Treatments that alter peripheral defocus are being considered for slowing the progression of myopia. New longitudinal data from the CLEERE Study show that peripheral defocus has little effect on children, suggesting that new treatments that change peripheral defocus may not be effective in the long-term against myopia.
Abstract
Purpose.
To investigate whether relative peripheral hyperopia is a risk factor for either the onset of myopia in children or the rate of myopic progression.
Methods.
The risk of myopia onset was assessed in 2043 nonmyopic third-grade children (mean age ± SD = 8.8 ± 0.52 years) participating in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study between 1995 and 2007, 324 of whom became myopic by the eighth grade. Progression analyses used data from 774 myopic children in grades 1 to 8. Foveal and relative peripheral refractive error 30° in the nasal visual field was measured annually by using cycloplegic autorefraction. Axial length was measured by A-scan ultrasonography.
Results.
The association between more hyperopic relative peripheral refractive error in the third grade and the risk of the onset of myopia by the eighth grade varied by ethnic group (Asian children odds ratio [OR] = 1.56, 95% confidence interval [CI] = 1.06–2.30; African-American children OR = 0.75, 95% CI = 0.58–0.96; Hispanics, Native Americans, and whites showed no significant association). Myopia progression was greater per diopter of more hyperopic relative peripheral refractive error, but only by a small amount (−0.024 D per year; P = 0.02). Axial elongation was unrelated to the average relative peripheral refractive error (P = 0.77), regardless of ethnicity.
Conclusions.
Relative peripheral hyperopia appears to exert little consistent influence on the risk of the onset of myopic refractive error, on the rate of myopia progression, or on axial elongation.
Numerous experiments show that chronic exposure to lenses simulating hyperopic refractive error accelerates axial growth in a predictable manner in various species, suggesting that defocus at the fovea influences eye growth.1–5 Greater amounts of accommodative lag from a deficient accommodative response is the putative analogous source of hyperopic defocus during near work in children.6–8 Several studies have shown that myopic children or those undergoing more rapid myopic progression spend more time in near work than nonmyopes or more slowly progressing myopes,9–13 yet the association between refractive error and near work is not always statistically significant.14–17 In addition, the potency of accommodative lag in stimulating human ocular growth is not clear. Hyperopic defocus is less related to the rate of emmetropization in infancy than is the level of accommodative effort expended by hyperopic infants.18 In children, many attempts to reduce accommodative lag through plus lens corrections at near have had only modest results in slowing myopic progression.12,19–23 However, other studies and subsamples of myopic children—for example, esophores at near with high accommodative lag—have shown a larger treatment effect of plus at near.24–26 Whether a high accommodative lag increases the risk of onset of myopia in nonmyopic children is also unclear. One study found increased accommodative lag 2 years before the onset of myopia and in the year of onset.7 Our own larger investigation showed no increase in accommodative lag before myopia onset in children who became myopic compared with children who were emmetropic.8
Recent investigations of the effects of hyperopic defocus on ocular growth have shifted their attention away from measures at the fovea and moved toward the retinal periphery. Ablation and occlusion experiments in primates have shown that manipulation of the more extensive peripheral visual environment can guide not only peripheral ocular growth, but also axial growth, suggesting that perhaps peripheral hyperopic defocus can act as a guide for foveal refractive error.27,28 Myopes have relative peripheral hyperopia more often than other refractive error groups, at least in the lateral visual field, because of their relatively less oblate ocular shape.29–35 This exposure to peripheral hyperopic defocus appears about 2 years before myopia onset, on average.36 The degree of risk for myopia onset from peripheral hyperopia has not yet been quantified, nor has it been adjusted for foveal refractive error or ethnicity. The purpose of this analysis was to use longitudinal data from the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study to evaluate the following hypotheses: (1) nonmyopic children with more relative peripheral hyperopia are at higher risk for becoming myopic, and (2) myopic children with more relative peripheral hyperopia have a faster rate of myopic progression.
