This study provides the first evidence that the murine cornea has a full range of lymphatic plasticity. Spontaneous lymphatic formation and regression occur in this unique tissue during a critical period after birth.
Abstract
Purpose.
Lymphatic dysfunctions are associated with many diseases, ranging from cancer metastasis to transplant rejection, for which there is little effective treatment. To date, there is no natural model with which to study lymphatic regression. This study was conducted to investigate whether murine cornea, an extensively exploited tissue for vascular studies, derives its lymphatic-free status from a natural regression mechanism. The differential behaviors between the lymphatic and blood vessels under normal development and inflammation conditions are also compared.
Methods.
Normal mouse eyeballs or whole-mount corneas encompassing the entire course of corneal development and maturation and adult inflamed corneas were used for immunofluorescent microscopic studies.
Results.
The data demonstrated, for the first time, that mouse cornea was endowed with a significant number of lymphatic vessels that underwent spontaneous formation and regression during a critical period after birth, which was not observed for blood vessels. Because lymphatic growth can be reactivated in the adult cornea after inflammatory stimulation, the cornea thereby becomes the first tissue ever identified to have a full range of lymphatic plasticity.
Conclusions.
These novel findings not only provide a new concept in defining the cornea and its related diseases, they also reveal a completely natural model with which to study both lymphatic regression and formation. It is hoped that further studies will divulge novel and potent pro- or anti-lymphatic factors to treat lymphatic disorders inside and outside the eye.
Lymphatic research signifies a new field of discovery and has attracted considerable attention during the past few years.1–5 Unlike blood vasculature, lymphatic vessels are not easily visible. The lymphatic network penetrates most tissues in the body, and its dysfunctions have been found in a broad spectrum of disorders, including transplant rejection, cancer metastasis, delayed wound healing, inflammatory and immune diseases, and lymphedema.1,3–8 To date, there is little effective treatment for lymphatic disorders; it is, therefore, a field with an urgent demand for new experimental models and therapeutic protocols.
The cornea, the tissue in the forefront of the visual pathway, provides a clear structure for the passage of light. It is also one of few adult tissues in the body normally devoid of any vessel types, blood or lymphatic. In addition, because of its transparent nature and accessible location, the murine cornea has been extensively exploited as a tool for vascular studies. As estimated by Judah Folkman, the grandfather of tumor vascular research, more than one-third of our basic knowledge on blood vessels is derived from studies with the cornea. More recently, the use of this unique tissue in the new area of lymphatic research has started to generate promising data.1 While most studies have focused on the prevention of lymphatic formation, little is known about how to induce lymphatic regression, which bears even more clinical significance in many diseases in which the already formed lymphatics play a major pathologic role. However, there is no natural model with which to study the processes of lymphatic regression to reveal pivotal mechanisms or factors for the development of new therapeutic strategies.
In this study, we investigated whether the murine cornea is devoid of lymphatic vessels because of a primary absence of lymphatic formation from the beginning of its embryogenesis or a secondary event of lymphatic regression, which occurs naturally as the cornea matures. In addition, the differential behavior between the blood and the lymphatic vessels under normal development and inflammation conditions was also examined.
Methods
Animals
Normal male and female mice (C57BL/6; Taconic Farms, Germantown, NY) were bred and maintained in the animal facilities of the University of California at Berkeley and the University of Louisville, Kentucky, in accordance with each institution's guidelines. All animal protocols were approved by the Animal Care and Use Committees.
Tissue Preparation
In all timed pregnancies, the plug date was designed as embryonic day (E) 0.5, and the date of birth was defined as postnatal day zero (P0). For prenatal stages, embryos were sampled at E10.5, E12.5, E14.5, E16.5, and E18.5; and for postnatal stages, ocular tissues were sampled at P0, P2, P6, P10, P14, P21, and 6 weeks of the adult age. For cross-sectional studies, the specimens were immersed in OCT compound (Tissue-Tek Compound; Sakura Finechemical, Torrance, CA) and frozen at −80°C for further studies; and for whole-mount assays, the specimens were fixed in 4% paraformaldehyde overnight at 4°C for further studies.
