This article describes innervation of the mouse cornea at different time points during development and reveals a novel behavior that is different from chick.
Abstract
Purpose.
Dense innervation of the cornea is important for maintaining its homeostasis and transparency. Although corneal nerves have been well studied in adults, little is known about mammalian corneal innervation during development. This study provides a detailed profile of nerves at various stages of mouse cornea development.
Methods.
Mouse heads and corneas were collected at various stages of development including embryonic days (E)12.5 to E16.5, postnatal days (P)0, P10, three weeks after birth, and the adult. Corneas were immunostained with an anti-neuron–specific β-tubulin antibody (TUJ1). Fluorescently labeled nerves in whole-mount tissues and sections were imaged and analyzed for their axonal projections during eye development.
Results.
The first nerve bundles appear at the periphery of the anterior portion of the eye by E12.5. Initial projection into the stroma occurs at E13.5 without formation of a pericorneal nerve ring. Between E13.5 and E16.5, nerve bundles project directly into the periphery of the presumptive cornea stroma. They branch repeatedly as they extend toward the cornea center and epithelium. Concomitantly, nerve bundles originating from four quadrants of the eye bifurcate into smaller branches that innervate the entire stroma. The first epithelial innervation occurs at E16.5. Epithelial nerves arrange into patterns that project toward the center subsequently forming a swirl at three weeks after birth, which becomes more pronounced in adults.
Conclusions.
Nerve bundles that arise from four quadrants of the eye innervate the mouse cornea. The nerve bundles directly innervate the stroma without forming a pericorneal nerve ring. Radial arrangement of epithelial nerves gradually becomes centrally oriented, subsequently forming a swirl pattern.
The vertebrate cornea is a transparent tissue at the anterior segment of the eye that is densely innervated by sensory and autonomic nerves. The majority of the cornea nerves are sensory1 and derive from the neural crest component of the trigeminal ganglion.2 Sympathetic and parasympathetic nerves, which respectively originate from the superior cervical and ciliary ganglia, contribute moderately to the cornea.1,3–5 The dense population of corneal nerves responds to irritation and pain,6 thus playing a critical role in protecting the cornea and the rest of the eye from the potentially harmful external environment. In addition, cornea nerves induce blinking reflexes that maintain proper hydration and also secrete neuropeptides that have a mitogenic effect on epithelial cells.7–9 Absence of corneal nerves results in neurotrophic keratitis, a clinical condition characterized by corneal anesthesia and desiccation, and abnormal epithelium metabolism (reviewed by Muller et al.10).
Innervation of the adult cornea has been widely studied in mammals.10–13 These studies revealed that there are no major differences in adult corneal innervation between species including mouse, rabbit, dog, cats, and humans. Nerve bundles traverse from the sclero-limbo region of the eye, enter the cornea periphery, and radially innervate the anterior third of the stroma and epithelium. Within the stroma, nerve bundles bifurcate into several smaller branches along the distance between the cornea periphery and center, as they project toward the surface epithelium. On penetrating the epithelial basal lamina, nerve bundles ramify into several smaller nerves (leashes) that form the subbasal plexus. Individual nerve endings project perpendicularly from the subbasal plexus and densely innervate the superficial epithelial layers. Despite the detailed description of adult mouse cornea innervation, there is a paucity of literature on how it is innervated during development. It is often inferred that development of mouse corneal innervation is similar to the pattern described in avian models.14
In the chick, cornea innervation begins when growth cones of presumptive corneal nerves arrive at the ventrotemporal side of the developing eye between E4 and E5. At this point, the nerves appear to be repelled such that instead of directly entering the cornea, they extend both dorsally and ventrally, forming a pericorneal nerve ring around its entire circumference.14,15 On completion of the nerve ring, axons begin to branch at regular intervals, radially innervating the stroma in the cornea periphery. Stromal nerves repeatedly bifurcate as they project toward the cornea center and epithelium.16,17
In this study, we examined innervation of the mouse cornea at various stages of development. Our results provide a detailed analysis of the development of mouse corneal innervation and reveal a novel pattern of cornea innervation that does not involve the formation of a pericorneal nerve ring, as seen in the chick embryo. The results provide a platform for future studies of corneal nerves in the mouse, a species often used as a model for human ocular defects.