Methods
Methods have been described in detail elsewhere.36 In brief, subjects were participants between 1995 and 2007 in the CLEERE Study, a cohort study of ocular component development and risk factors for the onset of myopia in children of various ethnic backgrounds. Each affiliated university's institutional review board (University of Alabama at Birmingham; University of California, Berkeley; University of Houston; The Ohio State University; Southern California College of Optometry, and the University of Arizona Department of Ophthalmology and Vision Science) approved the protocol and informed consent documents according to the tenets of the Declaration of Helsinki. Parents provided consent and children assent before the children were examined. Ethnic group was designated by a parent selecting from one of the following six ethnic designations: (corresponding to the categories used by the National Institutes of Health as of 1997 when ethnic data were first gathered): American Indian or Alaskan Native; Asian or Pacific Islander; black, not of Hispanic origin; Hispanic; white, not of Hispanic origin; other, or unknown. Any missing parent-reported ethnicity was filled in from investigator observation (2% of subjects). Investigator observation in the CLEERE study has been shown to have excellent agreement with parent-reported ethnicity.37
Trained and certified examiners measured central refractive error and peripheral refraction on the right eye of subjects with two validated autorefractors,38,39 (model R-1; Canon USA., Lake Success, NY; [no longer manufactured], used between 1989 and 2000, and model WR 5100-K; Grand Seiko Co., Hiroshima, Japan, used between 2001 and 2007). The subjects were tested after mydriasis and cycloplegia. When they had an iris color of grade 1 or 2,40 testing was done 30 minutes after 1 drop of proparacaine 0.5% and 2 drops of tropicamide 1%. When they had iris color darker than grade 2, testing was done 30 minutes after 1 drop of proparacaine 0.5% and 1 drop each of tropicamide 1% and cyclopentolate 1%.41 The subjects first fixated a reduced Snellen target through a +4.00-D Badal lens in primary gaze. Ten autorefractor measurements were made according to the standard CLEERE protocol for cycloplegic autorefraction.42 Immediately after measurement in primary gaze, the track holding the Snellen target was rotated 30° and placed before a front surface mirror on the patient's right. Five autorefraction measurements were then taken in peripheral gaze. Measurements of refractive error in peripheral gaze were obtained at all study visits beginning in 1995. Relative peripheral refractive error was calculated as the spherical equivalent of the average refraction in primary gaze subtracted from the spherical equivalent of the average refractive error in 30° temporal gaze (i.e., the autorefractor axis directed 30° in the nasal visual field of the subject's right eye). Axial ocular dimensions were measured by A-scan ultrasonography (model 820; Humphrey Instruments, Carl Zeiss Meditec, Inc., San Leandro, CA), consisting of five readings with a handheld probe on semiautomatic mode.
Risk of Myopia Onset Analysis
Subjects were 2043 nonmyopic third-grade children with a measurement of relative peripheral refractive error during that grade who returned for at least one subsequent follow-up visit. The mean age at the third grade visit ± SD was 8.8 ± 0.52 years. Of these subjects, 324 became myopic (−0.75 D or more myopic in each principal meridian) during follow-up between the third and the eighth grades. The distributions according to ethnicity and sex for the sample are shown in Table 1. The risk factors of interest were foveal refractive error and relative peripheral refractive (RPR) error. The spherical component of foveal refractive error (minus cylinder sign convention) of the 2043 nonmyopic children was analyzed as a continuous variable. Previous analysis has shown that this variable is the best single predictor of the onset of myopia by the eighth grade with an area under the receiver operating characteristic curve of 0.875.43 The relative peripheral refractive error of nonmyopic children was analyzed in two ways: (1) as a continuous variable and (2) dichotomized into two groups: one with relative peripheral hyperopia of any amount (i.e., RPR > 0.0 D, n = 661) and one without relative peripheral hyperopia (n = 1382). The mean ± SD values for foveal refractive error and RPR by sex and ethnicity are shown in Tables 1 and 2.
Table 1.
Foveal Refractive Error at Baseline by Sex and Ethnicity of the 2043 Nonmyopic Third-Grade Children in the Risk Analysis
| n (%) | P | Mean | SD | P | |
|---|---|---|---|---|---|
| Male | 986 (48.3) | 0.12 | +1.05 | 0.97 | 0.79 |
| Female | 1057 (51.7) | +1.06 | 0.96 | ||
| Native American | 296 (14.5) | +1.13 | 1.40 | ||
| Asian | 228 (11.2) | +0.87 | 0.67 | ||
| African American | 381 (18.6) | <0.0001 | +1.04 | 0.95 | <0.0001 |
| Hispanic | 484 (23.7) | +0.91 | 0.99 | ||
| White | 639 (31.3) | +1.19 | 0.74 | ||
| Other | 15 (0.7) | +1.57 | 1.59 |
Table 2.
Relative Peripheral Refractive Error and Peripheral Cylinder at Baseline by Sex and Ethnicity of the 2043 Nonmyopic Third-Grade Children in the Risk Analysis
| Mean RPR ± SD | P | Mean Peripheral Cylinder ± SD | P | > +0.00 D n (%) | P | |
|---|---|---|---|---|---|---|
| Male | −0.45 ± 0.97 | 0.20 | −2.47 ± 1.18 | 0.56 | 293 (30) | 0.014 |
| Female | −0.40 ± 1.00 | −2.50 ± 1.21 | 368 (35) | |||
| Native American | −0.53 ± 1.24 | −1.93 ± 0.79 | 105 (35) | |||
| Asian | −0.31 ± 0.86 | −2.62 ± 1.13 | 93 (41) | |||
| African American | −0.36 ± 1.09 | <0.0001 | −2.36 ± 1.14 | <0.0001 | 124 (33) | 0.0005 |
| Hispanic | −0.24 ± 0.87 | −2.42 ± 1.00 | 181 (37) | |||
| White | −0.60 ± 0.89 | −2.83 ± 1.42 | 156 (24) | |||
| Other | −0.73 ± 0.76 | −2.45 ± 0.99 | 2 (13) |
Numbers of subjects with a hyperopic RPR > +0.00 D are also given by sex and ethnicity.
