The authors show for the first time that recombinant myocilin interacts with the matricellular protein SPARC. It is proposed that the proteolytic processing of myocilin may modulate the interactions of myocilin with extracellular matrix and matricellular proteins such as SPARC and hevin, further supporting a functional role for this proteolytic processing.
Abstract
Purpose.
Myocilin is an extracellular glycoprotein with unknown function that is associated with glaucoma. Calpain II cleaves recombinant myocilin within the linker region of the protein, releasing the C-terminal olfactomedin domain from the N-terminal domain. The authors previously reported that myocilin interacts with the C-terminal region of hevin, a secretory glycoprotein belonging to the SPARC family of matricellular proteins. This study aims to investigate the interaction of myocilin with SPARC.
Methods.
Protein-protein interactions were evaluated by the yeast two-hybrid system. The positive interactions were confirmed by solid-phase binding assays using Ni-chelating HPLC purified recombinant proteins and coexpression of recombinant proteins in HEK-293T cells. Coexpression of myocilin, SPARC, and hevin in ocular tissues was identified by immunoflorescence microscopy, Western blot, and array-based gene profiling.
Results.
Yeast two-hybrid analyses showed that myocilin interacted with the highly conserved C-terminal extracellular calcium binding (EC) domain within SPARC and hevin. Solid-phase binding assays confirmed these interactions and showed that both myocilin and its C-terminal olfactomedin fragment interacted noncovalently with SPARC and a peptide containing the EC domain of SPARC. Full-length myocilin interacted with higher affinity with SPARC and its EC domain than the myocilin C-terminal fragment. Coexpression of the two recombinant proteins in HEK-293T cells also indicated their intracellular interaction.
Conclusions.
Recombinant myocilin and SPARC interact through their C-terminal domains. The data suggest that the proteolytic processing of myocilin modulates this interaction as well as the interactions of myocilin with other extracellular matrix and matricellular proteins, further supporting a functional role for this proteolytic cleavage.
Myocilin, a member of the olfactomedin family of proteins, is a secreted glycoprotein with an unidentified function that is expressed in different human tissues.1–5 It forms large extracellular aggregates linked by disulfide bonds.6–8 Mutations in the MYOCILIN (MYOC) gene are involved in different types of glaucoma.9–11 This protein is organized into three modular domains, each of which is encoded by one of its three exons. Recombinant myocilin undergoes an intracellular endoproteolytic cleavage in the middle of the polypeptide chain by calpain II, releasing two fragments that contain the N- and C-terminal domains.12,13 This cleavage occurs in the endoplasmic reticulum.12 The C-terminal fragment has been detected in both human and bovine ciliary body (CB), in aqueous humor and trabecular meshwork (TM),14–16 and in the eye angle of mice,17 indicating that this specific proteolysis also occurs in vivo.
It is believed that myocilin interactions may play key roles in the biological function of myocilin. The disulfide aggregates of this protein interact noncovalently, suggesting that myocilin complexes may organize into a reversible extracellular network sustained by noncovalent N-terminal interactions.16 It has been reported that myocilin interacts with flotillin-1, a structural protein of lipid rafts,18 as well as with extracellular proteins such as fibronectin,19 laminin,20,21 decorin,20 collagens,20 fibrillin-1,20 optimedin,22 and hevin (also known as SC1 and SPARC-like 1).23 Hevin is a matricellular protein with an unclear function that modulates cell shape and cell adhesion to different substrates in vitro, including fibronectin.24 This protein belongs to the secreted protein acidic and rich in cysteine (SPARC) family of matricellular proteins. The proteins in this family also include testicans 1–3,25 SMOC-126 and 2,27 thrombospondins 1 and 2, osteopontin, and tenascins C and X.28,29 Like myocilin, they present a modular design and share homologous C-terminal domains, including extracellular Ca2+-binding (EC) and follistatin-like domains. SPARC is a multifunctional protein that modulates cellular interactions with extracellular matrix (ECM) during embryogenesis and in adult tissues that continue to remodel.29,30 Furthermore, it binds components of the ECM, affects certain growth factors, alters the expression of matrix metalloproteinases, and has counteradhesive and antiangiogenic effects.25,31,32
We investigated the interaction of myocilin with the hevin-related protein SPARC. We show that myocilin interacts trough its C-terminal region with the EC domain of SPARC in a reversible fashion. Our data suggest that the specific cleavage of myocilin could modulate its interactions with SPARC and other extracellular proteins (e.g., laminin and fibronectin).
Methods
Yeast Two-Hybrid cDNAs
Recombinant bait cDNAs encoding myocilin (lacking the signal peptide) and the C-terminal domain of myocilin (amino acids 217–504) were PCR amplified using a myocilin cDNA clone14 as a template and the primer pairs 1 to 2 and 2 to 3 (Table 1), respectively. The following PCR conditions were used in all amplifications for yeast-two hybrid constructs: annealing at 55°C for 30 seconds and extension at 72°C for 3 minutes for 35 cycles. The resulting PCR products were fused to the GAL4 DNA binding domain by cloning into the EcoRI and BamHI restriction sites of the cloning vector (pAS2.1 vector; Clontech, Palo Alto, CA). Another vector (pACT2; Clontech) was used to clone prey cDNAs encoding the C-terminal regions of SPARC (amino acids 152–303) and hevin (amino acids 509–664). These molecules were amplified from cDNAs obtained from the Full-Length Mammalian Gene Collection (Invitrogen-GIBCO, Carlsbad, CA) encoding human hevin (GenBank Accession No. NM_004684, Invitrogen, 4939390) or SPARC (GenBank Accession No. NM_003118, Invitrogen, 3529445), using the primer pairs 4–5 and 6–7, respectively (Table 1). The obtained PCR products were subcloned into the EcoRI-XhoI and BamHI-XhoI restriction sites, respectively. The recombinant plasmids encoded the different proteins fused to the GAL4 activation domain. We used pVA3–1 [BD/murine p53 (codons 72–390) fusion; Clontech], pLAM5′-1 [BD/human laminin C (codons 66–230) fusion] and pTD1–1 [AD/SV40 large T antigen (codons 84–708) fusion; Clontech] as control cDNAs.
Table 1.
Oligonucleotide Primers Used to Generate cDNAs Encoding Myocilin, SPARC, and Hevin cDNAS Used in Yeast Two-Hybrid Assays and to Express Recombinant Proteins in HEK-293T Cells
| Primers | 5′→3′ Sequence | Restriction Site |
|---|---|---|
| Primers for Yeast Two-Hybrid Assays | ||
| 1 | AATAGAATTCACAGCTCAGCTCAGGAAGGCC | Eco-RI |
| 2 | TTATGGATCCTCACATCTTGGAGAGCTTGATGTC | Bam-HI |
| 3 | TTTTGAATTCTCTGGCTATCTCAGGAGTGG | Eco-RI |
| 4 | GGTGCTCGAGTCAAAACAAGAGATTTTCATC | Xho-I |
| 5 | AAATGAATTCTAAAATCTATTCCTACTTGTACGG | Eco-RI |
| 6 | TGTTCTCGAGTTAGATCACAAAGATCCTTGTCG | Xho-I |
| 7 | CAACGGATCCTAATCCCCCCTTGCCTGG | Bam-HI |
| Primers for Expression of Recombinant Proteins in HEK-293T Cells | ||
| 8 | GCCCTCTAGATGAGGGCCTGGATCTTCTTTCTCC | Xba-I |
| 9 | GCTCGGATCCAGGATCACAAGATCCTTGTCG | Bam-HI |
| 10 | GTGCAGGCAAGGGGGGATTGCCAAGGCCCTCCCGGC | — |
| 11 | GCCGGGAGGGCCTTGGCAATCCCCCCTTGCCTGGAC | — |
Sequences in bold italic type indicate restriction sites introduced in primers to facilitate subcloning of PCR products.