Methods
Animals
Wild-type C57/B6 mice were used for this study. Animal studies adhered to the Association for Research in Vision and Ophthalmology (ARVO) standards for the Use of Animals in Ophthalmic and vision research and were approved by the Institutional Animal Care and Use Committee (IACUC) of Rice University. Embryos were collected between embryonic days (E)12.5 and E16.5. Postnatal animals were collected at postnatal days (P)0, P10, and three weeks after birth. The number of eyes analyzed at each stage is as follows: E12.5 (n = 35), E13.5 (n = 15), E14.5 (n = 15), E15.5 (n = 10), E16.5 (n = 9), P0 (n = 4), P10 (n = 13), three weeks (n = 8), and adult (n = 6). Whole heads (E12.5–E15.5) or eyes (E16.5–adults) were collected in ice-cold Ringer's solution. Corneas were dissected from eyes and trimmed at the sclero-limbo region. To increase permeability of immunostaining reagents, trimmed corneas were incubated at room temperature in 1.5 mg/mL dispase (Atlanta Biologicals) for 15 to 30 minutes. In some corneas, the epithelial layer was removed after enzyme digestion and immunostained separately. All tissues were fixed overnight at 4°C using 4% paraformaldehyde (PFA) in phosphate-buffered solution (PBS), with the exception of isolated corneal epithelial layers which were fixed at room temperature for 2 hours or overnight at 4°C. All tissues were briefly rinsed in PBS containing 0.1% Tween (PBT) after fixation. Whole heads were sagittally cut in two halves.
Antibodies and Immunostaining
Whole-mount immunostaining of tissues followed standard protocols. Briefly, samples were washed in PBT, and then blocked in antibody buffer (PBT containing 0.1% BSA and 5% heat-inactivated sheep serum). Rabbit anti-neuron–specific β-tubulin (TUJ1) IgG (Covance, Richmond, CA) was used diluted at 1:500 to label all neurons. Following overnight incubation at 4°C in primary antibody solution, tissues were extensively rinsed in PBT, blocked for 1 hour at room temperature then incubated overnight at 4°C in secondary antibody (Alexa 594 goat anti-rabbit IgG, Invitrogen, Carlsbad, CA) diluted at 1:200 in antibody buffer. Tissues were rinsed and mounted in PBS for imaging. Some immunostained tissues were cryosectioned at 8 to 10 μm and counterstained with 4,6-diamidino-2-phenylindole (DAPI) to label all nuclei. Images of whole-mount embryos and corneas or their sections were captured using a CCD camera (Axiocam; Carl Zeiss AG, Oberkochen, Germany) mounted on a fluorescence microscope (AxioImager 2; Carl Zeiss AG) with a slider module (ApoTome; Carl Zeiss AG).
Classification of Innervation
Images of embryos between E12.5 and E15.5 were oriented so that the whisker pad was facing toward the right side of the image and lowered 45° below the horizontal midpoint (Fig. 1A). Using the whisker pad nerves as a reference point, a horizontal line was drawn across the eye bisecting it into dorsal (D) and ventral (V) regions. A perpendicular line was drawn intersecting the first line at the center and dividing the eye into temporal (T) and nasal (N) regions. The resulting quadrants were designated dorsal-temporal (DT), dorsal-nasal (DN), ventral-temporal (VT), and ventral-nasal (VN). Axons were analyzed and recorded based on their location in the designated quadrants.
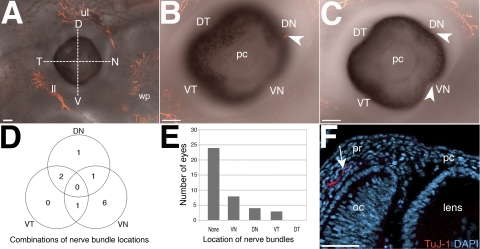
Figure 1.
Innervation of the anterior ocular region at E12.5. (A) An embryo showing innervation of the upper and lower eyelids, and the whisker pad but no innervation of the eye. To determine the positioning of nerve bundles before cornea innervation, the eye was divided into quadrants along the dorsal-ventral and temporal-nasal axes. (B) Innervation of the DN quadrant (arrowhead). (C) Innervation of the DN and VN quadrants (arrowheads). (D) Venn diagram summarizing the number and overlap of innervated quadrants. (E) Quantification of eye innervation and position of pioneer nerve bundles. (F) Cross-section through an E12.5 eye counterstained with DAPI showing a nerve bundle (arrow) in the periocular region projecting toward the presumptive cornea. Figure 1F was imaged from a similar location as the boxed region in Figure 2D. D, dorsal; N, nasal; V, ventral; T, temporal; DN, dorsal-nasal; VN, ventral-nasal; ll, lower eyelid; ul, upper eyelid; wp, whisker pad; pc, presumptive cornea; pr, periocular region; oc, optic cup. Scale bars: (A–C) 100 μm; (F) 50 μm.