The risk of myopia onset in nonmyopic children was analyzed as a function of foveal refractive error and RPR with a discrete time survival hazard model. Sex, ethnicity, and study site were covariates in the analysis. Life tables of hazard and survival probabilities were calculated. The hazard probability is the probability that a child will become myopic in a particular grade, given that he or she was not myopic before that grade. The survival probability is the probability that a child will remain nonmyopic up to and including a given grade. The chance that a child will become myopic before that grade equals the quantity (1 − the survival probability).
Association with Progression Analysis
Analysis of the association between foveal myopic progression and RPR was conducted using 774 myopic children (myopia of −0.75 D or more in each principal meridian) with at least one measurement of RPR at the beginning of a 1-year progression interval. The number of subjects by years of follow-up can be found in Table 3. The association between foveal myopic progression and RPR was analyzed by using multilevel modeling.44 This approach is a generalization of linear modeling that handles clustered, repeated measures within subjects. RPR was analyzed for its association with myopia progression in two separate models. Myopia progression over all visits was first modeled as a function of each subject's average RPR, and then myopia progression in each year of follow-up was modeled as a function of RPR at the beginning of that year. For each model, covariates included age, sex, study site, and ethnicity. All models were fitted with the MIXED procedure (SAS, ver. 9.2; SAS Institute, Cary, NC). An analysis was also performed regarding the consistency of any effects for RPR on myopia progression as a function of time since the onset of myopia. A significant interaction between years since myopia onset and RPR would indicate that effects varied with time since onset. Covariates in this analysis included years since myopia onset, age of myopia onset, sex, study site, and ethnicity. The sample size for this interaction analysis was lower (n = 487) because those with preexisting myopia were excluded from this analysis. Similar analyses to the two above were also done with axial length as the dependent variable.
Table 3.
The Number of Subjects by Years of Follow-up
| Years of Follow-up | n |
|---|---|
| 1 | 210 |
| 2 | 206 |
| 3 | 158 |
| 4 | 102 |
| 5 | 61 |
| 6 | 27 |
| 7 | 9 |
| 8 | 1 |
| Total | 774 |
Results
Risk of Onset
The average foveal refractive error in the third grade was slightly hyperopic (+1.05 ± 0.97 D) and the average RPR was slightly myopic (−0.43 ± 0.99 D). The average foveal refractive error and RPR varied by ethnicity but not by sex (Tables 1 and 2, ANOVA P < 0.0001 for ethnicity and P > 0.20 for sex). Whites and Native Americans had a more hyperopic average foveal refractive error and a more myopic average RPR than the other major ethnic groups. Asians and Hispanics had a less hyperopic foveal refractive error, while Asians, Hispanics, and African Americans had a less myopic RPR than whites and Native Americans. Each ethnic group had between 24% and 41% of children in the group with relative peripheral hyperopia >+0.00 D (Table 2). The group with relative peripheral hyperopia also contained a slightly higher percentage of girls (35% compared with 30%, P = 0.014; Table 2).
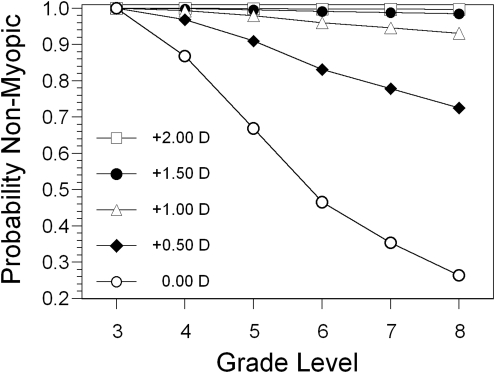
When the sample was analyzed as a whole across all ethnicities, the hazard probability odds ratio (OR) for the onset of myopia for foveal refractive error was 0.044 (95% confidence interval [CI] = 0.032–0.060), indicating a strong protective effect against myopia onset for each diopter of hyperopic refractive error (Table 4). In contrast, the OR for RPR as a continuous variable was not significant (0.89, 95% CI = 0.77–1.02). Table 4 also provides an analysis of RPR as a dichotomous variable (RPR > 0.0 D and RPR ≤ 0.0 D) in models with sex, study site, and ethnicity. Foveal refractive error was kept as a continuous variable in this analysis, because there was a significant negative correlation between foveal refractive error and RPR. As expected, less foveal hyperopia was associated with more peripheral hyperopia (r = −0.45, P < 0.0001). This association resulted in foveal refractive error being approximately 0.50 D less hyperopic when RPR was hyperopic (foveal refractive error = +0.72 D) than when RPR was not hyperopic (foveal refractive error = +1.21 D; P < 0.0001). When adjusted for foveal refractive error, dichotomized relative peripheral refractive error > +0.00 D was not a significant risk factor for the onset of myopia (hazard probability OR = 0.95, 95% CI = 0.73–1.23). The hazard probability OR for foveal refractive error remained statistically significant and nearly identical with its value when RPR was considered as a continuous variable (OR = 0.046, 95% CI = 0.034–0.062). The probabilities that a child would remain nonmyopic by a given grade, assuming various levels of foveal refractive error in the third grade, are shown in Figure 1.
