This study describes the elastin fiber system within the juxtacanalicular tissue of the inner and outer walls and within collector channel walls in low- and high-pressure perfused human eyes. The importance of the elastin fiber system, particularly in regard to the distensibility of the juxtacanalicular region under collector channels, is discussed.
Abstract
Purpose.
To determine the composition and investigate the elastin fiber system in the juxtacanalicular tissue adjacent to and between collector channel orifices in normal human eyes.
Methods.
Normal human eyes (71.0 ± 8.6 years; mean ± SD; n = 4) were perfusion fixed at low (10 mm Hg) and high pressure (20 mm Hg) with 3% paraformaldehyde/0.1 M phosphate buffer. Frontal serial sections were cut from paraffin blocks, and regions with and without collector channels were selected. Sections were stained using Weigert's resorcin-fuchsin stain with oxidation. Immunohistochemistry was performed using antibodies against elastin, fibrillin-1, and microfibrillar-associated protein-1/2.
Results.
Elastin, elaunin, and oxytalan fibers were identified within the juxtacanalicular tissue of the inner and outer walls in low- and high-pressure eyes. These fibers were found at collector channel orifices, between collector channels, and within collector channel walls. Fibrillin-1 was located at the base and lateral edges of Schlemm's canal endothelial cells. Microfibrillar-associated protein-1/2 was found with elastin-like fibers at the base of Schlemm's canal endothelium cells, in the juxtacanalicular tissue, and in the uveal region.
Conclusions.
Elastin, elaunin, oxytalan, and elastin-associated proteins fibrillin-1 and microfibrillar-associated protein-1/2 were identified within the juxtacanalicular tissue of the inner and outer walls and within collector channel walls of human eyes perfused at low and high pressure. No differences in labeling patterns for elastin, elaunin, and oxytalan were found in the juxtacanalicular tissue adjacent to or between collector channel orifices. The elastin fiber system appears to have a significant role in the support and distensibility of the juxtacanalicular region under collector channels.
The elastin fiber system (EFS) is important in many systems of the body, imparting support, resilience, and recoil to regions under biomechanical stress.1,2 Historically the EFS was classified into three components: elastin, elaunin, and oxytalan, using light microscopy stains and biochemical analysis. As technology evolved, electron microscopy defined elastin as having an amorphous central region surrounded by microfibrils, elaunin as a mixture of amorphous microfibril material, and oxytalan as mainly microfibrils.3 Complexity and variability of the EFS expanded with the identification of elastin-associated proteins: fibrillin-1 (FBN-1), fibrillin-2 (FBN-2), versican, latent TGFβ-binding proteins (LTBP 1–4), and microfibrillar-associated proteins (MFAP 1–4).4,5 In many regions of the body, FBN-1 and FBN-2 are laid down as a scaffold for elastin fibers.6 However, in periodontal membrane and the zonular fibers of the lens, FBN-1 exists alone, and elastin proteins are not present.7,8 Numerous pathologic conditions have alterations of the EFS system, including Marfan's syndrome, pseudoexfoliation syndrome, and emphysema.4,9–11
A major source of biomechanical stress in the anterior chamber of the eye is intraocular pressure. Head and eye movement and ocular pulse result in continuous pressure changes that require tissue deformation and recoil.12,13 The juxtacanalicular tissue (JCT) adjacent to Schlemm's canal (SC) is uniquely equipped to adjust for pressure changes within the conventional outflow pathway. The JCT, also known as the cribriform layer, is located between the outermost corneoscleral trabecular beam and SC endothelial cells.14,15 This region contains endothelial cells arranged in layers, commonly referred to as JCT cells. Within this region and located between the JCT cell layers is a complex system of elastin-like fibers known as the cribriform plexus.15 The cribriform plexus has a porelike or reticular appearance when viewed in a tangential orientation.16,17 The cribriform plexus extends from type A, B, and C tendons that originate from the ciliary muscle tips.17,18 Type A tendons anchor the longitudinal fibers of the ciliary muscle, type B tendons pass through the trabecular meshwork and anchor in the cornea, and type C tendons enter the fiber system at the outermost corneoscleral meshwork and make a 90° turn before joining the cribriform plexus. These elastin-like fibers have a small amount of elastin surrounded by a microfibrillar sheath with a periodic structure.14,17,19,20 Proteins identified in the sheath include FBN-1, MFAP-1, and type VI collagen.21–23 Extending from the sheath of the elastin-like fibers of the cribriform plexus are connecting fibrils that link to SC endothelial and JCT cells.20,24
Fluid flow through the trabecular meshwork is not uniform but appears segmental.25,26 Within segmental flow patterns are preferential fluid flow routes that occur in regions of the JCT underneath collector channels in human and animal eyes.25,27–29 In regions with elevated pressure, the JCT expands providing increased flow for aqueous humor. Areas of distension and loss of contact with underlying tissue have been observed in focal areas of SC and after laser treatment in monkey and human eyes.29–32 These “foamy” regions are characterized by endothelial cells that expand and extend into SC adjacent to collector channel orifices. It is unknown how or why preferential flow and “foamy regions” occur. One possible explanation is that differences in the distribution of extracellular matrix proteins adjacent to collector channel orifices may provide an environment that allows expansion of SC endothelium. The EFS provides both support and distensibility, which suggests that proteins within this group may have a functional role in formation of preferential flow regions. The aims of this study were to elucidate the composition of the EFS adjacent to collector channels, compare EFS underlying collector channels and the area between collector channels, and characterize changes to EFS under elevated pressure.
