This study highlights the importance of following up patients with glaucoma with more than one test.
Abstract
Purpose.
To compare glaucoma progression by functional and structural tests.
Methods.
The authors prospectively studied 33 glaucoma patients (55 eyes); 20 eyes (15 patients) had disc hemorrhage, and 35 eyes (18 patients) had exfoliation glaucoma. The following tests were performed at two baseline and three follow-up examinations: frequency doubling perimetry (FDT), 24-2 Humphrey visual fields (HVF), multifocal visual evoked potentials (mfVEP), and optical coherence tomography (OCT). To identify progression, the baseline measurements were averaged and compared to those obtained at the final examination. Stereophotographs of the optic disc were obtained at baseline and compared with those at the final examination.
Results.
Patients were followed up for 21.1 ± 1.8 months. For HVF there were significant changes in mean deviation (MD) in eight (14.5%) eyes but in pattern standard deviation (P/SD) in only two (3.6%) eyes. For FDT, there were significant changes in MD in 13 (23.6%) eyes. Five eyes showed changes in MD for HVF and FDT. For mfVEP, there was an increase in abnormal points in nine (16.4%) eyes. Six of these eyes did not show significant HVF or FDT changes. For OCT, RNFL average thickness values were significantly decreased in nine (16.4%) eyes. Nine (16.4%) eyes showed progression on stereophotography; four of these eyes did not show significant changes on OCT and functional tests.
Conclusions.
Each test showed evidence of progression in some eyes. However, agreement among tests and stereophotography regarding which eyes showed progression was poor, illustrating the importance of following up patients with a combination of functional and structural tests.
Glaucoma is defined as a progressive optic neuropathy that leads to a characteristic pattern of optic nerve head damage and visual field loss. Because documented progression may require modification of treatment strategies, determining progression is one of the most important and challenging aspects of management. Progression can be assessed with both structural and functional tests. For example, morphologic changes can be evaluated with stereophotography of the optic disc and optical coherence tomography (OCT), which allows quantitative measurement of the retinal nerve fiber layer. For functional testing, clinicians typically rely on standard automated perimetry (SAP), most commonly on changes in 24-2 Humphrey visual fields (HVF). It is commonly believed that SAP can detect glaucomatous changes only after extensive disc damage has occurred,1 although the situation is not that simple.2–4 This has led to the development of other tests of visual function designed to detect glaucomatous damage at earlier stages of the disease process. In this study, in addition to 24-2 Humphrey visual fields, we also used frequency doubling technology (FDT) perimetry and the multifocal visual evoked potential (mfVEP) technique to test visual function. The purpose of this study was to compare detection of progression as assessed by functional (HVF, FDT, mfVEP) and structural (OCT) tests and by stereophotography of the optic disc.
We enrolled patients with either exfoliation glaucoma or optic disc hemorrhage (DH), because these have been found to be important factors predisposing to glaucoma progression. Patients with exfoliation glaucoma present with higher intraocular pressure (IOP) and greater visual field loss and optic nerve damage than do patients with primary open-angle glaucoma. The prognosis for exfoliation glaucoma is worse than that for primary open-angle glaucoma because of higher IOP levels, rapid progression, and more severe optic nerve damage and visual field defects.5,6 The association between DH and glaucomatous damage has been known since the first report by Bjerrum more than 100 years ago.7,8 Visual field defects develop and progress more often if patients have had a DH.9,10
Subjects and Methods
Subjects
We recruited 33 glaucoma patients (10 men, 23 women), aged 34 to 80 years (mean, 65.3 ± 9.5) from the glaucoma practice of two of the authors (RR and JL). The patients were all experienced visual field takers. They had glaucomatous optic disc damage in either one or both eyes. Among the 55 eyes studied, 20 eyes of 15 patients had DH and 35 eyes of 18 patients had exfoliative glaucoma. Inclusion criteria were best-corrected visual acuity ≥20/40 and refractive errors not exceeding 6.00 diopters sphere or 2.00 diopters cylinder. Eyes with coexisting retinal disease, uveitis, or nonglaucomatous optic neuropathy were excluded. The study adhered to the tenets of the Declaration of Helsinki, and all participants gave written informed consent before enrollment.