Quantitative Analysis
Positions of major nerve bundles were recorded from digital images of similarly oriented corneas. Each innervated quadrant received a score of 1 and 0 for uninnervated quadrant.
Results
Early Innervation of the Eye
To determine the onset of sensory nerve projections into the anterior region of the eye, we immunostained mouse embryos starting at E11.5, corresponding to the time shortly after the formation of the lens vesicle and presumptive cornea epithelium.18 At this stage, trigeminal axons innervate the presumptive eyelids and the whisker pad, but no axons were observed in the anterior region of the eye (data not shown).
By E12.5, nerve bundles begin to appear at distinct locations in the periphery of the eye. To establish position identity of the pioneer axons, we partitioned each eye along the dorsal-ventral and temporal-nasal axes into four quadrants (VN, VT, DN, DT; Fig. 1A). Similar to E11.5, the majority of the embryos (24/35) have innervation of the upper and lower eyelid region and whisker pad, but not the eye (Fig. 1A). Nonetheless, some E12.5 embryos (11/35) exhibit an interesting variation in nerve staining along the edge of the eye. In some eyes, a single nerve bundle was present in the DN (Figs. 1B and 1C, arrowhead), VN (Fig. 1C, arrowhead) or VT (data not shown) quadrants. In other instances (Fig. 1D), a nerve bundle was present in each of the two quadrants of the same eye: DN and VN (1/35; Fig. 1C); VN and VT (1/35); and VT and DN (2/35). The VT quadrant was always innervated in combination with either the VN or DN quadrants. All three quadrants were never innervated in the same eye at E12.5. Figure 1E shows the number of times each quadrant was innervated. However, the majority of the eyes did not have innervation of any of the four quadrants (24/35). For the eyes that were innervated, the majority of the pioneer nerve bundles were located in the VN quadrant (8/35), followed by the DN quadrant (4/35), then the VT quadrant (3/35). Interestingly, the DT quadrant was never innervated at E12.5 (0/35). A representative cross-section through an innervated quadrant at E12.5 reveals a single nerve bundle in the periocular region projecting toward the adjacent presumptive cornea (Fig. 1F, arrow). Our results indicate that presumptive corneal nerves arrive in the periphery of the anterior surface of the eye as early as E12.5. The DN, VN, and VT quadrants are innervated before the DT quadrant. A single nerve bundle is located in each innervated quadrant of the eye.
Innervation of the Corneal Stroma
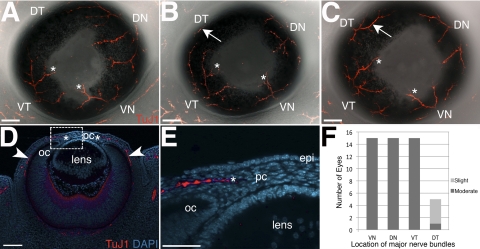
Whole-mount immunostaining of eyes at E13.5 reveals that several nerve bundles project from each innervated quadrant and extend toward the presumptive cornea. The nerve bundles in each quadrant are possibly branches of a single major nerve projecting from the posterior region of the eye that is not visible in whole-mount corneas at this stage. The DN, VN, and VT quadrants are consistently innervated (15/15; Figs. 2A, 2B, 2C, 2F). However, innervation of the DT quadrant appears to lag behind. The majority of E13.5 eyes (9/15) have no innervation of the DT quadrant (Fig. 2A), although a few eyes exhibit slight innervation of this quadrant (5/15; Fig. 2B, arrow; Fig. 2F). Only one eye showed more extended nerve bundles in the DT quadrant that were nearly equivalent in size to the other three quadrants (Fig. 2C, arrow; Fig. 2F). The tips of the main nerve bundle in the DN, VN, and VT quadrants innervate the periphery of the presumptive cornea (Figs. 2A–E, asterisk). Nerves from the DT quadrant never innervate the cornea at E13.5. Sections through E13.5 eyes show that nerves from the posterior region of the eye project along the optic cup toward the anterior (Fig. 2D, arrowheads). As they grow toward the presumptive cornea, nerve bundles project almost parallel to the epithelium and optic cup before innervating the stroma (Figs. 2D–E, asterisk). The results indicate that in the E13.5 mouse embryo, nerve bundles undergo several bifurcations as they extend from the periphery of the eye toward the cornea. Initial innervation of the cornea begins at this stage when nerve bundles project into the midstromal region.