Yeast Two-Hybrid Analysis
Protein-protein interaction analyses with the yeast two-hybrid assay were performed (Matchmaker-3 kit; Clontech). Saccharomyces cerevisiae CG1945 was cotransformed with the corresponding bait and prey recombinant plasmids following the manufacturer's recommendations (Clontech). For auxotrophic assays cotransformed clones were plated on selection medium (-Ade/-His/-Leu/-Trp/X-β-Gal) and incubated at 30°C for 8–21 days. For β-galactosidase activity assays cells were lysed by three freeze-thaw cycles in liquid nitrogen. The enzymatic activity was determined using the lysates and ONPG (ortho-nitrophenyl-β-galactopyranoside) as a chromogenic substrate (Sigma-Aldrich, St. Louis, MO), according to the manufacturer's instructions (Clontech). These assays were performed in triplicate.
Expression and Purification of Recombinant Proteins
The cDNAs encoding the different versions of recombinant myocilin and hevin, cloned in the pcDNA3.1 vector, were obtained as previously described.12,14,23 The cDNAs encoding SPARC and its EC domain were PCR amplified using the aforementioned SPARC cDNA clone as a template, and the primer pairs 8–9 (annealing at 50°C for 30 seconds, extension for 30 seconds) and 9–10 (annealing at 50°C for 30 seconds, extension 90 seconds; Table 1), respectively. The signal peptide of SPARC was obtained by PCR using the primer pair 8–11 (annealing at 60°C for 30 seconds, extension 90 seconds for 35 cycles; Table 1) and fused to the 5′ end of the EC domain as described previously.12 Recombinant proteins were transiently expressed in human embryonic kidney 293T (HEK-293T) cells bought from the American Type Culture Collection. These cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% fetal bovine serum as described previously.12,14 The recombinant proteins were directly purified from conditioned culture media by Ni-chelating high-performance liquid chromatography (HPLC).16 The purity of isolated proteins was assessed by SDS-PAGE with silver nitrate staining.33 The identity of the isolated recombinant proteins was confirmed by Western blot analyses using either anti-myc or anti-HA antibodies (Santa Cruz Biotechnology, Santa Cruz, CA).12,14
Solid-Phase Binding Assays
Microtiter wells (Microtest plate 96-well flat bottom; Sarstedt, Nümbrecht, Germany) were incubated with 50 μL of the different purified recombinant proteins, fibronectin, laminin, or decorin (all Sigma) [0.1 μM protein in coating buffer (50 mM NaCO3, pH 9.6)] for 12 hours at 4°C.16 The protein in the coating buffer was substituted for with BSA (Sigma) as a control for nonspecific binding. Wells were blocked for 3 hours with 200 μL of 10% BSA in coating buffer at 4°C. After washing 3 times with tris buffered saline (TBS)-Tween (25 mM tris-glycine, pH 7.5, 150 mM NaCl, 1% Tween-20), increasing concentrations (0–2.5 μM) of purified recombinant myocilin and its C-terminal fragment fused to the myc epitope at their C-terminal ends, dissolved in 50 μL of TBS-Tween containing 5% BSA, were added to each well and incubated for 2 hours under gentle agitation (100 rpm) at room temperature. Competitive binding analyses were performed by incubating increasing amounts of recombinant myocilin-Ct-HA (0.05–2 μM) to immobilized SPARC-EC in the presence myocilin-Ct-myc (competitor) at four myocilin-Ct-myc/myocilin-Ct-HA molar ratios (0, 0.5/1, 1/1, and 2/1). The competitive binding of myocilin-HA to immobilized SPARC-EC was tested at one concentration of myocilin-HA (0.1 μM) in the presence of myocilin-myc (competitor) at the aforementioned molar ratios. We also analyzed the competitive interaction of myocilin-Ct-HA with SPARC-EC in the presence of myocilin-myc (competitor) at myocilin-myc/myocilin-Ct-HA molar ratios of 0, 1/1, and 2/1. Wells were incubated with an anti-myc antibody, diluted at 1:300, for 2 hours at room temperature. A horseradish peroxidase-conjugated antibody against mouse IgG (Pierce, Rockford, IL) was diluted at 1:500. Colorimetric detection and protein concentration were determined in triplicate independent assays as described previously.16 Data were statistically analyzed with commercial software (SigmaStat 2.0; SPSS, Chicago, IL).
Coexpression of Myocilin and SPARC in HEK-293T Cells
To test the intracellular interaction of myocilin and SPARC, HEK-293T cells were transiently cotransfected with a SPARC-myc cDNA (200 ng) and increasing amounts (0–400 ng) of a second cDNA encoding myocilin-HA using reagent (SuperFect Transfection Reagent; Qiagen, Valencia, CA). In parallel control assays, SPARC-myc cDNA (200 ng) was cotransfected with increasing amounts (0–400 ng) of a cDNA encoding IGF-BP1-Myc, also cloned in the pcDNA3.1 vector. The total cDNA (400 ng) was kept constant in all transfections by adding the required amount of nonrecombinant cDNA (pcDNA3.1). Forty-eight hours after transfection the culture medium (CM) and the intracellular soluble (IC) fraction of cell lysates were collected.14,34 Similar amounts of proteins, quantified by the Bradford assay,35 in the CM and IC fractions were fractionated by SDS-PAGE (10% polyacrylamide). Recombinant proteins were detected by Western immunoblot using either anti-myc (1:400 dilution) or anti-HA (1:3000 dilution) antibodies. Using an anti-LDH antibody (AB-1222. Chemicon, diluted 1:5000), we verified by Western blot that similar amounts of protein were analyzed in the IC fractions (data not shown). Luminescent imaging film (LAS3000-mini; Fujifilm, Tokyo, Japan) was used for chemiluminescence detection. Proteins were quantified by densitometry in triplicated independent assays as described previously.14,34
Immunofluorescence Microscopy
For immunofluorescence analyses, semithin cryostat sections (0.5–1.0 μm thick) from bovine ciliary processes were used as previously described.36 The following antisera were tested: (1) an anti-peptide (R14T) antibody to myocilin4; (2) a commercial SPARC/osteonectin antibody (AON-5031; Hematologic Technologies, Essex Junction, VT); and (3) an antibody to SC1/hevin.37 Tissue sections were incubated at 37°C for 90 minutes with the above-mentioned primary antibodies (1:100). They were then rinsed in PBS and further incubated with the secondary antisera (100-fold–diluted rhodamine-conjugated goat anti-rabbit IgG) for 60 minutes After washing in PBS and mounting in a solution of glycerol mounting medium, the specimens were analyzed with a fluorescent microscope (Axioskop; Carl Zeiss, Göttingen, Germany). Photographs were taken using high-speed film (TMAX-400; Eastman Kodak, Rochester, NY). As a control, sections were stained with secondary antibodies alone or with BSA or normal serum.