Induction of Corneal Inflammation
The suture-induced inflammation model was used as described previously.9–11 Briefly, three 11–0 nylon sutures (AROSurgical, Newport Beach, CA) were placed in the central corneas of the adult mice at 6 weeks of age. Whole-mount corneas were sampled 7 days later for further studies.
Antibodies
The following primary and secondary antibodies were used: rabbit anti-mouse LYVE-1, rat anti-mouse F4/80 (Abcam, Cambridge, MA), rat anti-mouse CD31/PECAM-1 (BD PharMingen, San Diego, CA), rat anti-mouse LYVE-1 antibody (R&D Systems, Minneapolis, MN), rabbit anti-mouse Prox-1 antibody (a kind gift from Michael Detmar, Institute of Pharmaceutical Sciences, Swiss Federal Institute of Technology, Zurich, Switzerland), Cy3-conjugated donkey anti-rabbit IgG, and Alexa 488-conjugated donkey anti-rat IgG (Jackson ImmunoResearch, West Grove, PA). The isotype controls included normal rabbit IgG (Abcam), and purified rat IgG2a (BD PharMingen).
Immunofluorescent Microscopic Studies
Five-micrometer frozen sections or whole-mount tissues were used for immunofluorescent staining, as described previously.9,11–14 After series of incubations with the primary and secondary antibodies, the mounted samples were observed with a fluorescence deconvolution microscope (Carl Zeiss, München-Hallbergmoos, Germany) and photographed with a digital camera system (Axiocam, Carl Zeiss). For each antibody staining study, tissue samples from seven to ten mice were examined. For cross-sectional studies, at least five sections were analyzed for each tissue sample derived from each animal.
Vascular Quantification and Statistical Analysis
Corneal vessels were graded and analyzed using the ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) as described previously with some modifications.11,15,16 Basically, the vessel area was normalized to the total corneal area to obtain a percentage coverage score for each sample, and the total branching points and the vessel length were divided by the total number of lymphatic vessels to derive the average scores for each sample. Additionally, the largest diameter of the lymphatic vessels in each sample was also measured. Results were expressed as the mean ± SEM. Statistical analysis was performed by one-way ANOVA with statistical software (Prism; GraphPad Software, Inc., La Jolla, CA), and P < 0.05 was considered significant.
Results
LYVE-1 Expression in Embryonic Corneas
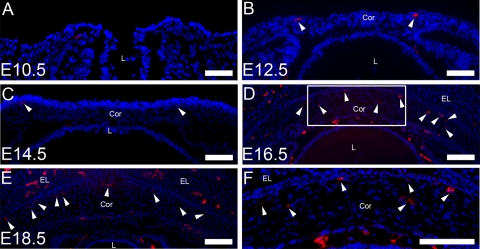
Given that corneal development in mice starts at around embryonic day (E) 10,17 we first examined the expression of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), a lymphatic marker,18 in the cross-sections of the embryonic corneas from E9.5 to E18.5. Immunofluorescence staining revealed that LYVE-1 was expressed in the corneas at E12.5 and thereafter (Figs. 1B–E). At late embryonic stages, prominent LYVE-1 signals were observed in the central corneas (Figs. 1D–F).
Figure 1.
LYVE-1 expression in embryonic corneas (A–F). Representative cross-sectional micrographs demonstrate LYVE-1+ signals detected in the mouse corneas at E12.5 (B) and thereafter (C–E), as indicated by the arrowheads. (F) Higher magnification view of the boxed area in (D) showing LYVE-1 expression in the central cornea. L, lens; Cor, cornea; EL, eyelid; E, embryonic. Red: LYVE-1; blue: DAPI (nuclei staining). Scale bars, 100 μm.