Table 4.
Hazard Probability ORs for the Risk of Onset of Myopia
| Variable | OR (95% CI) | P |
|---|---|---|
| Foveal refractive error | 0.044 (0.032–0.060) | <0.0001 |
| RPR (continuous) | 0.89 (0.77–1.02) | 0.10 |
| Foveal refractive error | 0.046 (0.034–0.062) | <0.0001 |
| RPR (>0.00 D) | 0.95 (0.73–1.23) | 0.69 |
RPR is treated either as a continuous variable or categorical (>0.00 D compared with ≤0.00 D as the reference group), as indicated. Models included foveal refraction and RPR as well as sex, ethnicity, and study site.
Figure 1.
The probability of remaining nonmyopic as a function of grade, given various levels of foveal refractive error in the third grade.
There were no significant two-way interactions between foveal refractive error, site, and sex. However, there was a statistically significant interaction between ethnicity and foveal refractive error (P for interaction = 0.030). Having more hyperopia at baseline was protective against the onset of myopia in all ethnic groups, but less so (i.e., ORs for foveal refractive error were slightly closer to 1.0) for Asians and more so for Native Americans. There was also a significant interaction between RPR (as a continuous variable) and ethnicity (P for interaction = 0.016). Each diopter of hyperopic RPR conferred a greater risk of myopia onset in Asian children, a lower risk in African American children, and no significant increase in Hispanic, Native American, or white children. There was no significant interaction between RPR as a categorical variable and ethnicity. Table 5 provides estimates of the ORs by ethnicity associated with a unit increase in foveal or RPR error.
Table 5.
ORs for the Onset of Myopia Associated with Unit Increases in Foveal Refractive Error and RPR as Continuous Variables by Ethnic Group
| Ethnic Group | OR (95% CI) | P |
|---|---|---|
| Foveal Refractive Error | ||
| Native American | 0.017 (0.006–0.052) | <0.0001 |
| Asian | 0.088 (0.048–0.16) | <0.0001 |
| African American | 0.037 (0.018–0.074) | <0.0001 |
| Hispanic | 0.058 (0.035–0.097) | <0.0001 |
| White | 0.028 (0.014–0.056) | <0.0001 |
| Relative Peripheral Refractive Error | ||
| Native American | 0.67 (0.42–1.05) | 0.082 |
| Asian | 1.56 (1.06–2.30) | 0.023 |
| African American | 0.75 (0.58–0.96) | 0.023 |
| Hispanic | 1.01 (0.72–1.42) | 0.95 |
| White | 0.88 (0.64–1.20) | 0.42 |
Models included foveal refraction and RPR as well as sex, ethnicity, and study site.
Association with Progression
The average annual rate of myopic progression for the sample as a whole was −0.38 ± 0.33 D per year. Progression of myopia was the dependent variable in multilevel modeling as a function of RPR, adjusted for age, sex, study site, and ethnicity. Selected coefficients for the models are shown in Table 6 (a negative sign indicates that the factor is associated with greater myopic progression). Girls progressed faster than boys by −0.090 D per year (P < 0.0001). Each year of increased age slowed progression by 0.033 D per year (P = 0.034). The more hyperopic an individual's average RPR, the greater the rate of myopic progression, but the magnitude of the association was very small, only −0.024 D of greater progression per year per diopter of hyperopic RPR (P = 0.020). RPR at the start of a 1-year interval had no effect on the rate of progression (P = 0.19). This effect was consistent across all ethnic groups. These effects of RPR were also consistent across years since myopia onset, as there was no significant interaction between time since myopia onset and average RPR (P for interaction = 0.70).
Table 6.
Estimates for Selected Coefficients in the Regression between Myopia Progression and Relative Peripheral Refraction, Age, and Sex
| Variable | Change in Foveal Refractive Error |
Change in AL |
||
|---|---|---|---|---|
| Coefficient | P | Coefficient | P | |
| Average RPR | −0.024 | 0.020 | 0.0015 | 0.77 |
| RPR at interval start | 0.012 | 0.19 | 0.0058 | 0.22 |
| Age | 0.033 | 0.034 | 0.046 | <0.0001 |
| Sex | −0.090 | <0.0001 | −0.024 | 0.030 |
Average RPR and RPR at the start of an interval were predictors in separate models. Coefficients for age and sex are from the model with average RPR. A negative sign indicates association with greater myopia progression or faster axial elongation. AL, axial length.