Gene Expression Profiling Analysis
Total RNA was isolated from distinct human ocular tissues using reagent (TRIzol; Invitrogen) according to the manufacturer's instructions. The ocular tissues were dissected from a pair of eyes of a 57-year-old donor (cadaver), obtained through the National Disease Research Interchange (Philadelphia, PA). The procedures conformed to the tenets of the Declaration of Helsinki. The RNA samples were further purified using cleanup columns (Qiagen RNeasy; Qiagen, Hilden, Germany). cRNA labeling and hybridization to the chip and array data analysis were carried out by the Yale Neuroscience Microarray Center (NIH Neuroscience Microarray Consortium) at the Keck Foundation at Yale University. The quality of RNA was determined with a bioanalyzer gel, and samples with an OD 260/280 > 1.8 were further processed for gene expression profiling (Illumina platform; Illumina, San Diego, CA). Single- and double-stranded cDNAs were synthesized and purified using an amplification kit beadchip (Illumina Total/Prep RNA; Illumina). An in vitro transcription reaction was then carried out in the presence of biotinylated UTP and CTP to produce biotin-labeled cRNA from the double-stranded cDNA and was then hybridized (beadchip HumanHT-12; Illumina), stained with streptavidin-Cy3, and the array was scanned (BeadArray reader; Illumina) and images were analyzed (Beadstudio software; Illumina). Quality control and data analyses were carried out according to the instructions provided by Illumina. Signals corresponding to myocilin SPARC and hevin were selected for the analysis.
Results
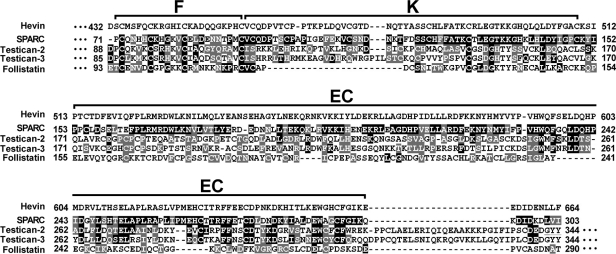
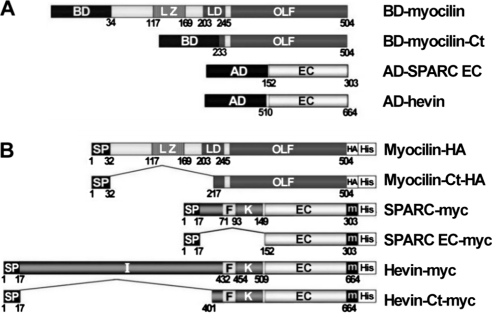
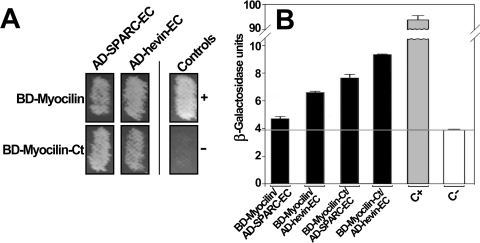
We have previously reported that myocilin interacts with the C-terminal region of hevin, which contains the follistatin, Kazal-like, and EC domains (amino acids 401–664).23 This region is highly conserved in the BM-40/SPARC/osteonectin family (Fig. 1). Interestingly, hevin shows the highest sequence similarity with SPARC (60.8% amino acid identity; 78% similarity; Fig. 1 and Table 2). These facts prompted us to initially test the possible interaction of SPARC with myocilin using the yeast two-hybrid system. To achieve this goal, we generated two sets of fusion proteins. The first set was composed of full-length myocilin and its C-terminal half (myocilin-Ct, amino acids 233–504), which resembles the fragment resulting from the proteolytic processing of the full-length protein by calpain II (Fig. 2A). The two myocilin versions were fused to the GAL4 DNA binding domain (Fig. 2A). The second set consisted of hybrids between sequences encoding the GAL4 DNA activation domain and the C-terminal EC domain of either SPARC (AD-SPARC-EC, amino acids 152–303) or hevin (AD-hevin-EC, amino acids 510–664; Fig. 2A). Histidine prototrophy was observed in yeast cotransformed with fusion plasmids encoding myocilin and either SPARC-EC or hevin-EC, as well as in yeast coexpressing myocilin-Ct and the C-terminal regions of SPARC or hevin (Fig. 3A). Histidine prototrophy was also detected in the positive control (pTD1–1-AD/pVA3–1BD cotransformation; Fig. 3A). As expected, yeast cotransformed with plasmids pTD1–1-AD and pLAM5–1-BD (negative control; Fig. 3A) was unable to grow in medium lacking His.
Figure 1.
Pairwise amino acid sequence alignment of the C-terminal region of hevin and members of the BM-40/SPARC/osteonectin family of proteins. The region of hevin shown in the alignment participates in the interaction with myocilin.23 The amino acids identical or similar to those of hevin are highlighted with a black or gray background, respectively. The numbers on the right and on the left correspond to the amino acid positions. The location of the follistatin-like (F), Kazal-like (K), and extracellular calcium binding (EC) domains are also indicated. Pairwise sequence alignments were generated using the ClustalW program.53
Table 2.
Amino Acid Sequence Identity and Similarity between the EC Regions of Hevin and Four Members of the BM-40/SPARC/Osteonectin Family of Proteins
| Hevin (Amino Acids 432–664) |
||
|---|---|---|
| Identity (%) | Similarity (%) | |
| SPARC (amino acids 71–303) | 60.8 | 78.0 |
| Testican-2 (amino acids 88–344) | 18.1 | 48.3 |
| Testican-3 (amino acids 85–344) | 17.7 | 43.1 |
| Follistatin (amino acids 93–290) | 7.8 | 28.9 |
Values were obtained by pairwise comparisons using ClustalW software.53
Figure 2.
Scheme of cDNA constructs of myocilin, SPARC, and hevin used for the two-hybrid analysis (A) or the production of recombinant proteins used in the solid-phase binding assays (B). The diagrams represent the primary amino acid structure of human myocilin, SPARC, and hevin. The numbers below the diagrams correspond to amino acid locations. AD, GAL4 activation domain; BD, GAL4 DNA binding domain; EC, extracellular calcium binding domain; F, follistatin domain; HA, HA epitope; His, histidine tail; K, kazal-like domain; LD, linker domain; LZ, leucine zipper; m, myc epitope; OLF, olfactomedin domain; SP, signal peptide.
Figure 3.
Yeast two-hybrid analyses of the interaction of human myocilin with the EC domain of SPARC. (A) Growth of cotransformed yeast on selective plates. Yeast strain CG1945 was cotransformed with the indicated myocilin and SPARC cDNA constructs and grown on selection plates lacking tryptophan, leucine, and histidine. As a positive control of myocilin interactions yeast were cotransformed with the cDNA constructs of myocilin and with the EC domain of hevin. (B) The relative interactions of myocilin with C-terminal fragments of SPARC or hevin were quantified by measurements of β-galactosidase activity in liquid cultures. Additional positive (C+) and negative (C−) controls of the interactions consisted of yeast cotransformed with plasmids pTD1–1-AD (AD/SV40 large T antigen fusion) and pVA3–1BD (BD/murine p53 fusion) or pTD1–1-AD and pLAM5–1-BD (BD/human laminin C fusion), respectively. The results are the mean ± SE of triplicate assays. AD, GAL4 activation domain; BD, GAL4 DNA binding domain.