Figure 2.
Projection of nerve bundles toward the presumptive cornea at E13.5. (A–C) Showing projection of nerve bundles from the DN, VN, and VT quadrants toward the presumptive cornea. Some nerve bundles innervate the cornea periphery (asterisks). Innervation of the DT quadrant ranges from (A) none, (B) slight (arrow), compared to other quadrants, and (C) moderate (arrow), compared to other quadrants. (D) Cross-section of an immunostained E13.5 eye counterstained with DAPI showing that presumptive cornea nerves project along the optic cup (arrowheads) toward the anterior region of the eye. (E) Close up of the boxed area in D showing innervation of the mid-stromal region (asterisk) of the presumptive cornea. (F) Quantification of innervated quadrants at E13.5. DN, dorsal-nasal; VN, ventral-nasal; VT, ventral-temporal; DT, dorsal-temporal; oc, optic cup; pc, presumptive cornea. Scale bars: (A–D), 100 μm; (E) 50 μm.
To examine the development of the stromal plexus and determine when nerve bundles begin to project toward and innervate the cornea epithelium, we immunostained corneas between E14.5 and E16.5. By E14.5 nerve bundles from the DN, VN, and VT quadrants appeared to extend further into the cornea than those from the DT quadrant. Only a few eyes (5/15) showed a single nerve bundle from the DT quadrant innervating the cornea periphery. Some of the smaller fascicles that branch from the nerve bundles innervate the cornea at different locations along the circumference. Between two to three nerve bundles each from the DN, VN, and VT quadrants innervate the cornea. Sections through the E14.5 cornea show that nerve bundles from the stromal plexus begin to project anteriorly toward the cornea surface (Fig. 3B).
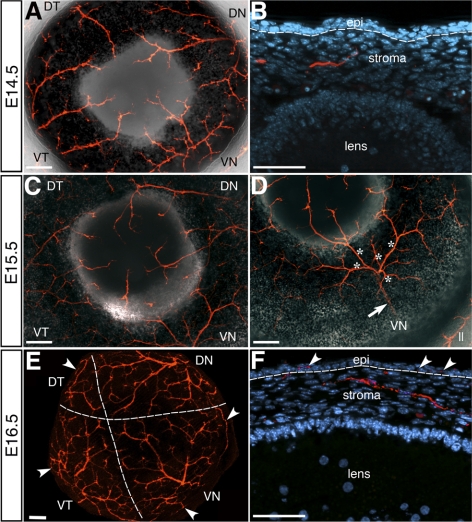
Figure 3.
Innervation of the mouse cornea stroma. Whole-mount staining of (A) E14.5 and (C) E15.5 and (E) E16.5 corneas showing progressive extension and branching of nerves as they innervate the entire cornea stroma. (D) Cornea in (C) imaged from an angle to show that a single major nerve bundle (arrow) from the posterior region of the eye bifurcates into several nerves (asterisks) in the VN quadrant. Cross-sections of (B) E14.5 showing that nerve bundles innervate only the anterior two-thirds of the stroma and project toward the epithelium, which is innervated by (F) E16.5 (arrowheads). Dotted lines demarcate in (E) the boundary of the area covered by nerves from each quadrant, and in (B) and (F) the epithelium/stroma boundary. DN, dorsal-nasal; VN, ventral-nasal; VT, ventral-temporal; DT, dorsal-temporal; oc, optic cup; st, stroma; epi, epithelium; ll, lower eyelid nerves. Scale bars: (A, C, D, E) 100 μm; (B, F) 50 μm.