Spontaneous Lymphatic Formation after Birth
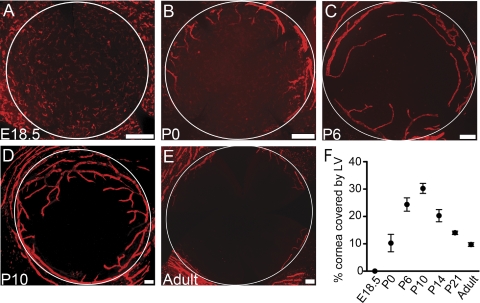
It becomes technically possible to study whole-mount flat corneas from late embryonic stages on; therefore, we next examined the whole-mount corneas of E18.5 and postnatal day (P) 0 to observe two-dimensional distributions of the LYVE-1 signals. Surprisingly, it was found that all the LYVE-1 signals at E18.5 were from single cells distributed across the entire cornea and its neighboring tissue of the conjunctiva. These cells also expressed F4/80, indicating a macrophage lineage (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5404/-/DCSupplemental). The newly developed lymphatic vessels were first observed in the periphery of the corneas at P0 (Fig. 2A), signifying onset of a spontaneous event of lymphatic formation.
Figure 2.
Spontaneous lymphatic vessel formation followed by regression in postnatal corneas (A–F). (A–E) Representative whole-mount micrographs demonstrating the gradual appearance and disappearance of lymphatic vessels in the corneas from E18.5 to the adult age. Red: LYVE-1. Scale bars, 200 μm. White circles: demarcation between the cornea and the conjunctiva. (F) Summarized data from repetitive experiments illustrating the trend of lymphatic changes over the time period. Results are expressed as the mean ± SEM. Statistical analysis was performed by one-way ANOVA (P < 0.0001). E, embryonic; P, postnatal; LV, lymphatic vessels.
Spontaneous Lymphatic Regression after Birth
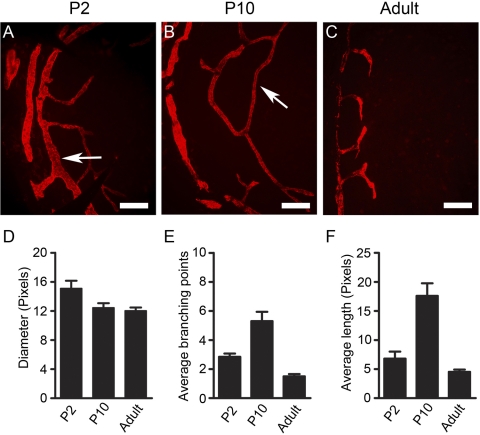
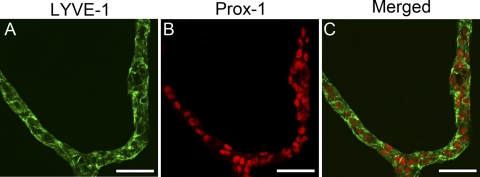
To understand whether this event of lymphatic formation continues and when it surrenders to a regression event before cornea maturation, we next examined the postnatal corneas up to the adult stage. These studies further revealed that the lymphatic vasculatures continue to grow into the central corneas, reaching a peak around P10 (Fig. 2D). Thereafter, the lymphatics started to regress and gradually receded to the limbal or peripheral areas around P21. This trend of lymphatic formation and regression gradually approaching the adult level (Fig. 2E) is summarized in Figure 2F. Furthermore, it was observed that the lymphatic vessels of the early stages had larger diameters, were longer, and had more branches (Fig. 3). We have confirmed that the LYVE-1+ vessels also expressed Prox-1, another lymphatic specific marker (Fig. 4).
Figure 3.
Comparison between early- and late-stage lymphatic vessels. (A–C) Representative whole-mount micrographs showing that the lymphatic vessels of the early stages had larger diameters, were longer, and had more branches as indicated by the arrows. (D–F) Summarized data from repetitive experiments. Results are expressed as the mean ± SEM. Statistical analysis was performed by one-way ANOVA. (D) P < 0.05. (E, F) P < 0.0001. Red: LYVE-1. Scale bars, 100 μm. P, postnatal.
Figure 4.
(A–C) Lymphatic origin of LYVE-1+ vessels in developing cornea. Representative whole-mount micrographs showing LYVE-1+ vessels in P6 corneas also expressed the Prox-1, another lymphatic specific marker. Green: LYVE-1; red: Prox-1. Scale bars, 50 μm.