Procedures
With the exception of stereophotography, the testing procedure involved two baseline (two tests within 30 days) and three follow-up examinations at 6-month intervals. The following tests were included in the study.
Stereophotography
Simultaneous stereophotographs of the optic disc were taken using a mydriatic fundus camera (3-Dx; Nidek, Aichi, Japan) and were viewed with a stereoviewer. Stereophotographs were obtained on all recruited patients at baseline and within 1 month of the final examination.
Multifocal VEP
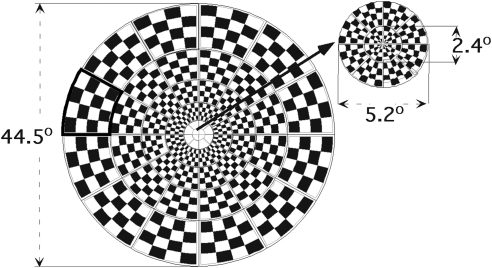
The mfVEPs were obtained from each eye using imaging software (VERIS 5.0; Electro-Diagnostic Imaging, San Mateo, CA). The stimulus display is shown in Figure 1. The dartboard pattern consisted of 60 sectors, each with a checkerboard pattern of 16 checks, eight white (luminance = 200 cd/m2) and eight black (luminance < 3 cd/m2). The dartboard pattern had a radius of 22.3° and was displayed on a black-and-white monitor driven at a frame rate of 75 Hz. The 16-element checkerboard of each sector had a probability of 0.5 of reversing on any pair of frame changes, and the pattern of reversals for each sector followed a pseudorandom (m) sequence. On every frame change (every 13.3 ms), each sector could reverse contrast or stay at the same contrast. A refractor/camera was used to refract the subjects and monitor eye position and stability throughout the test. Subjects fixated on the center of a black cross in the middle of the display. Segments contaminated by eye movements, loss of fixation, unsteady fixation, or external noise were discarded and rerecorded.
Figure 1.
The multifocal VEP stimulus display: the dartboard pattern consists of 60 sectors, each with a checkerboard pattern of 16 checks, eight white (200 cd/m2) and eight black (3 cd/m2). The entire display subtended a diameter of 44.5°, and the central 12 sectors fell within a diameter of 5.2° of the foveal center.
The details of the mfVEP recording and analysis have been published previously (see Ref. 11 for review). Briefly, three channels of recording were obtained with gold cup electrodes. Recording electrodes were placed at the inion, 4 cm above the inion, and two lateral locations up 1 cm and over 4 cm to the left and right side of the inion. In addition to the three channels that were recorded, data for three other channels were derived from the difference between pairs of electrodes, as previously described.11,12 Electrode impedance was <5 kW in all cases. Signals were amplified (preamplifier P511J; Grass Instruments, Quincy, MA), bandpass filtered from 3 to 100 Hz, and sampled at 1200 Hz. The mfVEPs were low-pass filtered using a sharp cutoff at 35 Hz and a fast Fourier transform technique. This and all other analyses were performed with programs written in computing software (MATLAB; The MathWorks, Inc., Natick, MA). The mfVEP records were processed, and an array of best channel responses were derived as previously described.11–13 Analyses were performed on the best responses from the six channels. The 60 responses selected for each eye defined the best array for that eye.13 The amplitude of each of the 60 local responses were compared with those of a normative group,14 and interocular (comparison of two eyes) and monocular probability plots, analogous to the HVF probability plots, were derived.11,15 The normative group consisted of 100 subjects with visual acuity ≥20/30 in each eye, normal HVFs, and no evidence of any ocular or systemic disease. They ranged in age from 21 to 92 years; the mean age was 49 ± 13.6 years.