Methods
Whole Eye Perfusion
Four pairs of human eyes (mean age 71.0 ± 8.6 years; n = 4) with no clinical history of eye disease were obtained from the Minnesota Lions' Eye Bank within 7.5 hours of death. One eye from each pair was perfused with cell culture medium (Dulbecco's Modified Essential Media [DMEM]; Mediatech, Manassas, VA,) at 10 mm Hg, and the fellow eye was perfused with DMEM at 20 mm Hg for 4 hours. Eyes were perfusion fixed at 10 and 20 mm Hg with 3% paraformaldehyde/0.1% Triton X-100/0.1 M phosphate buffer, pH 7.2, for 15 minutes followed by a 30 minute perfusion with 3% paraformaldehyde/0.1 M phosphate buffer, pH 7.2. The anterior segment was dissected from the eye, split into quadrants, and subdivided into smaller wedges. One wedge from each quadrant of each eye was processed for a total of 32 blocks (four blocks per eye in each of the 8 eyes). Each block was serially sectioned and examined on average for 364 μm per wedge. The 32 blocks contained a total of 63 collector channels with adjacent inner and outer walls of SC. The use of donor human eyes for this study was approved by the Mayo Clinic Institutional Review Board and conforms to the Declaration of Helsinki.
Light Microscopy
The elastic composition of regions with and without collector channels was examined and compared in eyes perfused at 10 and 20 mm Hg. One wedge from each quadrant was embedded in paraffin and serially sectioned at 7 μm in a frontal orientation (anterior to the limbus and proceeding posterior toward SC). Paraffin sections were stained with Weigert's resorcin-fuchsin stain with oxidation to identify elastin fibers.1 Tissue sections were deparaffinized and hydrated using descending concentrations of ethanol to phosphate-buffer saline (dH2O). The sections were oxidized in 10% oxone (Sigma, St. Louis, MO) for 40 minutes in a humidified chamber, rinsed in water, dipped 15 times each in 70% and 95% ethanol, and incubated in a staining solution (Weigert's resorcin-fuchsin staining solution; Electron Microscopy Sciences, Hatfield, PA) for 1 hour at room temperature. Sections were rinsed, differentiated in 1% acid alcohol, and rinsed again with water. Sections were counterstained for 30 seconds (Eosin Y/Phloxine solution; Richard-Allan Scientific, Kalamazoo, MI), dehydrated, cleared in xylene, and mounted in permount. Using this process, elastin stained deep purple-black, elaunin a medium pink-lavender, and oxytalan light lavender. Cell nuclei stained pink. Cell cytoplasm in some sections could be visualized as a lighter pink than the cell nuclei. Sections were examined and photographed using a microscope with a 100 × 1.45 NA oil objective lens (Zeiss Axioplan 2; Zeiss, Thornwood, NY).