Differential Behavior between the Lymphatic and Blood Vessels
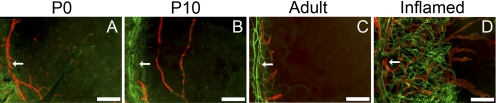
Because lymphatic and blood vessels accompany each other under many circumstances, we next explored, through a double-staining assay with antibodies against both CD31 (a panendothelial cell marker) and LYVE-1, whether the blood vessels also experienced similar dynamic changes during this period. The vascular structures that were CD31+ but LYVE-1− were defined as blood vessels. Results from these studies clearly demonstrated a dissociation phenomenon between the two vessel types: while the lymphatics underwent dramatic changes from formation to regression, the blood vessels constantly remained at the limbal or peripheral areas, as observed in the adult tissue (Figs. 5A–C). This novel finding and the fact that the lymphatics are not as easily visible to the naked eye as blood vessels may explain our long-time unawareness of their existence in the past. As a comparison, after the inflammatory stimulation in the adult cornea, both vessel types could be reactivated and developed into the central corneas in parallel, as demonstrated in Figure 5D.
Figure 5.
Differential behavior of lymphatic and blood vessels in the cornea. (A–C) Representative whole-mount micrographs demonstrating that though lymphatic vessels underwent dramatic changes from natural formation to regression, the blood vessels constantly remained at the peripheral or limbal areas. (D) However, at the adult age after inflammatory stimulation, both vessel types were reactivated and introduced into the cornea in parallel. Green: CD31; red: LYVE-1. Scale bars, 200 μm. Arrows: limbal peripheral vessels. P, postnatal.
Discussion
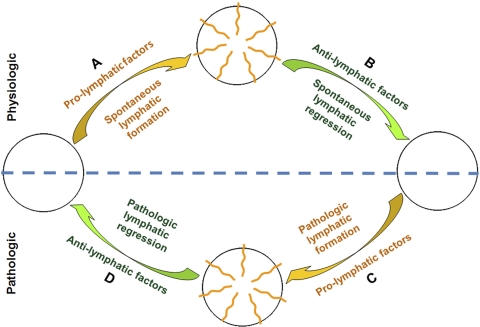
These results clearly demonstrate, for the first time, that the murine cornea is not born without lymphatic vessels. Instead, two dramatic and spontaneous events occur in this tissue before it finally develops into an immune-privileged site devoid of any vascular supply. Given that the lymphatics can reinvade the adult cornea after inflammatory stimulation, the cornea becomes the first tissue ever identified to have a full-range of plasticity in lymphatic vessels, whether this pertains to their physiologic growth and regression or to their pathologic redevelopment. The full range of lymphatic plasticity of the cornea is schematically illustrated in Figure 6 (phases A–D). Although it is yet to be determined how to induce pathologic lymphatic regression (phase D), further studies on the mechanisms underlying the natural regression processes (phase B) may yield important endogenous anti-lymphatic factors or insights into the development of novel therapeutic strategies. Allied to this anticipation is the accumulating evidence showing that molecular blockade of several pro-lymphatic factors critical for the embryonic lymphatic formation (phase A) also suppresses pathologic lymphatic growth (phase C). It is also speculated that the lymphatic status of the cornea is orchestrated and maintained by a group of pro- and anti-lymphatic factors already known or to be discovered; certain physiologic or pathologic stimulations will tip the balance in favor of lymphatic formation or regression. Further studies are required to reveal these important factors and the possible interactions between them.
Figure 6.
Schematic illustration summarizing the full range of lymphatic plasticity in the cornea under both physiologic (top half) and pathologic (bottom half) situations. Yellow: common pathways for lymphatic formation (phases A and C). Green: common pathways for lymphatic regression (phases B and D).
Our data also show that lymphatic coverage of the developing corneas peaks around P10, coinciding with the time of eyelid opening in mice.17 Eyelid opening is a critical event during the development of the eyeball and is associated with significant morphologic and molecular changes in the cornea, especially in rodents (such as the mouse), which are born with closed eyelids.19,20 Interestingly, it was also reported previously that lymphatic vessels were not detected in developing human corneas, in which eyelids are open with birth.21 Although lymphatic absence in human corneas requires further confirmation given that not all developmental stages were covered in that study, it is plausible to hypothesize that corneal lymphatic processes are associated with eyelid opening, warranting further investigation.