Two-hybrid interactions were initially verified and quantified, comparing their relative strength by measuring β-galactosidase activity in liquid yeast culture. In accordance with the previous assay, recombinant myocilin and its C-terminal fragment interacted with the C-terminal fragment of SPARC. The intensity of the interactions of the two versions of myocilin with SPARC-EC was slightly weaker than with hevin-EC (Fig. 3B).
Solid-Phase Binding Assays
To further confirm the interaction between myocilin and SPARC we carried out solid-phase binding assays using the purified recombinant proteins expressed in HEK-293T cells. Myocilin and its C-terminal fragment were expressed as recombinant proteins fused to the HA epitope at their C-terminal ends (Fig. 2B, myocilin-Ct-HA). The recombinant myocilin C-terminal fragment generated for these assays also coincided with the polypeptide produced by the proteolytic processing of the protein (amino acids 217–504). SPARC, hevin, and their respective C-terminal fragments incorporated the myc epitope at their C-terminal ends (Fig. 2B). The C-terminal SPARC polypeptide corresponded to the EC domain (SPARC-EC-myc), whereas that of hevin also incorporated the follistatin- and Kazal-like domains (hevin-Ct-myc). In addition, all the recombinant proteins were tagged with a 6xHis tail at their most C-terminal ends (Fig. 2B) and were purified by Ni-chelating HPLC as described previously.16 The identity of the proteins obtained was confirmed by Western blot analysis (Fig. 4A), and their purity, estimated by silver staining, was generally, higher than 80% (Fig. 4B). Recombinant hevin was not stained by silver nitrate because of its low concentration.
Figure 4.
SDS-PAGE analysis of recombinant proteins purified by Ni-chelating chromatography and used in the solid-phase binding assays. Culture media from HEK-293T cells transiently transfected with cDNA constructs encoding the different recombinant proteins were fractionated by Ni-chelating HPLC as described.16 Aliquots of the isolated proteins were fractionated by SDS-PAGE (10% acrylamide). After electrophoresis, proteins were either transferred to nitrocellulose membranes and detected by chemiluminiscence using anti-myc or anti-HA antibodies (1:500)12,14 (A) or detected by silver nitrate staining (B).
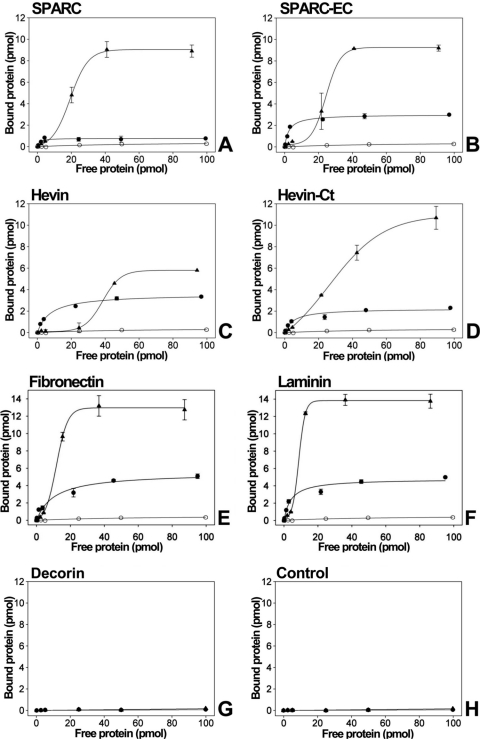
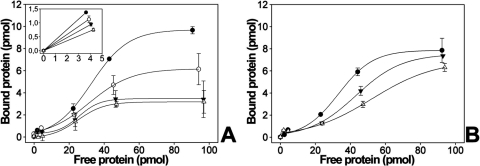
Microtiter plates were coated with either recombinant SPARC-myc (Fig. 5A) or the SPARC-EC-myc fragment (0.1 μM protein; Fig. 5B) and incubated with increasing concentrations, up to 100 pmol, of either myocilin-HA or myocilin-Ct-HA. The amount of immobilized proteins estimated by Western blot was higher than 95%. The two versions of myocilin showed a concentration-dependent and saturable binding to immobilized SPARC and SPARC EC (Figs. 5A, 5B). In parallel assays we also analyzed the interaction of myocilin and its C-terminal fragment with either immobilized hevin-myc (Fig. 5C) or hevin-Ct-myc (Fig. 5D). Again, we observed a concentration-dependent and saturable interaction of the two versions of myocilin with the two versions of hevin (Figs. 5C, 5D). Interestingly, myocilin showed hyperbolic saturation curves with the four immobilized proteins while its C-terminal fragment fitted a sigmoid with increased maximal binding compared with full-length myocilin, ranging from approximately 10 times (myocilin-Ct versus SPARC; Fig. 5A) to 2 times (myocilin-Ct versus hevin; Fig. 5B). The apparent affinities of the interactions were estimated as the amount of protein required for half-maximal binding (½Bmax; Table 3). The affinity of noncovalent binding of myocilin to SPARC and hevin was of a similar order of magnitude to that of the myocilin-myocilin interactions (½Bmax ranging from 1.1 to 6.7; Table 3). Myocilin-Ct-HA showed reduced interaction affinity with SPARC, hevin, and the C-terminal fragments of these two proteins (½Bmax ranging from 19.4 to 38.2 pmol; Table 3). These data corroborate the noncovalent interaction between myocilin and SPARC and support that it is mediated by the olfactomedin and EC domains of these proteins. In addition, they indicate that the C-terminal fragment of myocilin interacts in a positive cooperative fashion, and with reduced affinity, with both SPARC and hevin as well as with the C-terminal fragments of these two matricellular proteins. The positive cooperativity was also suggested by rough estimates of Hill coefficients obtained from the slope of Hill plots, which clearly exceeded 1. We are aware that the experimental design followed is not well suited to derive reliable Hill coefficients. Further analyses are required to confirm this point.
Figure 5.
Analysis of myocilin-SPARC interactions by solid-phase binding assays. Binding of recombinant myocilin-HA (●) and myocilin-Ct-HA (▴) to either SPARC-myc (A) or the EC domain of SPARC-myc (B) immobilized in 96-well microtiter plates. The interaction of the two versions of myocilin with hevin-myc (C), the C-terminal region of hevin-myc (D), fibronectin (E), laminin (F), and decorin (G) was also tested. As a negative control of the assay recombinant myocilin was added to BSA-blocked wells, which did not contain any other immobilized protein (H). The bound proteins were detected using an anti-HA antibody. The data shown in (H) represent the background (baseline) binding of the two versions of recombinant myocilin in all the interaction assays, which for simplicity is included only in this panel. Values in all graphs correspond to total binding. Error bars represent the SE of triplicate experiments.
Table 3.
Estimation of the Affinities (1/2 Bmax) of the Interactions between Myocilin and Other Proteins from Solid-Phase Binding Assays Shown in Figures 5 and 6
| Interaction | 1/2 Bmax (pmol) |
|---|---|
| Myocilin vs. | |
| Hevin | 6.7 |
| Hevin-Ct | 4.9 |
| SPARC | 1.1 |
| SPARC-EC | 2.5 |
| Fibronectin | 9.5 |
| Laminin | 4.4 |
| Myocilin* | 3.2 |
| Myocilin-Ct vs. | |
| Hevin | 38.2 |
| Hevin-Ct | 31.4 |
| SPARC | 19.4 |
| SPARC-EC | 24.1 |
| Fibronectin | 12.1 |
| Laminin | 8.8 |
1/2 Bmax represents the amount of protein required for half-maximal binding.