Immunohistochemistry
Paraffin tissue sections 7 μm thick from perfused eyes were mounted on glass slides (Superfrost/Plus; Fisher, Pittsburgh, PA), incubated at 60°C for 2 hours, deparaffinized with xylene, and rehydrated through a descending series of ethanols (100%, 95%, 80%, 70%) and rinsed with PBS. To enhance epitope labeling, antigen retrieval was performed using either a hot or cold method. In the hot method, tissue was incubated at 95°C for 15 minutes in 1mM EDTA, pH 8.0, followed by a 15-minute incubation at room temperature. In the cold method, slides were immersed in a solution containing 6 M guanidine HCl, 50 mM dithiothreitol, and 20 mM Tris, pH 8.0, for 15 minutes, washed in 20 mM Tris, pH 8.0, and treated with 100 mM iodoacetamide in the dark for 15 minutes. Both antigen retrieval methods were followed by a water rinse before incubation in 1% BSA/0.3% Triton X-100 in PBS for 60 minutes (blocking buffer). Sections were incubated with guinea pig anti-elastin (AbCam, Cambridge, MA), rabbit anti-FBN-1 (Elastin Products Company, Owensville, MO), or rabbit anti-MFAP-1/2 (Sigma) antibodies. Specificity was checked using human skin sections as a positive control. Skin contains all three fibers; elastin, elaunin, and oxytalan. The immunofluorescent labeling was checked against the known locations of the elastin, elaunin, and oxytalan found using Weigert's stain. For control slides, negative serum of the corresponding primary antibody or blocking buffer without Triton X-100 was used. Secondary antibodies (Alexa Fluor 488, 546, and 647; Invitrogen, Carlsbad, CA) were used to label the primary antibodies. Nuclei were stained with 4′,6-diamidino-2-phenylindole in mounting medium (Vectashield; Vector Laboratories, Burlingame, CA). Labeled tissue sections were examined and photographed with a confocal microscope (Zeiss Confocal LSM 510; Carl Zeiss).
Results
Elastin-like Fibers Adjacent to and between Collector Channel Orifices
Weigert's resorcin-fuchsin stain with oxidation enables characterization of elastin, elaunin, and oxytalan. Analysis of elastin-like fibers within the JCT revealed elastin fibers traveling in proximity to the innermost trabecular beam (Fig. 1). Some elastin fibers split and branched at various angles becoming elaunin. The elaunin fibers surrounded the JCT cells giving them a starlike appearance. Elastin was also found at the base of SC cells (Fig. 2A). Elaunin appeared frequently as a “strut” in a T-formation branching from fibers in the JCT to the base of SC cells. In some areas the elastin/elaunin network took on a ladder-like appearance at the base of the SC cells on both the inner and outer walls. This same ladder-like appearance of the elastin/elaunin network was found in the walls of the collector channels and at the collector channel orifices (Fig. 2B).
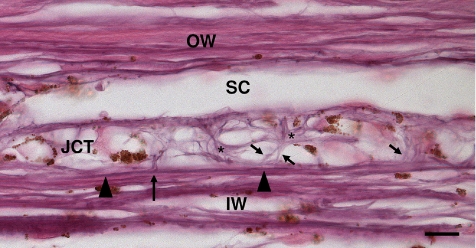
Figure 1.
Elastin fiber system on the inner and outer wall of SC between collector channels under high pressure (20 mm Hg). The elastin fibers traveling adjacent to the innermost beam (arrowheads) splits into elastin (long arrow) and elaunin (short arrows). These fibers surround the JCT cells giving them a starlike appearance (black asterisks). IW, inner wall; JCT, juxtacanalicular tissue; OW, outer wall; SC, Schlemm's canal. Scale bar, 10 μm.
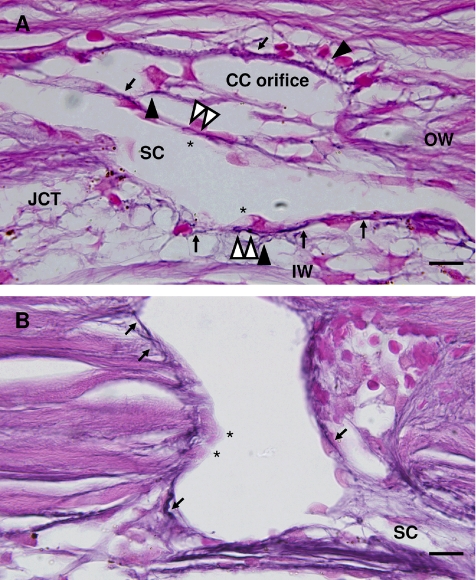
Figure 2.
Elastin and elaunin encircle the base of SC and collector channel cells. (A) Elastin and elaunin adjacent to the base of SC endothelium on the inner wall, outer wall, and at the base of collector channel orifice cells (black arrows). Ladder-like appearance of elastin/elaunin (white arrowheads) at base of cells (asterisks). Elaunin struts extend from JCT region (black arrowheads). (B) Elastin and elaunin are visible on both the left and right sides of collector channel orifice (arrows). Arrows on right side indicate elastin/elaunin at cell base. Out-of-plane cell nuclei are indicated by asterisks. CC, collector channel; IW, inner wall; OW, outer wall; SC, Schlemm's canal. Scale bar, 10 μm.