The average increase in axial length for the myopic sample as a whole was 0.20 ± 0.20 mm. Change in axial length was also considered the dependent variable in multilevel modeling as a function of relative peripheral refractive error, adjusted for age, sex, study site, and ethnicity. The coefficients for this model are also shown in Table 6. A negative sign indicates that the factor was associated with a greater increase in axial length. Axial elongation was more rapid in girls than in boys by 0.024 mm (P = 0.030). One year's increase in age slowed axial elongation by 0.046 mm per year (P < 0.0001). Subjects' average RPR was not related to the rate of axial elongation (P = 0.77) nor was a more hyperopic RPR at the start of a 1-year interval (P = 0.22). As with progression of myopia, this lack of effect of RPR on axial elongation was consistent across all ethnic groups and years since myopia onset.
Planes of focus other than the relative peripheral spherical equivalent were also considered—namely, the peripheral most hyperopic meridian and the peripheral least hyperopic meridian, as well as the amount of peripheral cylinder. The two meridians were each considered relative to the central spherical equivalent. Neither meridian had any significant effect on the risk of myopia onset, either as a categorical or as a continuous variable (all P > 0.10). Peripheral cylinder was also not related to the risk of onset (P = 0.66). Each meridian and the amount of peripheral cylinder had significant effects on the rate of myopia progression, but the results were as inconsistent as those reported for RPR. More hyperopia in the most hyperopic meridian at the beginning of an interval was associated with less myopia progression by 0.02 D per year (P = 0.007), but the relative refraction in the most hyperopic meridian averaged over all intervals was not associated with myopia progression (P = 0.32). More hyperopia in the least hyperopic meridian at the beginning of an interval was not associated with myopia progression (P = 0.79), but more hyperopia in the least hyperopic meridian averaged over all intervals was associated with more myopia progression by 0.03 D per year (P = 0.002). Less peripheral cylinder, whether at the beginning of an interval or averaged over all intervals, was associated with more myopia progression, but only by 0.03 D per year (P = 0.003). These analyses suggest that peripheral defocus exerts little consistent influence on the risk of developing myopia or its rate of progression.
Discussion
In this longitudinal study of a multiethnic cohort of school-age children, we found a less hyperopic foveal refractive error in the third grade to be a significant risk factor for the onset of myopia by the eighth grade, as we reported in the mostly white Orinda subset of the current cohort.43 Interestingly, this risk varied by ethnic group. Asian children had the least reduction in risk of myopia onset from a more hyperopic sphere and Native American children the greatest reduction in risk. Adjusted for foveal refractive error, RPR was not a significant risk factor for the onset of myopia in the cohort as a whole. The interaction analysis suggested the lack of effect overall was the result of Asian children having a significantly higher risk of onset, African-American children a lower risk of onset, and Hispanics, Native Americans, and whites having no effect from a more hyperopic RPR. Asian children were exposed to a more hyperopic RPR before the onset of myopia more often than other ethnic groups (Table 2).36 In contrast, African-American children were the only ethnic group where the average relative peripheral refractive error 3 or fewer years before the onset of their myopia was less hyperopic than that of children who remained emmetropic.36 Interpreting this interaction between RPR and ethnicity as “causative” in one group and “protective” in another is difficult when theory suggests the that the RPR's effect should be consistent with respect to the sign of defocus. There was no ethnicity interaction when RPR was dichotomized, nor was there an effect of ethnicity on the very small association between RPR and myopia progression. The more conservative interpretation of CLEERE results may be that the effects of ethnicity are more idiosyncratic than causative with respect to RPR and myopia.