Estimated from published data.16
As an additional positive control, the interaction of myocilin with two ECM proteins, fibronectin and laminin, which have been shown to interact with it,19–21 was also analyzed by solid-phase binding assays. Myocilin and its C-terminal fragment interacted noncovalently with the two proteins in a manner similar to that described for SPARC and hevin (Figs. 5E, 5F). The estimated binding affinities of myocilin to fibronectin (9.5 pmol) and laminin (4.4 pmol) were in the same range observed for the interactions of myocilin with SPARC and hevin (Table 3). Myocilin-Ct-myc also showed reduced affinity (Table 3) and increased maximal binding (Figs. 5E, 5F) in the interaction with fibronectin and laminin. Under these conditions the two versions of recombinant myocilin did not bind significantly either to decorin, a related ECM molecule, or to BSA-blocked wells, showing the specificity of the assay (Figs. 5G–E).
To confirm the specificity of the interactions, we performed competitive solid-phase binding assays. Because of the large amounts of recombinant proteins required, only the competitive binding of myocilin-Ct-HA and myocilin-HA to SPARC-EC was assayed. Increasing amounts of recombinant myocilin-Ct-HA (0.05-μM) were incubated with immobilized SPARC-EC in the presence of myocilin-Ct-myc (competitor) at four different myocilin-Ct-myc/myocilin-Ct-HA molar ratios (0, 0.5/1, 1/1, and 2/1; Fig. 6A). The competitive binding of myocilin-HA to immobilized SPARC-EC was tested only at one myocilin-HA concentration (0.1 μM) in the presence of myocilin-myc (competitor) at the aforementioned molar ratios. We observed that the two competitors decreased the binding of the two versions of myocilin to immobilized SPARC-EC in a dose-dependent manner (Fig. 6A). Myocilin-myc also displaced binding of myocilin-Ct-HA to SPAR-EC (Fig. 6B). These data support the specificity of the interactions.
Figure 6.
Competitive binding of recombinant myocilin to immobilized SPARC-EC. (A) Binding of recombinant myocilin-Ct-HA to immobilized SPARC-EC in the presence of myocilin-Ct-myc (competitor) at myocilin-Ct-myc/myocilin-Ct-HA molar ratios of 0 (●), 0.5/1 (○), 1/1 (▾), and 2/1 (▿). The insert shows the competitive binding of myocilin-HA (0.1 μM) to immobilized SPARC-EC in the presence of myocilin-myc (competitor) at the three molar ratios indicated above. (B) Binding of myocilin-Ct-HA to SPARC-EC in the presence of myocilin-myc (competitor) at myocilin-myc/myocilin-Ct-HA molar ratios of 0 (●), 1/1 (▾), and 2/1 (▿).
Coexpression of Recombinant Myocilin and SPARC in HEK-293T Cells
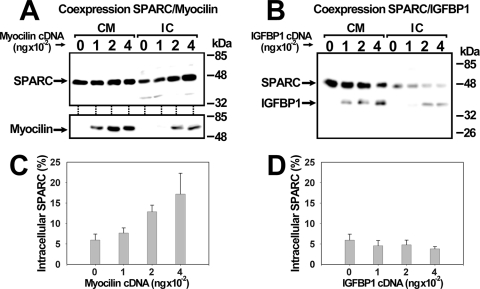
Previous evidence indicates that myocilin and hevin interact intracelularly in the secretory pathway.23 We reasoned that a similar interaction could occur between myocilin and SPARC. To test this hypothesis, we analyzed the intracellular behavior of a constant amount of SPARC-myc coexpressed with increasing amounts of myocilin-HA in HEK-293T cells (Fig. 7). SPARC-myc accumulated intracellularly (from 5% to 15%) as the amount of recombinant myocilin-HA increased in the intracellular fraction (Figs. 7A and 7C). However, intracellular SPARC-myc remained constant at 5% when it was coexpressed with increasing amounts of IGF-BP1-Myc, a recombinant protein used as control (Figs. 7B and 7D). These data indicate that the two proteins interact intracellularly in the secretory pathway.
Figure 7.
Intracellular interaction of recombinant myocilin and SPARC tested by coexpression in HEK-293T cells. (A) HEK-293T cells were transiently cotransfected with a cDNA encoding SPARC-myc (200 ng) and increasing amounts (0–400 ng) of a cDNA encoding myocilin-HA. (B) As a control, HEK-293T cells were transiently cotransfected with the cDNA encoding SPARC-myc (200 ng) and increasing amounts (0–400 ng) of an IGF-BP1-Myc cDNA. The two recombinant proteins were detected in the culture medium (CM) and intracellular fraction (IC) by Western blot using anti-myc (SPARC or IGF-BP1) and anti-HA (myocilin) antibodies. Percentage of intracellular SPARC-myc coexpressed with myocilin (C) or IGF-BP1-myc (D). Values were calculated by densitometry of bands in fractions from (A) and (B), respectively, and represent mean ± SE of three independent assays.
Coexpression of Myocilin, SPARC, and Hevin in Ocular Tissues
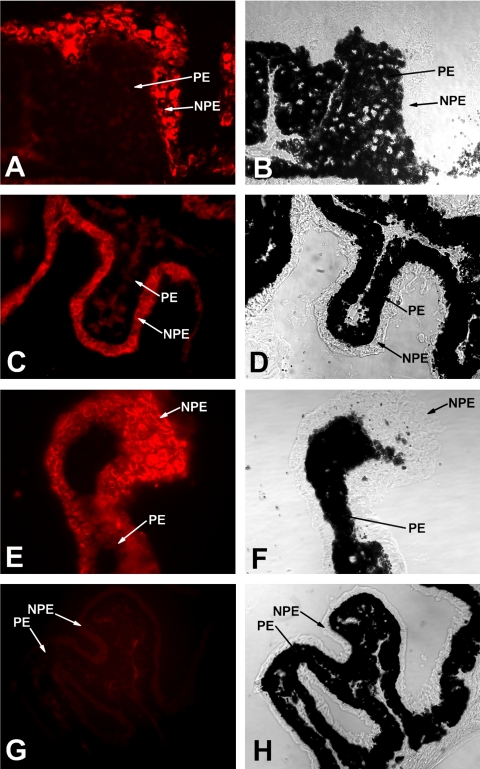
The coexpression of myocilin, SPARC, and hevin in ocular tissues was investigated by immunofluorescence, Western blot, and microarray gene profiling. The immunofluorescence of semi-thin frozen sections of bovine ciliary processes using antibodies against myocilin, SPARC or hevin showed a clear labeling of the NPE layer with the three antibodies (Fig. 8). The abundance of melanin in the PE does not enable us to completely rule out the presence of three proteins in this layer.
Figure 8.
Cellular distribution of myocilin, SPARC, and hevin in the bovine ocular ciliary epithelium. Cryostat sections of bovine ciliary processes were stained with polyclonal antibodies to myocilin (A, B), SPARC (C, D), and hevin (E, F). The immunolabeling of these proteins is confined preferentially along the nonpigmented cell layer of the ciliary epithelium. The strong presence of melanin granules in the pigmented cells masked a possible presence of these proteins in this cell layer. In the presence of normal serum or in the absence of the primary antibody no significant label was detected (G, H). NPE, nonpigmented epithelium; PE, pigmented epithelium. (B), (D), (F), and (H) are phase contrast micrographs of (A), (C), (E), and (G), respectively. Magnification: (A–F) ×600; (G–H) ×400.