In addition to elastin and elaunin, oxytalan fibers were visible within the JCT (Fig. 3). These smaller fibers, approximately 1–3 μm in length, were identified in the JCT adjacent to and between collector channels and on both the inner and outer walls of SC. Extending from elaunin and elastin fibers, oxytalan appeared as splayed double- or triple-pronged fine strands. Examination of multiple sections did not reveal discernible differences at the light level in the pattern of these fibers adjacent to or between collector channels.
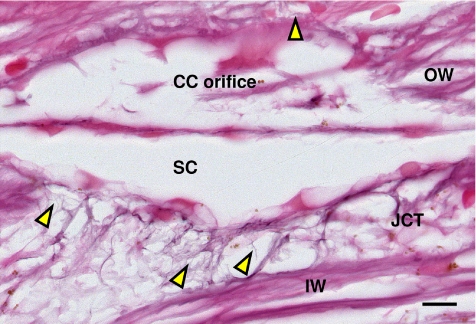
Figure 3.
Oxytalan fibers in JCT of the inner wall. Oxytalan fibers (1–3 μm) found extending from elastin and elaunin fibers in the JCT of the inner and outer wall and in the wall of the collector channel orifice (yellow arrowhead). CC, collector channel; IW, inner wall; OW, outer wall; JCT, juxtacanalicular tissue; SC, Schlemm's canal. Scale bar, 10 μm.
Appearance of the Elastin Fiber System under Elevated Pressure
Examination of the JCT/SC region under collector channels in eyes perfused at high pressure (20 mm Hg) showed an expanded JCT region when compared to the same region in eyes perfused at low pressure (10 mm Hg) (Fig. 4). The inner wall of SC was like a roof supported by elastic and elaunin struts. At low pressure (10 mm Hg), the struts relax or bow and the JCT appears narrow. When pressure increases (20 mm Hg), the struts distend and stretch and the JCT area expands. In high-pressure eyes, elastin and elaunin fibers were elongated and appeared column-like adjacent to SC. Oxytalan fibers were easily visible as elongated splayed fibers (Fig. 4A). At low pressure the elastin and elaunin fibers within the nonexpanded JCT were bowed, compact, and appeared shortened (Fig. 4B). Oxytalan fibers were not readily apparent.
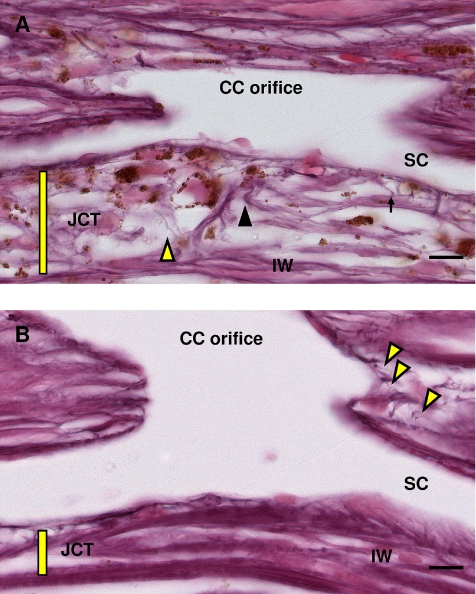
Figure 4.
Elastin fiber system at high and low pressure. (A) At high pressure (20 mm Hg), elastin (black arrowhead), elaunin (arrow), and oxytalan (yellow arrowhead) are visible in the JCT tissue (yellow bar) and in the collector channel walls. (B) At low pressure (10 mm Hg), elastin and elaunin are visible, but oxytalan is less apparent because of compact JCT (yellow bar). Oxytalan can be seen in the collector channel wall (yellow arrowheads). Note expansion of JCT in high pressure (A) compared with low pressure (B). CC, collector channel; IW, inner wall; JCT, juxtacanalicular tissue; SC, Schlemm's canal. Scale bar, 10 μm.