Only one peripheral point was assessed in CLEERE. This limitation must be kept in mind when considering that exposure to peripheral defocus may vary by quadrant. The variation may be great enough to result in an average relative peripheral myopia in the vertical meridian.45 However, the purpose of the analysis was to evaluate whether relative peripheral hyperopia affected the risk of myopia onset or the rate of myopia progression. Selection of the temporal retinal quadrant (nasal field), one with relative peripheral hyperopia, therefore seems a reasonable choice. Relative peripheral refractive errors were also measured without spectacles. Low myopic corrections have little effect on RPR.46 A moderate myopic spectacle correction, −3.00 D or more myopic, has been reported to increase relative peripheral hyperopia by 0.75 D or more, depending on eccentricity and retinal quadrant.46,47 Therefore, spectacle wear would not be expected to affect the progression rate of low myopes, but might be hypothesized to add to the progression rate of moderate to high myopes. The current analysis would suggest that any additional progression from wear of a moderate myopic spectacle correction would be minimal. Another consideration is that the criterion for myopia onset is conservative, −0.75 D in each principal meridian. A less conservative criterion would capture children showing myopic tendencies earlier in development (but may include more future nonmyopes) and a more conservative one would include few false positives but would exclude new cases with low amounts of myopia. We evaluated the effect of two different criteria for myopia onset: any minus spherical equivalent and a −1.25-D spherical equivalent. When myopia onset was any minus spherical equivalent, the P-values for RPR were 0.11 (RPR continuous) and 0.77 (RPR categorical), virtually the same as currently found in Table 4. When the criterion was −1.25-D spherical equivalent, the P-values for RPR were 0.07 and 0.77 (RPR continuous and categorical, respectively). While a P-value of 0.07 approaches significance, the OR was 0.87 (95% CI = 0.74–1.01), in the same protective direction as the value of 0.95 (95% CI = 0.73–1.23) in Table 4 and contrary to the hyperopic defocus theory. The negative results for RPR appear to be robust across criteria for myopia onset.
These data do not suggest a major role for peripheral refractive error in myopia onset or progression in children. The −0.024 D of additional myopia progression per year per diopter of hyperopic RPR would require 10 years to add a measurable change to myopic refractive error that was attributable to RPR. Exposure to a more hyperopic RPR at the start of a 1-year progression interval had no significant effect on the rate of myopia progression. Similarly, RPR appeared to have no significant effect on the rate of axial elongation. This negative result for the periphery is consistent with other results that suggest minimal effects of foveal defocus on refractive error.8,18,23 These results differ from a previous report of an increased risk of myopia onset in a sample of adult pilots when they had more relative peripheral hyperopia (type I skiagram).48 One reason for the discrepancy might be that the previous study was done in adults. Perhaps RPR is a significant risk factor for myopia onset in subjects older than the children in the present study. Another explanation may be that the undefined refractive error grouping in the previous study did not adjust adequately for central refractive error. The risk attributed to RPR by Hoogerheide et al.48 may have been the risk due to foveal refractive error; hyperopic RPR may have been a correlate of less foveal hyperopia.
The established concept of local control, that manipulating the visual environment in one portion of the visual field influences only the refractive state for the corresponding retinal area, could argue against peripheral defocus affecting refractive error at another location such as the fovea.49 However, peripheral refraction is generally fit with monotonic functions,45 indicating that relative peripheral hyperopia is not confined to one location but rather begins adjacent to the fovea and increases with field angle. The 30° peripheral location in CLEERE was chosen more for making the degree of RPR easily detectable than for any presumed effect of that eccentricity. Form deprivation beyond 37° (18.5° eccentricity) and lens-induced defocus beyond 31° (15.5° eccentricity) was sufficient to influence foveal refractions in monkeys.27,50 While the 30° eccentricity in the present study was within this treated range, it is unclear what level of defocus at what retinal eccentricity might influence human foveal refraction. Another possible explanation for our negative result may be the limited magnitude of the peripheral defocus; ±2 SD from values in Table 2 would be on the order of ±2.00 D. Whether this amount is enough to influence peripheral or foveal growth is an open question. Foveal defocus from accommodative lag on the order of a 0.50-D difference from emmetropes has been hypothesized to promote myopia.7,8 The threshold for blur to drive eye growth in the periphery, if one exists, may be greater than what is needed at the fovea. Peripheral form deprivation is effective in influencing foveal refractive error in monkeys,27,51 as is a moderate level of peripheral defocus from annular lenses, on the order of 3 D.50
Center-distance bifocal contact lenses, orthokeratology, or custom-designed spectacles can reduce relative peripheral hyperopia.52–54 The lack of a relationship between RPR and myopia risk suggests that there may be little therapeutic value in doing so. Despite this prediction, results from clinical evaluations of overnight orthokeratology and a specialty spectacle design indicate slower myopic progression compared with conventional corrections.54–56 Alternatively, bifocal contact lenses or orthokeratology may affect myopic progression because of their bifocal effect on accomodation rather than their effect on peripheral defocus. Also, hyperopic RPR may have little influence on the risk of myopia onset or on rates of progression, yet better peripheral image quality could still be beneficial as an inhibitor of ocular growth. If “stop” is different from “go,” then good peripheral image quality may be beneficial even if peripheral defocus is not harmful. Recent animal data show that growth signals such as hyperopic defocus do not sum equally but may be outweighed by stop signals such as a clear retinal image or myopic defocus.4,57 As noted above, it may also be the case that ordinary levels of RPR do not influence foveal refractive error, yet more extreme manipulations may show some benefit. It is not known what the refractive error characteristics of the retinal periphery must be to influence growth: myopic, plano spherical equivalent, or nonastigmatic. Making peripheral defocus myopic seems less likely to be beneficial; the current analysis would have found an association between myopic RPR and less progression if that were the case. In addition, some of the retinal periphery is already relatively myopic on average in the vertical meridian, a finding seen in both myopes and nonmyopes.45 Predictions aside, clinical trials in which the level of peripheral defocus is varied, such as corneal reshaping or bifocal contact lens wear, would provide a valuable perspective on the potential for the retinal periphery to affect the risk of myopia onset or the rate of myopia progression at the fovea.