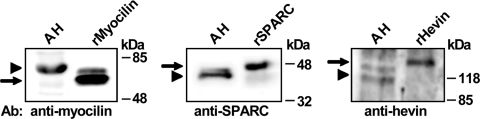
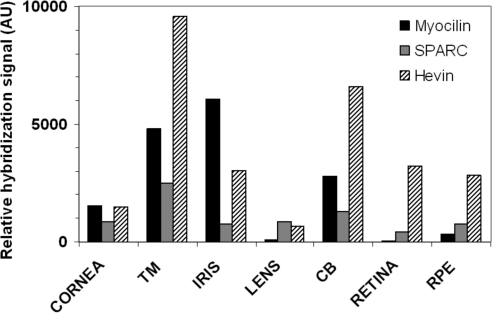
Western blot analysis revealed the presence of the three proteins in the bovine aqueous humor (Fig. 9). According to previous reports, the anti-myocilin R14T antibody recognized a band with an apparent slightly higher molecular weight than that of the recombinant human protein.14 The SPARC antibody detected a polypeptide of approximately 43 kDa in the aqueous humor. Recombinant SPARC, which contained the myc- and HA- epitopes, had a somewhat higher molecular weight than the bovine protein. Finally, bovine hevin was detected as a doublet of around 118 kDa,37 which was close to the molecular weight of the recombinant human protein which also carried the two epitopes. In addition, the gene expression profiling for myocilin, SPARC, and hevin among the human ocular tissues was determined by microarray analysis and showed that SPARC and hevin were expressed in all the tissues. However, these genes were coexpressed abundantly with myocilin, predominantly in the TM and iris and CB (Fig. 10).
Figure 9.
Western blot analysis of myocilin, SPARC, and hevin in bovine aqueous humor. The proteins in bovine aqueous humor (100 μg of total protein) were separated on a 12% SDS-PAGE, transferred onto a nitrocellulose membrane, and probed against specific antibodies to myocilin (R14T),4 SPARC (AON-5031, Hematologic Technologies), and hevin (S5075, Sigma) as described previously.34 Specific bands of the corresponding molecular mass for myocilin, SPARC, and hevin were detected. Purified recombinant proteins were analyzed in parallel as a positive control. The control immunodetection performed only with horseradish peroxidase-conjugated secondary antibody against mouse IgG (Pierce) diluted at 1:1000, was negative (data not shown). Arrows indicate the position of recombinant proteins, and the arrowheads proteins present in the aqueous humor. rMyocilin, recombinant human myocilin; rSPARC, recombinant human SPARC; rHevin, recombinant human hevin.
Figure 10.
Gene expression profiling of myocilin, SPARC, and hevin in ocular tissues of a human eye donor. The indicated tissues were dissected, and their RNA was isolated and analyzed on a microarray platform. The relative hybridization signal obtained for each gene was normalized with internal controls and expressed as arbitrary units. AU, arbitrary units.
Discussion
The biological function of myocilin has remained elusive for more than a decade. As an approach to address this question, different groups have attempted to identify proteins that interact with myocilin.
We have previously reported that myocilin interacts with the C-terminal region of hevin,23 a member of the SPARC family of matricellular proteins. Among the members of this family, the C-terminal region of hevin and SPARC share the highest sequence similarity (60.8%). Based on these facts, we reasoned that myocilin may also interact with SPARC. The interaction between the C-terminal regions of the two proteins was initially evidenced by the two-hybrid assay and was further supported by the solid-phase binding assays using purified recombinant proteins and the coexpression in HEK-293T cells. The relative interactions in yeast, estimated by liquid β-galactosidase activity measurements, indicate that the binding of the myocilin C-terminal fragment to the C-terminal fragments of SPARC and hevin apparently increases with respect to the entire myocilin. In contrast, the solid-phase binding assays indicate that full-length myocilin exhibits higher affinities than myocilin-Ct in the interaction with SPARC or hevin. This discrepancy can be explained if we bear in mind that there is not direct correlation between β-galactosidase activity and the Kd of an interaction.38 In fact, in the yeast two-hybrid system, the tested interactions possibly depend on factors such as the orientation of the fused protein and either the GAL-4 activation or the binding domains, the reporter gene used,38 or the expression levels of the two-hybrid proteins.39
The minimal C-terminal SPARC fragment that interacted with myocilin contained the EC domain, thus revealing that it is involved in the interaction with the olfactomedin domain of myocilin. It is also worth noting that the C-terminal fragment of myocilin used in all binding assays coincides with that resulting after the proteolytic processing of the protein by calpain II.12,14 Therefore, the differential binding properties of full-length myocilin and its C-terminal fragment suggest that the specific cleavage of myocilin might regulate its interaction with the matricellular proteins SPARC and hevin, as well as with the ECM proteins fibronectin and laminin, as has been reported for myocilin self-aggregation.16
The typical saturable hyperbolic curves of the interaction of myocilin with the two versions of both SPARC and hevin, as well as with fibronectin and laminin, suggest the absence of cooperativity. This is in contrast with the sigmoidal binding of the myocilin C-terminal fragment to the same proteins, indicating the possible existence of more than one binding site and positive cooperativity. This type of cooperativity may be due to interactions between the binding sites or to the existence of different binding constants. The C-terminal fragment of myocilin also showed increased maximal binding compared with full-length myocilin. This may be due to its smaller molecular size because it is able to interact with a higher number of binding sites. Overall, these data reveal the interaction of myocilin with SPARC through their C-terminal regions and further support the previously reported interaction with hevin.23 On the other hand, the specific intracellular accumulation of SPARC, which correlates with the amount of myocilin coexpressed in HEK-293T cells, indicates the existence of an interaction between the two recombinant proteins early in the secretory pathway.
Our data also show that myocilin, SPARC, and hevin are coexpressed in different ocular tissues, which is a condition required for the in vivo interaction of these molecules. However, additional experiments are required to demonstrate that the interactions among these molecules actually occur in vivo. In accordance with these results, it has been reported that SPARC is present with myocilin in ocular tissues such as the trabecular meshwork,40 aqueous humor and vitreous,41 nonpigmented epithelial cells,42 and the smooth muscle43 of the ciliary body.
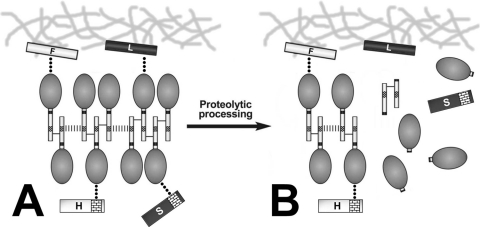
We previously proposed that extracellular myocilin may form a dynamic molecular network composed of disulfide aggregates that are linked in a reversible way by noncovalent interactions through the N-terminal domain of myocilin16 (Fig. 11A). The data presented herein allow the completion of this model. We hypothesize that the molecular myocilin network may also bind noncovalently, through the C-terminal domain of myocilin monomers, with the EC domains of both SPARC and hevin (Fig. 11A). Furthermore, the myocilin network can also interact with fibronectin and laminin in the ECM, acting as a linker between matricellular and ECM proteins. These features resemble those of matricellular proteins, a functional group of nonhomologous and secreted proteins that mediate the interactions between cells and the extracellular matrix.31 Thus, we also speculate that myocilin might function as a putative matricellular protein. This issue has also been discussed previously.44
Figure 11.