Localization of FBN-1 and MFAP-1/2 in Relation to Elastin
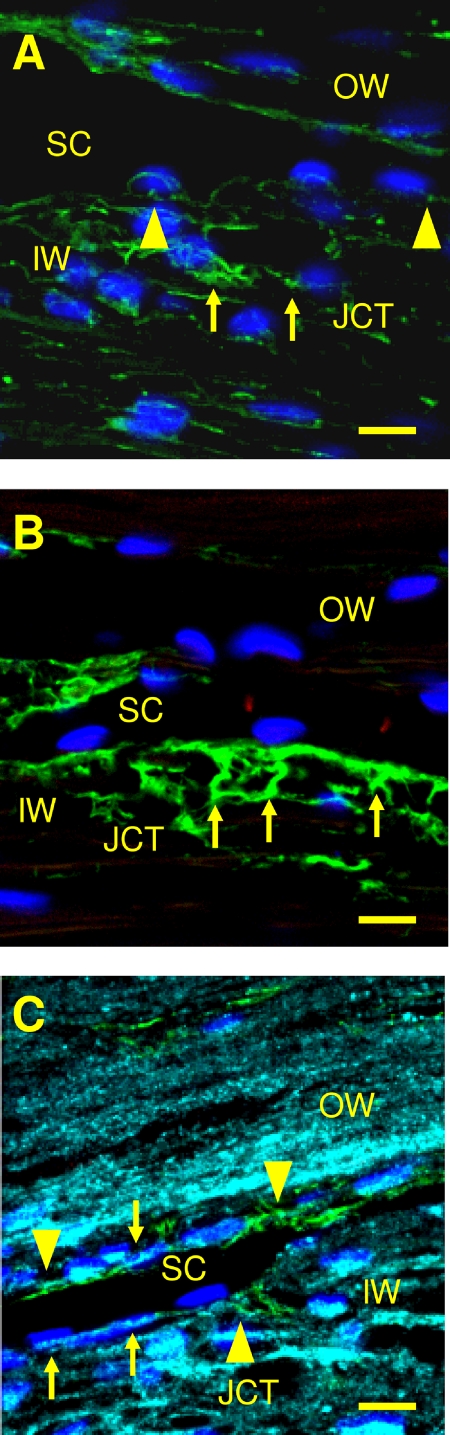
In addition to elastin-like fibers, the EFS contains elastin-associated proteins. Previous reports have identified FBN-1 and MFAP-1 in the JCT.21,22 To verify the presence of FBN-1 and MFAP-1/2, immunohistochemistry was performed on normal trabecular meshwork tissue. In the JCT/SC region, FBN-1 was identified at the base and on the lateral borders of SC cells on the inner and outer wall (Fig. 5A). FBN-1 was found in patches and some fibers surrounding JCT cells. Fine fibers with splayed ends were not observed. MFAP-1/2 was located at the base of SC cells and corresponded in size and appearance to the T-formation of the elaunin struts (Fig. 5B). MFAP-1/2 was also observed in the trabecular meshwork uveal region, which appeared to terminate on the surface of and between the trabecular beams. Elastin staining appeared as patches and streaks within the JCT and at the base of SC endothelial cells in the inner and outer walls. Elastin and MFAP-1/2 staining localized to the same areas in some JCT/SC sections (Fig. 5C). Elastin labeling was also found at the base of SC between cells in an abbreviated ladder-like appearance. This ladder-like appearance was similar to the ladder-like appearance of elastin observed with Weigert's resorcin-fuchsin stain (compare Fig. 2 with Fig. 5C).
Figure 5.
Elastin and elastin-associated proteins in the JCT and SC region. (A) FBN-1 (green, Alexa Fluor 488) labels in discrete areas adjacent to Schlemm's canal endothelial cells (arrowheads) and some fibers surrounding JCT cells (arrows). (B) MFAP-1/2 (arrows) stains strutlike fibers (green, Alexa Fluor 488) extending from the JCT region to base of SC cells. (C) Elastin (pseudocolored aqua, Alexa Fluor 647) at base of SC endothelium and JCT cells (arrows). Some elastin is observed adjacent to and in the same regions as MFAP-1/2 fibers (green, Alexa Fluor 488) in the inner wall and outer wall (arrowheads). CC, collector channel; IW, inner wall; JCT, juxtacanalicular tissue; SC, Schlemm's canal. Scale bar, 10 μm.
Discussion
Recent studies from several laboratories including ours suggests that preferential fluid flow pathways exist within the trabecular meshwork underneath collector channel orifices.25,27–29 Associated with these preferential pathways is an expansion of the trabecular meshwork within the JCT. What the expanded trabecular meshwork region is composed of and how it expands is unknown. This study demonstrates that elastin, elaunin, and oxytalan fibers are found in the JCT region at the base of SC endothelium, in the inner and outer walls of SC, and in the orifice and walls of the collector channels. Under pressure, expanded JCT regions contain elastin, elaunin, and oxytalan fibers that are elongated, forming pillars that appear to support and control the expansion process. The presence and elongation of elastin, elaunin, and oxytalan fibers within the preferential flow pathways suggests a complex role of the EFS system in outflow resistance and intraocular pressure regulation.
