchi A, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 inhibits terminal differentiation of myogenic cells by suppressing the transcriptional activity of MyoD and myogenin. Experimental Cell Research. 1997;230:342–351. doi: 10.1006/excr.1996.3432. [DOI] [PubMed] [Google Scholar]
- Teboul L, Gaillard D, Staccini L, Inadera H, Amri EZ, Grimaldi PA. Thiazolidinediones and fatty acids convert myogenic cells into adipose-like cells. Journal of Biological Chemistry. 1995;270:28183–28187. doi: 10.1074/jbc.270.47.28183. [DOI] [PubMed] [Google Scholar]
- Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N. Generation of different fates from multipotent muscle stem cells. Development. 2002;129:2987–2995. doi: 10.1242/dev.129.12.2987. [DOI] [PubMed] [Google Scholar]
- Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, Cummins J, Epperly M, Qu-Petersen Z, Huard J. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640–646. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol. 2002;30:1339–1345. doi: 10.1016/S0301-472X(02)00954-2. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43922. [DOI] [PubMed] [Google Scholar]
- McKinney-Freeman SL, Jackson KA, Camargo FD, Ferrari G, Mavilio F, Goodell MA. Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proc Natl Acad Sci U S A. 2002;99:1341–1346. doi: 10.1073/pnas.032438799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka SM, Jackson KA, Kienstra KA, Majesky MW, Goodell MA, Hirschi KK. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI200316157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Chen JC, Goldhamer DJ. Transcriptional mechanisms regulating MyoD expression in the mouse. Cell Tissue Res. 1999;296:213–219. doi: 10.1007/s004410051282. [DOI] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D'Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, Bottinelli R, Cossu G. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, Mora M, De Angelis MG, Fiocco R, Cossu G, Vescovi AL. Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci. 2000;3:986–991. doi: 10.1038/79924. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop. 1980:294–307. [PubMed] [Google Scholar]
- Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988;106:2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, Freilinger M, Hoger H, Elbe-Burger A, Wachtler F. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 1999;199:391–396. doi: 10.1007/s004290050237. [DOI] [PubMed] [Google Scholar]
- Corti S, Strazzer S, Del Bo R, Salani S, Bossolasco P, Fortunato F, Locatelli F, Soligo D, Moggio M, Ciscato P, Prelle A, Borsotti C, Bresolin N, Scarlato G, Comi GP. A Subpopulation of Murine Bone Marrow Cells Fully Differentiates along the Myogenic Pathway and Participates in Muscle Repair in the mdx Dystrophic Mouse. Exp Cell Res. 2002;277:74–85. doi: 10.1006/excr.2002.5543. [DOI] [PubMed] [Google Scholar]
- Partridge TA. Stem cell route to neuromuscular therapies. Muscle Nerve. 2003;27:133–141. doi: 10.1002/mus.10243. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, Blau HM. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992;356:435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- Yao SN, Kurachi K. Implanted myoblasts not only fuse with myofibers but also survive as muscle precursor cells. J Cell Sci. 1993;105 ( Pt 4):957–963. doi: 10.1242/jcs.105.4.957. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Camirand G, Pisati F, Belicchi M, Rossi B, Colombo F, El Fahime M, Caron NJ, Issekutz AC, Constantin G, Tremblay JP, Bresolin N. Identification of a putative pathway for the muscle homing of stem cells in a muscular dystrophy model. J Cell Biol. 2003;162:511–520. doi: 10.1083/jcb.200210006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrente Y, El Fahime E, Caron NJ, Del Bo R, Belicchi M, Pisati F, Tremblay JP, Bresolin N. Tumor necrosis factor-alpha (TNF-alpha) stimulates chemotactic response in mouse myogenic cells. Cell Transplant. 2003;12:91–100. doi: 10.3727/000000003783985115. [DOI] [PubMed] [Google Scholar]
- El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res. 2000;258:279–287. doi: 10.1006/excr.2000.4962. [DOI] [PubMed] [Google Scholar]
- Qu Z, Huard J. Matching host muscle and donor myoblasts for myosin heavy chain improves myoblast transfer therapy. Gene Ther. 2000;7:428–437. doi: 10.1038/sj.gt.3301103. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Bennett RR, Muskiewicz KR, Meyerrose T, Nolta JA, Gilgoff I, Stein J, Chan YM, Lidov HG, Bonnemann CG, Von Moers A, Morris GE, Den Dunnen JT, Chamberlain JS, Kunkel LM, Weinberg K. Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest. 2002;110:807–814. doi: 10.1172/JCI200216098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RE, Rankin LL, Greene EA, Boxhorn LK, Johnson SE, Taylor RG, Pierce PR. Desmin is present in proliferating rat muscle satellite cells but not in bovine muscle satellite cells. Journal of Cellular Physiology. 1991;149:525–535. doi: 10.1002/jcp.1041490323. [DOI] [PubMed] [Google Scholar]
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138:311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]