At E15.5, nerve bundles from the DN, VN, and VT quadrants continue to branch as they project closer to the cornea center (Fig. 3C). Nerve bundles from the DT quadrant still lag behind, only innervating the cornea periphery in half of the corneas (5/10). In the five corneas that are innervated in the DT quadrant, between one to two nerve bundles are visible. The other quadrants are each innervated by three to five nerve bundles that project into the cornea periphery. Interestingly, the major nerve that bifurcates into the nerve bundles of the VN quadrant becomes visible when the eye is oriented at an angle (Fig. 3D, arrow). In this case the single major nerve bundle bifurcates into several branches (Fig. 3D, asterisks) that innervate the anterior region of the eye including the cornea. Cross-section through the cornea at this stage reveals that nerve bundles subdivide into smaller branches and extend closer to the epithelium (data not shown).
By E16.5, major nerve bundles from all quadrants have undergone several bifurcations to form smaller branches that cover most of the cornea stroma (Fig. 3E). The dotted lines demarcate the approximate area innervated by nerve bundles from each quadrant. The DT quadrant appears to contribute relatively fewer nerves to the cornea, which is compensated by longer and more extensive nerve branching from the DN, VN, and VT nerve bundles. At this stage, several large nerve bundles and numerous smaller branches project through the cornea periphery. Some of the nerve branches project along the cornea circumference (Fig. 3E, arrowheads). The earliest innervation of the cornea epithelium is observed at E16.5 (Fig. 3F, arrowheads). Therefore, continuous branching of the nerve bundles from the four quadrants results in the formation of the stromal plexus and innervation of the anterior two-thirds of the entire cornea stroma, with visibly less contribution from the DT quadrant.
Innervation of the Corneal Epithelium and Formation of the Subbasal Nerve Swirl
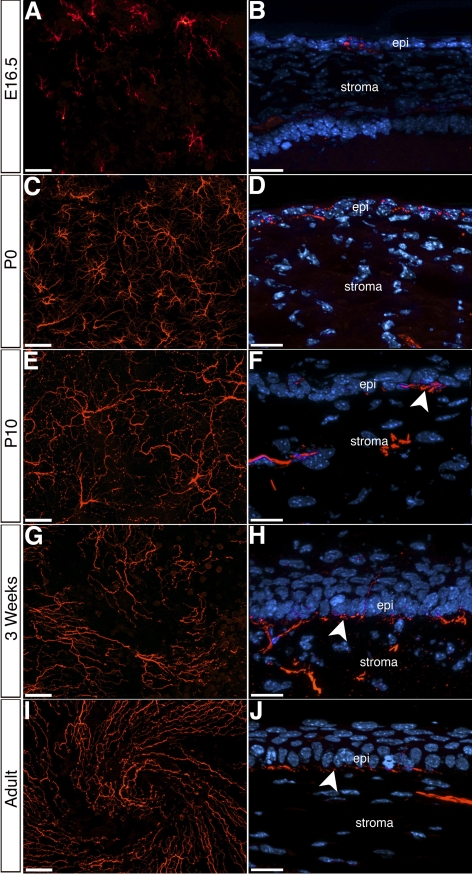
In the adult mouse cornea, subbasal nerves orient toward the center where they form a distinct swirl pattern.19,20 To determine when the subbasal nerves form the swirl pattern, we examined the innervation of the cornea epithelium at E16.5 and subsequent stages of eye development. To avoid interference of stromal nerve staining, we enzymatically isolated the epithelium from the rest of the cornea. Whole-mount immunostaining of E16.5 cornea epithelium demonstrates that each nerve bundle subdivides into several small branches that radially spread from the point of entry (Fig. 4A). Cross-section of the cornea at this stage shows that the 1 to 2 cell thick epithelium is directly innervated (Fig. 4B).
Figure 4.
Innervation of the mouse corneal epithelium. (A, C, E, G, I) Whole-mount immunostaining of cornea epithelia showing the distribution of epithelial nerves at the corneal apex during different stages of development and in adult. (B, D, F, H, J) Cross-sections of corneas at corresponding time points, showing innervation of the epithelium and the formation of the subbasal plexus (arrowheads). epi, epithelium; st, stroma. Scale bars: (A, C, E, G, I) 50 μm; (B, D, F, H, J) 20 μm.