Previous light microscopy studies found elastin and elastin-like fibers in the JCT region.14,17 Using a combination of Gömöri stain for reticulum and resorcin-fuchsin without oxidation, Rohen et al.17 identified elastin and elaunin within the cribiform plexus. Oxytalan was not identified. With transmission electron microscopy, Lutjen-Drecoll and Rohen14 confirmed the presence of elastin and elaunin within the cribiform plexus and described connecting fibrils in the JCT. Because of their location within the JCT, the connecting fibrils were later identified as oxytalan.14 In contrast, antibodies made to elastin did not detect elastin in the JCT region.33 The discrepancy between Murphy et al.33 and previous studies could be due to the inability of the antibody to recognize elastin in fixed tissue. Our study, using resorcin-fuchsin with oxidation, showed elastin and elaunin and identified numerous oxytalan fibers either as single fibers or in bundles within the JCT region. In addition, FBN-1, which was previously localized to oxytalan fibers in the zonular fibers of the lens,34 was observed at the base and edges of SC endothelial cells, similar to oxytalan fibers identified in the JCT/SC region. The oxytalan/FBN-1 patches present at lateral edges of SC may be where connecting fibrils insert or join with SC cells. However, it is apparent that the oxytalan fibers are not solely composed of FBN-1 because of the punctate staining pattern found with immunolabeling compared with the fiber-like appearance with Weigert's resorcin-fuchsin stain. The oxytalan/FBN-1 network may contribute to the repetitive nature of adjustment and fine distensibility required in the JCT/SC region, much like the function of the zonular fibers of the lens.
Although analysis of the EFS in anterior segments has concentrated mainly on regions between collector channels, this study concentrated on the composition of the EFS under collector channels. Our hypothesis was that if the EFS was altered or even absent adjacent to collector channels, this change would allow/enhance the formation of expanded regions of SC endothelium, thus contributing to the formation of preferential flow pathways. This was not the case. Elastin, elaunin, and oxytalan fibers were not marginalized to regions adjacent to the orifice, nor were they absent from the orifice regions. Clustering or concentration of any one fiber type was not observed at or adjacent to the orifice. The homogeneous pattern of elastin, elaunin, and oxytalan underneath and between collector channels suggests they are not the only players in the formation of preferential flow pathways. The EFS family and associated proteins comprise 34 molecules,35 and it is plausible that other EFS molecules may be differentially expressed in preferential flow pathways. One method to discriminate important molecules involved in preferential flow pathways may be to perform comparative studies in animal species that demonstrate the “wash-out” effect. In “wash-out,” prolonged perfusion causes separation of the JCT, JCT and endothelium, or endothelium alone, thus increasing the outflow facility.36,37 Humans do not have “wash-out”; this is partly because of the more complex EFS structure that anchors the inner wall.37 Comparative studies at the light microscope level using Weigert's resorcin-fuchsin stain with oxidation and immunohistochemistry may further clarify the differences observed in the EFS between species.
The EFS in the JCT region appears to be responsive to pressure. Under normal pressure, elastin/elaunin fibers appear as compact structures that encircle the base of SC cells and link to fibers that surround the JCT cells. Similar structures are apparent in scanning electron micrographs in which SC endothelial cells are absent after H7 treatment.38 Under elevated pressure, the elastin/elaunin struts in the JCT straighten and become the columns between the layers of the cribriform plexus and the elastin/elaunin fibers encircling the base of SC. The expansion appears to effectively increase the JCT region under collector channels and to a lesser extent between collector channels. Although collector channels have been thought to be passive structures conducting aqueous humor to the episcleral veins, the identification of oxytalan extending from elaunin and elastin fibers into the extracellular matrix within collector channel walls suggests the EFS may provide patency within collector channels when they are subjected to normal and elevated pressure.