Furthermore, this study also bears two other broad implications. One is that the cornea is a representative of several alymphatic tissues. Our results indicate that other tissues traditionally believed to be alymphatic, such as brain and cartilage, may also be endowed with lymphatic vessels during their development. Further investigation on these tissues may yield a new concept for their physiology and pathogenesis. The other is that strong evidence supports that the lymphatic vessels can behave independently or differently from blood vessels under certain circumstances. Unlike blood vessels, which have been studied extensively in the past, lymphatic research is still at its pioneering phase, with many questions yet to be answered. It is still being debated whether and how the lymphatic vessels depend on blood vessels. Our results thus provide a new conceptual framework for future investigations on this topic.
In summary, this study not only reveals a completely natural model to study the processes of lymphatic formation and regression, it also opens the possibility of carrying out further investigations in broad disciplines. It is hopeful that future studies using this natural model will reveal potent endogenous pro-lymphatic or anti-lymphatic therapeutic factors to treat lymphatic diseases, both inside and outside the eye.
Supplementary Material
Acknowledgments
The authors thank Jeffrey LeDue (University of California, Berkeley) for his excellent technical assistance with the immunofluorescence microscopic studies and Michael Detmar (Swiss Federal Institute of Technology, Zurich, Switzerland) for providing the Prox-1 antibody.
Footnotes
Supported in part by research grants from the National Institutes of Health, the Department of Defense, the University of California at Berkeley (LC), and the National Natural Science Foundation of China (HZ).
Disclosure: H. Zhang, None; X. Hu, None; J. Tse, None; F. Tilahun, None; M. Qiu, None; L. Chen, None
References
- 1. Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009;42:66–76 [PMC free article] [PubMed] [Google Scholar]
- 2. Folkman J, Kaipainen A. Genes tell lymphatics to sprout or not. Nat Immunol. 2004;5:11–12 [DOI] [PubMed] [Google Scholar]
- 3. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953 [DOI] [PubMed] [Google Scholar]
- 4. Brown P. Lymphatic system: unlocking the drains. Nature. 2005;436:456–458 [DOI] [PubMed] [Google Scholar]
- 5. Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci. 2008;1131:225–234 [DOI] [PubMed] [Google Scholar]
- 7. Radhakrishnan K, Rockson SG. The clinical spectrum of lymphatic disease. Ann N Y Acad Sci. 2008;1131:155–184 [DOI] [PubMed] [Google Scholar]
- 8. Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55:122–145 [DOI] [PubMed] [Google Scholar]
- 9. Chen L, Cursiefen C, Barabino S, Zhang Q, Dana MR. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Invest Ophthalmol Vis Sci. 2005;46:4536–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815 [DOI] [PubMed] [Google Scholar]
- 11. Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010;51:2436–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang H, Hara M, Seki K, Fukuda K, Nishida T. Eyelid fusion and epithelial differentiation at the ocular surface during mouse embryonic development. Jpn J Ophthalmol. 2005;49:195– 204 [DOI] [PubMed] [Google Scholar]
- 13. Chen L, Huq S, Gardner H, de Fougerolles AR, Barabino S, Dana MR. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch Ophthalmol. 2007;125:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang H, Tse J, Hu X, et al. Novel discovery of LYVE-1 expression in the hyaloid vascular system. Invest Ophthalmol Vis Sci. 2010;51:6157–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith RS, John SWM, Nishina PM, Sundberg JP. Systematic Evaluation of the Mouse Eye: Anatomy, Pathology, and Biomethods (Research Methods for Mutant Mice). Boca Raton, FL: CRC Press; 2002 [Google Scholar]
- 18. Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zieske JD. Corneal development associated with eyelid opening. Int J Dev Biol. 2004;48:903–911 [DOI] [PubMed] [Google Scholar]
- 20. Kao WW, Xia Y, Liu CY, Saika S. Signaling pathways in morphogenesis of cornea and eyelid. Ocul Surf. 2008;6:9–23 [PubMed] [Google Scholar]
- 21. Cursiefen C, Rummelt C, Junemann A, et al. Absence of blood and lymphatic vessels in the developing human cornea. Cornea. 2006;25:722–726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.