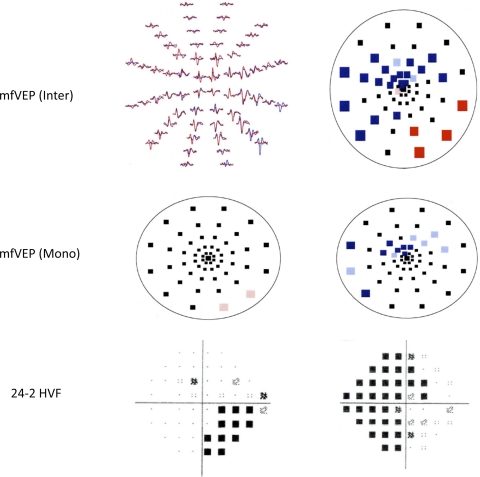
Figure 2 shows sample mfVEP responses, monocular and interocular probability plots, and HVF results obtained from both eyes of a patient with bilateral DH.
Figure 2.
mfVEP interocular (right, top) and monocular (middle) probability plots and 24-2 HVF test results (bottom) for a patient with bilateral DH. Colored squares: significant difference at greater than the 5% (desaturated) or 1% (saturated) level. Left, top: mfVEP responses obtained from the right eye (blue) and left eye (red) of the patient.
Humphrey Visual Field Testing
SAP was performed with the HVF (Humphrey Visual Field Analyzer; Humphrey-Zeiss, San Francisco, CA) using the 24-2 SITA standard program. The criteria for reliable HVF results were <20% fixation losses, <25% false positives, and <25% false negatives. Mean deviation (MD) and pattern SD (PSD) values were analyzed.
FDT Perimetry
FDT perimetry was performed (Humphrey Matrix Perimeter; Carl Zeiss Meditec, Humphrey Division, Dublin, CA) using the 24-2 ZEST test strategy. The reliability criteria for the FDT test were <20% fixation losses, <25% false positives, and <25% false negatives. MD and PSD values were analyzed.
Optical Coherence Tomography
Retinal nerve fiber layer (RNFL) thickness values were obtained with OCT using the Fast RNFL Thickness acquisition protocol (Stratus OCT model 3000; Carl Zeiss Meditec) with version 4.0 software. If the diameter of the patient's pupil was <3 mm, it was dilated with 0.5% tropicamide before taking OCT scans. Retinal thickness was determined at 256 points in a three-sequential circular scan of 3.4 mm around the center of the optic disc. RNFL total thickness was evaluated from the average scan. Superior and inferior quadrant RNFL thickness measurements were analyzed.
Identifying Progression
Given that there was no control group in this study, we used the Bland-Altman method to establish a criterion for progression.16 This method has been used for identifying change in repeated visual acuity measurements in patients.17–20 Our cutoff for identifying progression was the limit of agreement for test-retest variability. This was defined as ±1.96 SD of the differences between paired (i.e., baseline) measurements of tested eyes. This approach ensures that, in the absence of true progression (assuming test-retest variability to be normally distributed, normality was tested with the D'Agostino-Pearson K2 omnibus test; assuming homoscedasticity, it was tested by visual inspection of plots of the residuals), only 5% of the measured differences will exceed this level; in other words, the approach fixes the specificity of the procedure at 95%. Because the limits of agreement are estimates, the 95% confidence intervals (CIs) or tolerance limits for the upper and lower limits were also calculated. The CIs take into account the uncertainty of the estimates of the limits of agreement. They are shown in each Bland-Altman plot to indicate the possible range for progression and false positives. To identify progression, the two baseline measurements for the right eyes were averaged, and these values were compared with those obtained at the final examination. The same procedure was followed for the left eyes. In addition to the points satisfying significant progression, those showing significant “improvement” were also tabulated. This analysis was performed for MD and PSD values for FDT and HVF, number of abnormal points with probability values of 1% and 5% for mfVEP, and average RNFL thickness value for superior and inferior quadrants for the OCT.