Model of the interactions of myocilin with matricellular (SPARC and hevin) and ECM (fibronectin and laminin) proteins. (A) For simplicity only a myocilin dimer, trimer, and tetramer are shown. Myocilin monomers are linked by disulfide bonds (short black lines), and the covalent aggregates interact through noncovalent forces (dashed lines) in the N-terminal domain (rectangle linked to the oval). Full-length myocilin aggregates interact non covalently (dots) through the C-terminal olfactomedin domain (oval) with the EC domains (brick pattern) of the matricellular proteins SPARC (S) and hevin (H). In addition, other molecules of the myocilin network may interact in a similar fashion with the extracellular matrix (ECM) proteins fibronectin (F) and laminin (L). This model suggests that the extracellular and dynamic molecular network of myocilin links the ECM with matricellular proteins such as SPARC and hevin in a reversible fashion. (B) Activation of the intracellular proteolytic processing of myocilin reduces the myocilin network. The released C-terminal fragment interacts with SPARC and hevin with lower affinity than full-length myocilin. The extracellular presence of the N-terminal fragment decreases because it is mainly retained intracellularly.12 Modified with permission from Aroca-Aguilar JD, Martínez-Redondo F, Sánchez-Sánchez F, Coca-Prados M, Escribano J. Functional role of proteolytic processing of recombinant myocilin in self-aggregation. Invest Ophthalmol Vis Sci. 2010;51:72–78.
There are interesting functional parallelisms between myocilin, SPARC, and hevin related to cell adhesion and proteolytic processing. Along these lines, hevin and SPARC exhibit a strong antiadhesive activity toward attachment of endothelial cells to fibronectin in vitro,24 as well as antispreading and focal adhesion-labilizing activity.45,46 Similarly, myocilin blocks the adhesion of cultured TM cells onto fibronectin and induces the loss of actin stress fibers and focal adhesions,47,48 but it promotes substrate adhesion, spreading, and formation of focal contacts in podocytes and mesangial cells.44 Regarding the proteolytic processing of these proteins, a 29 kDa N-terminal SPARC fragment has been detected in both human and bovine ocular tissues, including the ciliary body and aqueous humor.41 In addition, hevin is present in different mouse tissues, including the eye, as a 55-kDa fragment that probably contains the C-terminal FS-EC domains.37,49 Finally, a C-terminal hevin peptide has also been detected in Engelbreth-Holm-Swarm murine sarcoma cells,50 which may also occur for tissue-derived SPARC.51,52 Altogether, these data suggest that the three proteins may play coordinated roles in cell adhesion and that proteolytic processing may be a common theme that possibly regulates their interactions and, therefore, their biological activities. Further work is required to confirm these hypotheses.
In short, we show that myocilin interacts with SPARC and hevin, and we provide further support for the functional role of the proteolytic processing of myocilin in regulating its molecular interactions. Furthermore, our data highlight the function of myocilin as an extracellular protein that may link ECM molecules such as fibronectin and laminin with matricellular proteins (e.g., SPARC and hevin), opening the way for future investigations.
Footnotes
This work was presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2010.
Supported in part by research grants from the Spanish Ministry of Science and Innovation, the Regional Ministry of Health and the Regional Ministry of Science and Technology of the Board of the Communities of Castilla–La Mancha and Instituto de Salud Carlos III (SAF2008-02228, GCS-2006_C/12, PAI-05-002, and PCI08-0036; and RD07/0062/0014; JE); National Institutes of Health Grant EY00785 for core facilities; Research to Prevent Blindness; and the Fundación María Cristina Masaveu Paterson. MC-P is Catedrático Rafael del Pino en la Fundación de Investigación Oftalmológica, Instituto Oftalmológico Fernández-Vega, Oviedo, Spain.
Disclosure: J.-D. Aroca-Aguilar, None; F. Sánchez-Sánchez, None; S. Ghosh, None; A. Fernández-Navarro, None; M. Coca-Prados, None; J. Escribano, None
References
- 1. Escribano J, Ortego J, Coca-Prados M. Isolation and characterization of cell-specific cDNA clones from a subtractive library of the ocular ciliary body of a single normal human donor: transcription and synthesis of plasma proteins. J Biochem (Tokyo). 1995;118:921–931 [DOI] [PubMed] [Google Scholar]
- 2. Kubota R, Noda S, Wang Y, et al. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: molecular cloning, tissue expression, and chromosomal mapping. Genomics. 1997;41:360–369 [DOI] [PubMed] [Google Scholar]
- 3. Ortego J, Escribano J, Coca-Prados M. Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Lett. 1997;413:349–353 [DOI] [PubMed] [Google Scholar]
- 4. Huang W, Jaroszewski J, Ortego J, Escribano J, Coca-Prados M. Expression of the TIGR gene in the iris, ciliary body, and trabecular meshwork of the human eye. Ophthalmic Genet. 2000;21:155–169 [PubMed] [Google Scholar]
- 5. Karali A, Russell P, Stefani FH, Tamm ER. Localization of myocilin/trabecular meshwork–inducible glucocorticoid response protein in the human eye. Invest Ophthalmol Vis Sci. 2000;41:729–740 [PubMed] [Google Scholar]
- 6. Fautsch MP, Johnson DH. Characterization of myocilin-myocilin interactions. Invest Ophthalmol Vis Sci. 2001;42:2324–2331 [PubMed] [Google Scholar]
- 7. Fautsch MP, Vrabel AM, Peterson SL, Johnson DH. In vitro and in vivo characterization of disulfide bond use in myocilin complex formation. Mol Vis. 2004;10:417–425 [PubMed] [Google Scholar]
- 8. Russell P, Tamm ER, Grehn FJ, Picht G, Johnson M. The presence and properties of myocilin in the aqueous humor. Invest Ophthalmol Vis Sci. 2001;42:983–986 [PubMed] [Google Scholar]
- 9. Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670 [DOI] [PubMed] [Google Scholar]
- 10. Fingert JH, Heon E, Liebmann JM, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905 [DOI] [PubMed] [Google Scholar]
- 11. Campos-Mollo E, Sanchez-Sanchez F, Lopez-Garrido MP, Lopez-Sanchez E, Lopez-Martinez F, Escribano J. MYOC gene mutations in Spanish patients with autosomal dominant primary open-angle glaucoma: a founder effect in southeast Spain. Mol Vis. 2007;13:1666–1673 [PubMed] [Google Scholar]
- 12. Sanchez-Sanchez F, Martinez-Redondo F, Aroca-Aguilar JD, Coca-Prados M, Escribano J. Characterization of the intracellular proteolytic cleavage of myocilin and identification of calpain II as a myocilin-processing protease. J Biol Chem. 2007;282:27810–27824 [DOI] [PubMed] [Google Scholar]
- 13. Coca-Prados M, Escribano J. New perspectives in aqueous humor secretion and in glaucoma: the ciliary body as a multifunctional neuroendocrine gland. Prog Retin Eye Res. 2007;26:239–262 [DOI] [PubMed] [Google Scholar]