At P0, epithelial nerves extend further compared with E16.5 from the point of entry and subdivide into smaller fascicles (Fig. 4C). Cross-sections of corneas at this stage reveal that the epithelium is two to three cells thick, and small nerves innervate all these layers (Fig. 4D). At P10, nerves cover the entire surface of the cornea epithelium and ramify at several locations (Fig. 4E). In the cross-section, nerves appear to form a subbasal plexus from which individual strands perpendicularly grow through the three to four cells thick epithelium and toward the superficial layer (Fig. 4F). At three weeks after birth, subbasal nerves begin to orient toward the center, where they start to form a swirl pattern (Fig. 4G). The cornea epithelium is stratified and approximately four to five cells thick at this stage. The subbasal plexus is clearly visible below the basal epithelial layer (Fig. 4H, arrowhead). Nerves branching from the subbasal plexus penetrate all layers of the epithelium and extend upwards into the superficial apical layer. In adults, subbasal nerves orient toward the center and the swirl pattern is distinct (Fig. 4I). Cross-section of adult cornea reveals a nerve pattern that is similar to three weeks after birth (Fig. 4J). These results suggest that the cornea epithelial cells are innervated before rearrangement of nerves to form the swirl pattern.
Discussion
The mouse is widely used to model genetic defects that reflect human ocular disorders. Surprisingly, there is only anecdotal information on development of mouse cornea innervation. It is inferred that mouse cornea innervation is similar to avian radial innervation after nerve ring formation. In this study, the authors performed a detailed analysis of mouse cornea innervation during development and show that nerve bundles grow directly into the cornea without forming a pericorneal nerve ring. Nerve bundles from four quadrants innervate the cornea, with the DT quadrant contributing relatively fewer nerves. The nerve bundles bifurcate into smaller branches that cover the entire stroma by E16.5. The cornea epithelium is first innervated at E16.5, subsequently forming a swirl pattern in the subbasal nerve plexus at about three weeks after birth.
Positioning of Major Corneal Nerves
During development, trigeminal sensory nerves innervate several tissues including the eyelids, eye, and maxillo-mandibular processes.21 Unlike chicks where presumptive corneal nerves always project from the ventrotemporal region, we find that the positioning varied from embryo to embryo in mice. Although the majority of embryos did not show innervation at E12.5, nerves mostly projected from the DN and VN quadrants first, since the VT quadrant was always innervated in combination with either of the two. This pattern was maintained during subsequent stages of development resulting in a large portion of the cornea stroma being innervated by nerve bundles from the DN, VN, and VT quadrants. Although fewer nerves grew into the stroma from the DT quadrant, it was apparent that nerves from the DN, VN, and VT quadrants compensated for this shortfall. The number of nerve bundles innervating each quadrant was not rigorously quantified in this study, but in the 30-day-old rat cornea, large stromal nerves and their branches are equally distributed in all quadrants.22 Nonetheless, the number of nerve bundles entering the rat cornea in each quadrant is unclear. Interestingly, immunostaining of adult human cornea nerves reveal an even distribution of major stromal nerve bundles that radially innervate the cornea along the entire cornea circumference.10,12 This suggests that similar to chick,16,17 stromal nerves are equally distributed along the circumference of the human cornea. Our results suggest that the distribution of mouse corneal nerves differs from the chick and human, with the DT quadrant contributing relatively fewer nerves to the stroma.
Development of the Stromal Plexus
In mouse, the presumptive cornea stroma remains uninnervated until E13.5 when nerve bundles project into the cornea periphery. Interestingly, unlike the chick14,15 where nerve bundles form a pericorneal nerve ring before cornea innervation, nerves grew directly into the mouse cornea. By E15.5, approximately 9 to 17 nerve bundles project into the cornea stroma. We also found that nerve bundles from the VN quadrant originate from a single major nerve projecting from the posterior region of the eye. Since a single nerve bundle is present in each of the innervated quadrants at E12.5, it is possible that one major nerve from the posterior eye gives rise to the nerve bundles in one quadrant, suggesting that four major nerves, one in each quadrant, give rise to all the nerve bundles that innervate the anterior portion of the eye, including the cornea. In chick, four to five major nerve bundles (personal observation) from the VT region form the pericorneal nerve ring from which approximately 44 branches radially innervate the cornea.16 In humans, approximately 30 nerves project into the stroma at three months after birth and that number increases to approximately 40 by ten months.23 The number of major nerve bundles is not known for humans and it is also not clear whether a pericorneal nerve ring forms before cornea innervation. The radial innervation of the adult human cornea is similar to chick,12 suggesting the presence of a pericorneal nerve ring from which nerves project. Different numbers of radial nerves have been reported in adult human corneas, ranging from 44 to 80.11,12,24 In the rat, the first cholinesterase-positive nerves are visible at E19.25 At P0, 12 to 15 nerve bundles enter the rat cornea periphery at regular intervals and radially innervate the stroma.13 Similar to chicks,16 humans,24 and rats,13 in the mouse, nerve bundles enter the cornea at the midstromal level and after several bifurcations, smaller branches innervate the anterior stroma as they grew toward the surface.