To identify progression using the stereophotographs of the optic disc, two glaucoma specialists (RR, JML) independently viewed each pair of stereophotographs masked to both patient data and temporal sequence of the images. A third glaucoma specialist (CGVM) reviewed the sets of photographs in which there was disagreement regarding adjudication.21
Results
Thirty-three patients (55 eyes) completed the two baseline visits (baseline 1 and baseline 2) within a 30-day period. Because test-retest variability is a major limitation in assessing visual function in glaucoma, retest repeatability was assessed using Bland-Altman plots. The mean difference for all was close to 0, with the exception of the superior quadrant thickness values for the OD for OCT (mean, −2.41) and the mfVEP monocular analysis for the left eye (mean, 1.86). In addition, to determine whether there was proportional bias, regression analyses were performed. The trend lines had slopes close to zero, with P > 0.05.
Identification of Progression
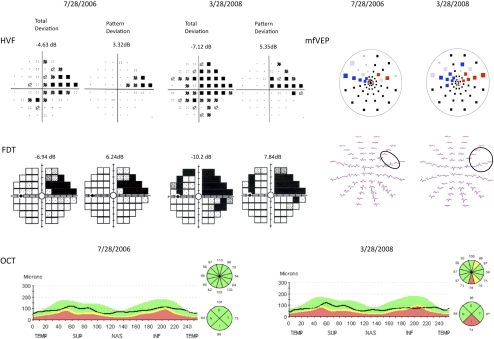
The 33 patients were followed up for an average of 21 months (21.1± 1.8 months; range, 18–26 months). An example of progression in a patient's left eye is shown in Figure 3. At baseline (July 28, 2006) the HVF and FDT total deviation and pattern deviation probability plots show a superior visual field defect for the left eye. The mfVEP interocular probability plot also shows a cluster of abnormal points in the superior field for the left eye (red). However, the OCT superior and inferior quadrant RNFL thickness is within normal limits. After 20 months (March 28, 2008), there appears to be progression on all four tests.
Figure 3.
Follow- up HVF, FDT, mfVEP, and OCT results for the left eye of a patient with bilateral exfoliation glaucoma. For each test, results at baseline are on the left and results at the final visit are on the right.
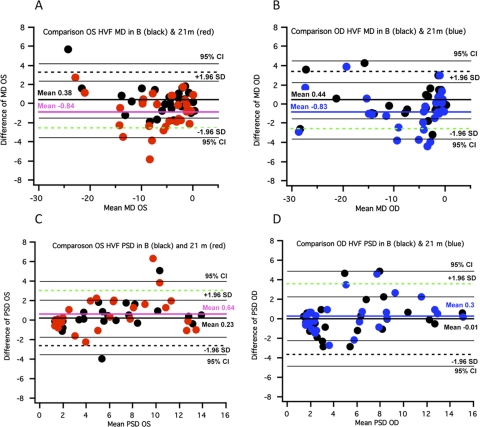
For all 33 patients (55 eyes), we compared the results at the final visit (mean, 21 months) with those obtained at baseline to determine whether the data fell outside the baseline limits of agreement. For example, we considered the MD values of the HVF test in Figure 4 for the left (Fig. 4A) and right (Fig. 4B) eyes. In this figure, and in Figures 5 to 7, the deviations from the mean baseline value are shown; each plot shows the results for the baseline measures (black dots) or the results at a mean of 21 months (colored dots). Four of the 27 data points for the left eye and six of the data points for the right eye fall below the dashed green line, representing the 95% limit of agreement, our definition of progression. Two of these data points for the left eye and four for the right eye fall below the 95% CI for the limit of agreement. For the PSD values, three of the data points fall above (i.e., the direction of a worsening of the field) the limit of agreement, two for OS and one for OD. Only one data point for OS falls above the 95% CI for the limit of agreement. In summary, for the HVF test, we found significant changes (values outside the limits of agreement) in MD and PSD values, indicating progression in 10 (18.2%) eyes for MD but in only three (5.5%) eyes for PSD (Figs. 4A–D).
Figure 4.