Footnotes
Supported by NIH/NEI Grants U10-EY08893 and R24-EY014792, the Ohio Lions Eye Research Foundation, and the E. F. Wildermuth Foundation.
Disclosure: D.O. Mutti, None; L.T. Sinnott, None; G.L. Mitchell, None; L.A. Jones-Jordan, None; M.L. Moeschberger, None; S.A. Cotter, None; R.N. Kleinstein, None; R.E. Manny, None; J.D. Twelker, None; K. Zadnik, None
References
- 1. Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error, and eye growth in chickens. Vision Res. 1988;28:639–657 [DOI] [PubMed] [Google Scholar]
- 2. Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435 [DOI] [PubMed] [Google Scholar]
- 3. Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus). Vision Res. 1999;39:189–206 [DOI] [PubMed] [Google Scholar]
- 4. Norton TT, Siegwart JT, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–4837 [DOI] [PubMed] [Google Scholar]
- 6. Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34:690–694 [PubMed] [Google Scholar]
- 7. Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005;82:273–278 [DOI] [PubMed] [Google Scholar]
- 8. Mutti DO, Mitchell GL, Hayes JR, et al. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47:837–846 [DOI] [PubMed] [Google Scholar]
- 9. Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–3640 [PubMed] [Google Scholar]
- 10. Zylbermann R, Landau D, Berson D. The influence of study habits on myopia in Jewish teenagers. J Pediatr Ophthalmol Strabismus. 1993;30:319–322 [DOI] [PubMed] [Google Scholar]
- 11. Hepsen IF, Evereklioglu C, Bayramlar H. The effect of reading and near-work on the development of myopia in emmetropic boys: a prospective, controlled, three-year follow-up study. Vision Res. 2001;41:2511–2520 [DOI] [PubMed] [Google Scholar]
- 12. Pärssinen O, Hemminki E, Klemetti A. Effect of spectacle use and accommodation on myopic progression: final results of a three-year randomised clinical trial among schoolchildren. Br J Ophthalmol. 1989;73:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kinge B, Midelfart A, Jacobsen G, Rystad J. The influence of near-work on development of myopia among university students: a three-year longitudinal study among engineering students in Norway. Acta Ophthalmol Scand. 2000;78:26–29 [DOI] [PubMed] [Google Scholar]
- 14. Saw SM, Tan SB, Fung D, et al. IQ and the association with myopia in children. Invest Ophthalmol Vis Sci. 2004;45:2943–2948 [DOI] [PubMed] [Google Scholar]
- 15. Saw SM, Shankar A, Tan SB, et al. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47:1839–1844 [DOI] [PubMed] [Google Scholar]
- 16. Jones LA, Sinnott LT, Mutti DO, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285 [DOI] [PubMed] [Google Scholar]
- 18. Mutti DO, Mitchell GL, Jones LA, et al. Accommodation, acuity, and their relationship to emmetropization in infants. Optom Vis Sci. 2009;68:666–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grosvenor T, Perrigin DM, Perrigin J, Maslovitz B. Houston Myopia Control Study: a randomized clinical trial. Part II. Final report by the patient care team. Am J Optom Physiol Opt. 1987;64:482–498 [PubMed] [Google Scholar]
- 20. Jensen H. Myopia progression in young school children: a prospective study of myopia progression and the effect of a trial with bifocal lenses and beta blocker eye drops. Acta Opthalmol Suppl. 1991;1–79 [PubMed] [Google Scholar]
- 21. Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77:395–401 [DOI] [PubMed] [Google Scholar]
- 22. Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002;43:2852–2858 [PubMed] [Google Scholar]
- 23. Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500 [DOI] [PubMed] [Google Scholar]
- 24. Oakley KH, Young FA. Bifocal control of myopia. Am J Optom Physiol Opt. 1975;52:758–764 [DOI] [PubMed] [Google Scholar]
- 25. Leung JT, Brown B. Progression of myopia in Hong Kong Chinese schoolchildren is slowed by wearing progressive lenses. Optom Vis Sci. 1999;76:346–354 [DOI] [PubMed] [Google Scholar]
- 26. Gwiazda JE, Hyman L, Norton TT, et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci. 2004;45:2143–2151 [DOI] [PubMed] [Google Scholar]
- 27. Smith EL, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith EL, Ramamirtham R, Qiao-Grider Y, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48:3914–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferree CE, Rand G, Hardy C. Refraction for the peripheral field of vision. Arch Ophthalmol. 1931;5:717–731 [Google Scholar]