Epithelial Innervation and Formation of Subbasal Swirl
Innervation of the adult cornea epithelium has been well studied in mice19,20,27 and humans.12,24,26 Taken together, these studies demonstrate that stromal nerves project through the Bowman's membrane and form small nerve bundles (leashes) that further bifurcate into individual terminals that innervate the epithelium. Innervation of the cornea epithelium is first seen between 46 to 49 days gestation in Rhesus macaque28 and at 5 months in humans.23 In the adult human,29 mouse,19,20 and rat,30 epithelial nerves form a swirl pattern near the apex of the cornea.
Our results show that the mouse embryonic cornea epithelium is innervated by E16.5. The highly organized mouse cornea epithelium innervation develops in two steps. First, stromal nerves ramify into radial patterns at the points of entry that directly innervate the developing epithelial layer. Second, concomitant with stratification of the cornea epithelium, nerves arrange into leashes that project toward the cornea center, initiating a swirl pattern at approximately three weeks after birth. The factors regulating the projection of subbasal nerves toward the central cornea remain unknown. Interestingly, epithelial cells similarly migrate toward the central cornea and form a swirl pattern at the apex.31,32 Therefore, it has been postulated that epithelial cell migration guides the nerves into a similar pattern. However, the epithelial swirl does not form until after four weeks,32 indicating that the swirl pattern of nerves is not dependent on epithelial cell migration. Our results confirm previous observations by Leiper et al.20 showing that centripetal projection of nerves is independent of epithelial cell migration.
The present study is the first to reveal that development of mouse corneal innervation differs from chick. Unlike chick, rat, and humans, where the stroma is radially innervated at regular intervals, mouse stromal innervation is by nerve bundles from four quadrants, which branch irregularly to cover the entire cornea. Our results may be used to guide future studies of mouse corneal nerves and provide insight into cornea development, wound healing, and disease.
Acknowledgments
The authors thank Marianne Bronner and Shannon Griswold for helpful comments on the manuscript.
Footnotes
Supported by National Institutes of Health Grant EY018050 (PYL).
Disclosure: C.C. McKenna, None; P.Y. Lwigale, None.
References
- 1. Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Invest Ophthalmol Vis Sci. 1989;30:461–472 [PubMed] [Google Scholar]
- 2. Lwigale PY. Embryonic origin of avian corneal sensory nerves. Dev Biol. 2001;239:323–337 [DOI] [PubMed] [Google Scholar]
- 3. Marfurt CF, Jones MA, Thrasher K. Parasympathetic innervation of the rat cornea. Exp Eye Res. 1998;66:437–448 [DOI] [PubMed] [Google Scholar]
- 4. Morgan C, DeGroat WC, Jannetta PJ. Sympathetic innervation of the cornea from the superior cervical ganglion. An HRP study in the cat. J Auton Nerv Syst. 1987;20:179–183 [DOI] [PubMed] [Google Scholar]
- 5. Tervo T. Demonstration of adrenergic nerve fibres in the nasociliary but not the ophthalmic nerve of the rat. Exp Eye Res. 1978;27:607–613 [DOI] [PubMed] [Google Scholar]
- 6. Belmonte C, Gallar J. Corneal nociceptors. In: Belmonte C, Cervero F. eds. Neurobiology of Nociceptors. Oxford: Oxford University Press; 1996:146–183 [Google Scholar]
- 7. Baker KS, Anderson SC, Romanowski EG, Thoft RA, SundarRaj N. Trigeminal ganglion neurons affect corneal epithelial phenotype. Influence on type VII collagen expression in vitro. Invest Ophthalmol Vis Sci. 1993;34:137–144 [PubMed] [Google Scholar]