The labyrinth of fibers seen in the JCT region, adjacent to SC endothelium, and in collector channel walls suggests that a greater potential for distensibility than previously thought exists in this region. We have previously reported that under collector channels, the JCT can expand by up to 200%.28 Interestingly, both elastin and FBN-1 microfibrils are capable of stretching up to two times their original length.39,40 This web of fibers links to the fenestrated substrate composed, in part, of elaunin and elastin encircling SC endothelium. This would allow for the formation of “foamy regions” by distension of cells on this network. In addition, the EFS may also function to prevent overextension and separation of the SC endothelium and the JCT both adjacent to CCs and between CCs. Tracer outflow patterns have also been noted to increase adjacent to CCs under increasing pressure conditions.37 More study of CCs is warranted because narrowing of the orifices and/or the channels could cause changes in outflow facility.41
Increases in the number of microfibrils have been demonstrated in primary open-angle glaucoma.14 In addition, alterations in elastic fibers have been found in the optic nerve head of glaucomatous eyes.42 This leaves open the possibility that glaucoma in part may be a degeneration or alteration in the elastin-like fibers of the JCT, collector channels, and/or the optic nerve head. The EFS appears to have two major functions: a key connection between the elastin-like tendons of the ciliary body, JCT cells, and SC endothelium, and as a support structure in the anchorage, expansion, and control of distension within the inner wall. It is a distinct possibility that because of this interconnectivity, the cribriform plexus, the JCT, and SC endothelium may function as a “biomechanical unit” to modulate aqueous outflow by expansion and distension in response to discrete and overt changes in pressure through elastin-like fibers. Alteration of the EFS may affect the distensibility observed in this region, influencing aqueous outflow and elevating intraocular pressure.
Footnotes
Supported in part by National Institutes of Health research Grants EY 07065 and EY 15736; Mayo Foundation, Rochester, Minnesota; and Research to Prevent Blindness, New York, New York. (MPF is a recipient of a Lew R. Wasserman Merit Award, and the Department of Ophthalmology, Mayo Clinic, is the recipient of an unrestricted grant).
Disclosure: C.R. Hann, None; M.P. Fautsch, None
References
- 1. Montes GS. Distribution of oxytalan, elaunin and elastic fibres in tissues. J Brazil Assoc Advance Sci. 1992;44:224–233 [Google Scholar]
- 2. Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7:1208–1218 [PubMed] [Google Scholar]
- 3. Ghadially FN. Extracellular matrix (extracellular components). Ultrastructural Pathology of the Cell and Matrix. 3rd ed London: Butterworth; 1988:1252–1258 [Google Scholar]
- 4. Koenders MMJF, Wismans RG, Starcher B, Hamel BCJ, Dekhuij-zen RPN, van Kuppevelt TH. Fibrillin-I staining anomalies are associated with increased staining for TGF-B and elastic fibre degradation: new clues to the pathogenesis of emphysema. J Pathol. 2009;218:446–457 [DOI] [PubMed] [Google Scholar]
- 5. Kielty CM, Michael J., Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828 [DOI] [PubMed] [Google Scholar]
- 6. Kielty CM, Shuttleworth CA. Fibrillin-containing microfibrils: structure and function in health and disease. Int J Biochem Cell Biol. 1995;27:747–760 [DOI] [PubMed] [Google Scholar]
- 7. Sawada T, Sugawara Y, Asai T, et al. Immunohistochemical characterization of elastic system fibers in rat molar periodontal ligament. J Histochem Cytochem. 2006;54:1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wheatley HM, Traboulsi EI, Flowers BE, et al. Immunohistochemical localization of fibrillin in human ocular tissues: relevance to the Marfan syndrome. Arch Ophthalmol. 1995;113:103–109 [DOI] [PubMed] [Google Scholar]
- 9. Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum Mol Genet. 1995;4:1799–1809 [DOI] [PubMed] [Google Scholar]
- 10. Ritch R, Schlotzer-Schrehardt U, Konstas AGP. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res. 2003;22:253–275 [DOI] [PubMed] [Google Scholar]
- 11. Lonnqvist L, Karttunen L, Rantamaki T, Kielty C, Raghunath M, Peltonen L. A point mutation creating an extra N-glycosylation site in fibrillin-1 results in neonatal Marfan syndrome. Genomics. 1996;36:468–475 [DOI] [PubMed] [Google Scholar]
- 12. Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82:637–640 [DOI] [PubMed] [Google Scholar]
- 13. Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13:421–438 [DOI] [PubMed] [Google Scholar]
- 14. Lutjen-Drecoll E, Rohen JW. Morphology of aqueous outflow pathways in normal and glaucomatous eyes. In: Ritch R, Shields MB, Krupin T. eds. The Glaucomas. St. Louis: Mosby;1996:89–102 [Google Scholar]