- 14. Aroca-Aguilar JD, Sanchez-Sanchez F, Ghosh S, Coca-Prados M, Escribano J. Myocilin mutations causing glaucoma inhibit the intracellular endoproteolytic cleavage of myocilin between amino acids Arg226 and Ile227. J Biol Chem. 2005;280:21043–21051 [DOI] [PubMed] [Google Scholar]
- 15. Ezzat MK, Howell KG, Bahler CK, et al. Characterization of monoclonal antibodies against the glaucoma-associated protein myocilin. Exp Eye Res. 2008;87:376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aroca-Aguilar JD, Martinez-Redondo F, Sanchez-Sanchez F, Coca-Prados M, Escribano J. Functional role of proteolytic processing of recombinant myocilin in self-aggregation. Invest Ophthalmol Vis Sci. 2010;51:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Y, Grinchuk O, Tomarev SI. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1932–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joe MK, Sohn S, Choi YR, Park H, Kee C. Identification of flotillin-1 as a protein interacting with myocilin: implications for the pathogenesis of primary open-angle glaucoma. Biochem Biophys Res Commun. 2005;336:1201–1206 [DOI] [PubMed] [Google Scholar]
- 19. Filla MS, Liu X, Nguyen TD, et al. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest Ophthalmol Vis Sci. 2002;43:151–161 [PubMed] [Google Scholar]
- 20. Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43:1068–1076 [PubMed] [Google Scholar]
- 21. Fautsch MP, Vrabel AM, Johnson DH. The identification of myocilin-associated proteins in the human trabecular meshwork. Exp Eye Res. 2006;82:1046–1052 [DOI] [PubMed] [Google Scholar]
- 22. Torrado M, Trivedi R, Zinovieva R, Karavanova I, Tomarev SI. Optimedin: a novel olfactomedin-related protein that interacts with myocilin. Hum Mol Genet. 2002;11:1291–1301 [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Aroca-Aguilar JD, Ghosh S, Sanchez-Sanchez F, Escribano J, Coca-Prados M. Interaction of myocilin with the C-terminal region of hevin. Biochem Biophys Res Commun. 2006;339:797–804 [DOI] [PubMed] [Google Scholar]
- 24. Girard JP, Springer TA. Modulation of endothelial cell adhesion by hevin, an acidic protein associated with high endothelial venules. J Biol Chem. 1996;271:4511–4517 [DOI] [PubMed] [Google Scholar]
- 25. Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827 [DOI] [PubMed] [Google Scholar]
- 26. Vannahme C, Smyth N, Miosge N, et al. Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J Biol Chem. 2002;277:37977–37986 [DOI] [PubMed] [Google Scholar]
- 27. Vannahme C, Gosling S, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem J. 2003;373:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–173 [PubMed] [Google Scholar]
- 29. Yan Q, Sage EH. SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem. 1999;47:1495–1506 [DOI] [PubMed] [Google Scholar]
- 30. Motamed K, Sage EH. SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J Cell Biochem. 1998;70:543–552 [DOI] [PubMed] [Google Scholar]
- 31. Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616 [DOI] [PubMed] [Google Scholar]
- 32. Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Switzer RC, III, Merril CR, Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979;98:231–237 [DOI] [PubMed] [Google Scholar]
- 34. Aroca-Aguilar JD, Sanchez-Sanchez F, Martinez-Redondo F, Coca-Prados M, Escribano J. Heterozygous expression of myocilin glaucoma mutants increases secretion of the mutant forms and reduces extracellular processed myocilin. Mol Vis. 2008;14:2097–2108 [PMC free article] [PubMed] [Google Scholar]
- 35. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254 [DOI] [PubMed] [Google Scholar]
- 36. Ghosh S, Freitag AC, Martin-Vasallo P, Coca-Prados M. Cellular distribution and differential gene expression of the three alpha subunit isoforms of the Na,K-ATPase in the ocular ciliary epithelium. J Biol Chem. 1990;265:2935–2940 [PubMed] [Google Scholar]
- 37. Hambrock HO, Nitsche DP, Hansen U, et al. SC1/hevin: an extracellular calcium-modulated protein that binds collagen I. J Biol Chem. 2003;278:11351–11358 [DOI] [PubMed] [Google Scholar]
- 38. Estojak J, Brent R, Golemis EA. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995;15:5820–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu J, Kahn CR. Analysis of a peptide hormone-receptor interaction in the yeast two-hybrid system. Proc Natl Acad Sci U S A. 1997;94:13063–13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rhee DJ, Haddadin RI, Kang MH, Oh DJ. Matricellular proteins in the trabecular meshwork. Exp Eye Res. 2009;88:694–703 [DOI] [PubMed] [Google Scholar]
- 41. Yan Q, Clark JI, Sage EH. Expression and characterization of SPARC in human lens and in the aqueous and vitreous humors. Exp Eye Res. 2000;71:81–90 [DOI] [PubMed] [Google Scholar]
- 42. Gilbert RE, Cox AJ, Kelly DJ, et al. Localization of secreted protein acidic and rich in cysteine (SPARC) expression in the rat eye. Connect Tissue Res. 1999;40:295–303 [DOI] [PubMed] [Google Scholar]
- 43. Rhee DJ, Fariss RN, Brekken R, Sage EH, Russell P. The matricellular protein SPARC is expressed in human trabecular meshwork. Exp Eye Res. 2003;77:601–607 [DOI] [PubMed] [Google Scholar]
- 44. Goldwich A, Scholz M, Tamm ER. Myocilin promotes substrate adhesion, spreading and formation of focal contacts in podocytes and mesangial cells. Histochem Cell Biol. 2009;131:167–180 [DOI] [PubMed] [Google Scholar]
- 45. Lane TF, Sage EH. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990;111:3065–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murphy-Ullrich JE, Lane TF, Pallero MA, Sage EH. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand. J Cell Biochem. 1995;57:341–350 [DOI] [PubMed] [Google Scholar]
- 47. Wentz-Hunter K, Kubota R, Shen X, Yue BY. Extracellular myocilin affects activity of human trabecular meshwork cells. J Cell Physiol. 2004;200:45–52 [DOI] [PubMed] [Google Scholar]
- 48. Peters DM, Herbert K, Biddick B, Peterson JA. Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp Cell Res. 2005;303:218–228 [DOI] [PubMed] [Google Scholar]
- 49. Yao HY, Cheng CY, Fan BJ, et al. [Polymorphisms of myocilin and optineurin in primary open angle glaucoma patients]. Zhonghua Yi Xue Za Zhi. 2006;86:554–559 [PubMed] [Google Scholar]
- 50. Mann K, Deutzmann R, Paulsson M, Timpl R. Solubilization of protein BM-40 from a basement membrane tumor with chelating agents and evidence for its identity with osteonectin and SPARC. FEBS Lett. 1987;218:167–172 [DOI] [PubMed] [Google Scholar]
- 51. Tyree B. The partial degradation of osteonectin by a bone-derived metalloprotease enhances binding to type I collagen. J Bone Miner Res. 1989;4:877–883 [DOI] [PubMed] [Google Scholar]
- 52. Otsuka K, Yao KL, Wasi S, et al. Biosynthesis of osteonectin by fetal porcine calvarial cells in vitro. J Biol Chem. 1984;259:9805–9812 [PubMed] [Google Scholar]
- 53. Thompson JD, Higgins DG, Gibson TJ. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]