- 8. Reid TW, Murphy CJ, Iwahashi CK, Foster BA, Mannis MJ. Stimulation of epithelial cell growth by the neuropeptide substance P. J Cell Biochem. 1993;52:476–485 [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neurotrophic influences on corneal epithelial cells. Exp Eye Res. 1994;59:597–605 [DOI] [PubMed] [Google Scholar]
- 10. Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542 [DOI] [PubMed] [Google Scholar]
- 11. Zander E, Weddell G. Observations on the innervation of the cornea. J Anat. 1951;85:68–99 [PMC free article] [PubMed] [Google Scholar]
- 12. Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 90:478–492 [DOI] [PubMed] [Google Scholar]
- 13. Jones MA, Marfurt CF. Calcitonin gene-related peptide and corneal innervation—a developmental study in the Rat. J Comp Neurol. 1991;313:132–150 [DOI] [PubMed] [Google Scholar]
- 14. Bee JA. The development and pattern of innervation of the avian cornea. Dev Biol. 1982;92:5–15 [DOI] [PubMed] [Google Scholar]
- 15. Lwigale PY, Bronner-Fraser M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev Biol. 2007;306:750–759 [DOI] [PubMed] [Google Scholar]
- 16. Riley NC, Lwigale PY, Conrad GW. Specificity of corneal nerve positions during embryogenesis. Mol Vis. 2001;7:297–304 [PubMed] [Google Scholar]
- 17. Bee JA, Hay RA, Lamb EM, Devore JJ, Conrad GW. Positional specificity of corneal nerves during development. Invest Ophthalmol Vis Sci. 1986;27:38–43 [PubMed] [Google Scholar]
- 18. Pei Y, Rhodin J. The prenatal development of the mouse eye. Anat Rec. 1970;168:105–125 [DOI] [PubMed] [Google Scholar]
- 19. Yu CQ, Rosenblatt MI. Transgenic corneal neurofluorescence in mice: a new model for in vivo investigation of nerve structure and regeneration. Invest Ophthalmol Vis Sci. 2007;48:1535–1542 [DOI] [PubMed] [Google Scholar]
- 20. Leiper LJ, Ou J, Walczysko P, et al. Control of patterns of corneal innervation by Pax6. Invest Ophthalmol Vis Sci. 2009;50:1122–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arvidson B. Retrograde axonal transport of horseradish peroxidase from cornea to trigeminal ganglion. Acta Neuropathol. 1977;38:49–52 [DOI] [PubMed] [Google Scholar]
- 22. Ishida N, Delcerro M, Rao GN, Mathe M, Aquavella JV. Corneal stromal innervation. A quantitative analysis of distribution. Ophthalmic Res. 1984;16:139–144 [DOI] [PubMed] [Google Scholar]
- 23. Kitano S. An embryological study on the human corneal nerves. Jpn J Ophthalmol. 1957;1:48–55 [Google Scholar]
- 24. Al-Aqaba M, Fares U, Suleman H, Lowe J, Dua HS. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010;94:784–789 [DOI] [PubMed] [Google Scholar]
- 25. Tervo K, Tervo T, Palkama A. Pre- and postnatal development of catecholamine-containing and cholinesterase-positive nerves of rat cornea and iris. Anat Embryol (Berlin). 1978;154:253–265 [DOI] [PubMed] [Google Scholar]
- 26. Muller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38:985–994 [PubMed] [Google Scholar]
- 27. de Castro F, Silos-Santiago I, de Armentia ML, Barbacid M, Belmonte C. Corneal innervation and sensitivity to noxious stimuli in trkA knockout mice. Eur J Neurosci. 1998;10:146–152 [DOI] [PubMed] [Google Scholar]
- 28. Ozanics V, Rayborn M, Sagun D. Observations on the morphology of the developing primate cornea: epithelium, its innervation and anterior stroma. J Morphol. 1977;153:263–297 [DOI] [PubMed] [Google Scholar]
- 29. Patel DV, McGhee CN. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005;46:4485–4488 [DOI] [PubMed] [Google Scholar]
- 30. Dvorscak L, Marfurt CF. Age-related changes in rat corneal epithelial nerve density. Invest Ophthalmol Vis Sci. 2008;49:910–916 [DOI] [PubMed] [Google Scholar]
- 31. Collinson JM, Morris L, Reid AI, et al. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn. 2002;224:432–440 [DOI] [PubMed] [Google Scholar]
- 32. Nagasaki T, Zhao J. Centripetal movement of corneal epithelial cells in the normal adult mouse. Invest Ophthalmol Vis Sci. 2003;44:558–566 [DOI] [PubMed] [Google Scholar]