- 30. Rempt F, Hoogerheide J, Hoogenboom W. Peripheral retinoscopy and the skiagram. Ophthalmologica. 1971;162:1–10 [DOI] [PubMed] [Google Scholar]
- 31. Millodot M. Effect of ametropia on peripheral refraction. Am J Optom Physiol Opt. 1981;58:691–695 [DOI] [PubMed] [Google Scholar]
- 32. Charman WN, Jennings JAM. Ametropia and peripheral refraction. Am J Optom Physiol Opt. 1982;59:992–993 [DOI] [PubMed] [Google Scholar]
- 33. Mutti DO, Sholtz RI, Friedman NE, Zadnik K. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41:1022–1030 [PubMed] [Google Scholar]
- 34. Logan NS, Gilmartin B, Wildsoet CF, Dunne MCM. Posterior retinal contour in adult human anisomyopia. Invest Ophthalmol Vis Sci. 2004;45:2152–2162 [DOI] [PubMed] [Google Scholar]
- 35. Atchison DA, Pritchard N, Schmid KL, et al. Shape of the retinal surface in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2005;46:2698–2707 [DOI] [PubMed] [Google Scholar]
- 36. Mutti DO, Hayes JR, Mitchell GL, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48:2510–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones LA, Mitchell GL, Zadnik K. The CLEERE Study Group. Agreement between parent-reported and clinician-assessed race in the CLEERE Study. Control Clin Trials. 2001;22:98S [Google Scholar]
- 38. McBrien NA, Millodot M. Clinical evaluation of the Canon Autoref R-1. Am J Optom Physiol Opt. 1985;62:786–792 [DOI] [PubMed] [Google Scholar]
- 39. Davies LN, Mallen EA, Wolffsohn JS, Gilmartin B. Clinical evaluation of the Shin-Nippon NVision-K 5001/Grand Seiko WR-5100K autorefractor. Optom Vis Sci. 2003;80:320–324 [DOI] [PubMed] [Google Scholar]
- 40. Seddon JM, Sahagian CR, Glynn RJ, et al. Evaluation of an iris color classification system. Invest Ophthalmol Vis Sci. 1990;31:1592–1598 [PubMed] [Google Scholar]
- 41. Kleinstein RN, Mutti DO, Manny RE, Shin JA, Zadnik K. Cycloplegia in African-American children. Optom Vis Sci. 1999;76:102–107 [DOI] [PubMed] [Google Scholar]
- 42. Zadnik K, Mutti DO, Friedman NE, Adams AJ. Initial cross-sectional results from the Orinda Longitudinal Study of Myopia. Optom Vis Sci. 1993;70:750–758 [DOI] [PubMed] [Google Scholar]
- 43. Zadnik K, Mutti DO, Friedman NE, et al. Ocular predictors of the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 1999;40:1936–1943 [PubMed] [Google Scholar]
- 44. Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London: Sage Publications, Ltd.; 1999:272 [Google Scholar]
- 45. Atchison DA, Pritchard N, Schmid KL. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vision Res. 2006;46:1450–1458 [DOI] [PubMed] [Google Scholar]
- 46. Lin Z, Martinez A, Chen X, et al. Peripheral defocus with single-vision spectacle lenses in myopic children. Optom Vis Sci. 2010;87:4–9 [DOI] [PubMed] [Google Scholar]
- 47. Tabernero J, Vazquez D, Seidemann A, Uttenweiler D, Schaeffel F. Effects of myopic spectacle correction and radial refractive gradient spectacles on peripheral refraction. Vision Res. 2009;49:2176–2186 [DOI] [PubMed] [Google Scholar]
- 48. Hoogerheide J, Rempt F, Hoogenboom WPH. Acquired myopia in young pilots. Ophthalmologica. 1971;163:209–215 [DOI] [PubMed] [Google Scholar]
- 49. Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77 [DOI] [PubMed] [Google Scholar]
- 50. Smith EL, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith EL, Huang J, Hung LF, et al. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50:5057–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aller TA, Wildsoet C. Bifocal soft contact lenses as a possible myopia control treatment: a case report involving identical twins. Clin Exp Optom. 2008;91:394–399 [DOI] [PubMed] [Google Scholar]
- 53. Mathur A, Atchison DA. Effect of orthokeratology on peripheral aberrations of the eye. Optom Vis Sci. 2009;86:E476–E484 [DOI] [PubMed] [Google Scholar]
- 54. Sankaridurg P, Donovan L, Varnas S, et al. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optom Vis Sci. 2010;87:631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30:71–80 [DOI] [PubMed] [Google Scholar]
- 56. Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009;93:1181–1185 [DOI] [PubMed] [Google Scholar]
- 57. Zhu X, Winawer JA, Wallman J. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44:2818–2827 [DOI] [PubMed] [Google Scholar]