- 15. Rohen JW, Lutjen-Drecoll E. Age changes of the trabecular meshwork in human and monkey eyes. In: Bredt H, Rohen JW. eds. Aging and Development. Stuttgart, Germany: Schattauer;1971:1–36 [PubMed] [Google Scholar]
- 16. Flocks M. The anatomy of the trabecular meshwork as seen in tangential sections. Arch Ophthal. 1956;56:708–718 [DOI] [PubMed] [Google Scholar]
- 17. Rohen JW, Futa R, Lutjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981;21:574–585 [PubMed] [Google Scholar]
- 18. Rohen JW. Morphology and pathology of the trabecular meshwork. In: Smelser GK. ed. The Structure of the Eye. New York: Academic Press;1961:335–341 [Google Scholar]
- 19. Gong H, Trinkaus-Randall V, Freddo TF. Ultrastructural immunocytochemical localization of elastin in normal human trabecular meshwork. Curr Eye Res. 1989;8:1071–1082 [DOI] [PubMed] [Google Scholar]
- 20. Lutjen-Drecoll E, Futa R, Rohen JW. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1981;21:563–573 [PubMed] [Google Scholar]
- 21. Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43:1068–1076 [PubMed] [Google Scholar]
- 22. Ueda J, Yue BYJT. Distribution of myocilin and extracellular matrix components in the corneoscleral meshwork of human eyes. Invest Ophthalmol Vis Sci. 2003;44:4772–4779 [DOI] [PubMed] [Google Scholar]
- 23. Lutjen-Drecoll E, Rittig M, Rauterberg J, Jander R, Mollenhauer J. Immunomicroscopical study of type VI collagen in the trabecular meshwork of normal and glaucomatous eyes. Exp Eye Res. 1989;48:139–147 [DOI] [PubMed] [Google Scholar]
- 24. Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E, Birke M. Biochemical and morphological analysis of basement membrane component expression in corneoscleral and cribriform human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:794–801 [DOI] [PubMed] [Google Scholar]
- 25. Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49:5346–5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88:656–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hann CR, Bahler CK, Johnson DH. Cationic ferritin and segmental flow through the trabecular meshwork. Invest Ophthalmol Vis Sci. 2005;46:1–7 [DOI] [PubMed] [Google Scholar]
- 28. Hann CR, Fautsch MP. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest Ophthalmol Vis Sci. 2009;50:1692–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melamed S, Epstein DL. Alterations of aqueous humour outflow following argon laser trabeculoplasty in monkeys. Br J Ophthalmol. 1987;71:776–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee WR, Grierson I. Relationships between intraocular pressure and the morphology of the outflow apparatus. Trans Ophthalmol Soc UK. 1974;94:430–449 [PubMed] [Google Scholar]
- 31. Svedbergh B. Protrusions of the inner wall of Schlemm's canal. Am J Ophthalmol. 1976;82:875–882 [DOI] [PubMed] [Google Scholar]
- 32. Johnson DH. Histologic findings after argon laser trabeculoplasty in glaucomatous eyes. Exp Eye Res. 2007;85:557–562 [DOI] [PubMed] [Google Scholar]
- 33. Murphy CG, Yun AJ, Newsome DA, Alvarado JA. Localization of extracellular proteins of the human trabecular meshwork by indirect immunofluorescence. Am J Ophthalmol. 1987;104:33–43 [DOI] [PubMed] [Google Scholar]
- 34. Ashworth JL, Kielty CM, McLeod D. Fibrillin and the eye. Br J Ophthalmol. 2000;84:1312–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kielty CM. Elastic fibres in health and disease. Exp Rev Mol Med. 2006;8:1–23 [DOI] [PubMed] [Google Scholar]
- 36. Scott PA, Overby DR, Freddo TF, Gong H. Comparative studies between species that do and do not exhibit the washout effect. Exp Eye Res. 2007;84:435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong H, Freddo TF. The washout phenomenon in aqueous outflow—why does it matter? Exp Eye Res. 2009;88:729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bahler CK, Hann CR, Fautsch MP, Johnson DH. Pharmacologic disruption of Schlemm's canal cells and outflow facility in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2004;45:2246–2254 [DOI] [PubMed] [Google Scholar]
- 39. Roach MR, Song SH. Arterial elastin as seen with scanning electron microscopy: a review. Scanning Microsc. 1988;2:994–1004 [PubMed] [Google Scholar]
- 40. Sherratt MJ, Baldock C, Hoston JL, et al. Fibrillin microfibrils are stiff reinforcing fibers in compliant tissues. J Mol Biol. 2003;332:183–193 [DOI] [PubMed] [Google Scholar]
- 41. Gong H, Freddo TF, Zhang Y. New morphological findings in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2007;48:E–Abstract 2079 [Google Scholar]
- 42. Quigley HA, Brown A, Dorman-Pease ME. Alterations in elastin of the optic nerve head in human and experimental glaucoma. Br J Ophthalmol. 1991;75:552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]