Bland-Altman plots for HVF MD and PSD results for 55 eyes at baseline and 21 months. (A, B) Plots of the difference in MD values for the final visit (mean, 21 months; red and blue dots) versus the baseline visit (black dots) for left (red) and for right (blue) eyes. (C, D) Plots of the differences in PSD values for the final visit versus the baseline visit. Green dashed line: −1.96 SD for baseline data and limits of agreement. Black dashed lines: +1.96 SD for baseline data and limits of agreement. Thin black lines: 95% CI of the limits of agreement.
Figure 5.
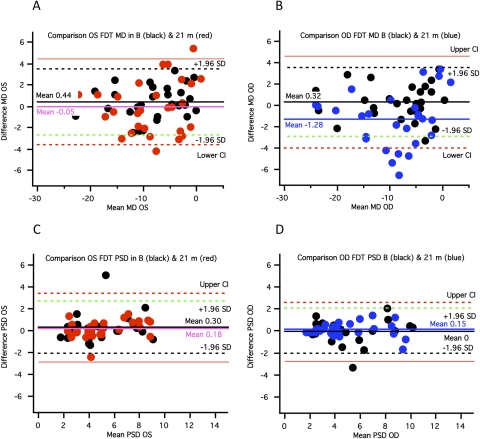
Bland-Altman plots for FDT MD and PSD results for 55 eyes at baseline and 21 months. (A, B) Plots of the difference in MD values for the final visit (mean, 21 months; red and blue dots) versus the baseline visit (black dots) for left (red) and for right (blue) eyes. (C, D) Plots of the differences in PSD values for the final visit versus the baseline visit. Green dashed line: −1.96 SD for baseline data and limits of agreement. Black dashed lines: +1.96 SD for baseline data and limits of agreement. Thin black lines: 95% CI of the limits of agreement.
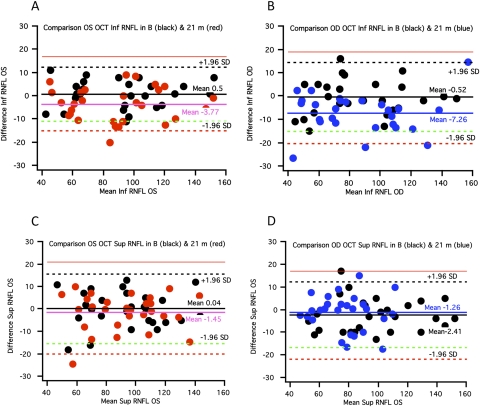
Figure 7.
Bland-Altman plots for OCT RNFL thickness results for 55 eyes at baseline and 21 months. Plots of the difference in (A, B) inferior quadrant area values and (C, D) superior quadrant area values for the final versus the baseline visit.
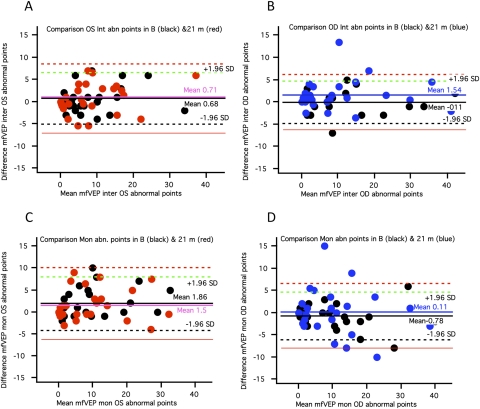
Figure 5 shows a similar analysis for the FDT test. For FDT, there were significant changes in MD in 13 (23.6%) eyes but no change consistent with progression in PSD values (Figs. 5A–D). Six eyes showed changes consistent with progression in MD for both FDT and HVF tests. Results for the mfVEP are shown in Figure 6. There was a significant increase in the number of abnormal points in nine eyes (16.4%). However, 6 of 9 eyes did not show changes consistent with progression for either HVF or FDT MD and PSD values.
Figure 6.
Bland-Altman plots for mfVEP abnormal points for 55 eyes at baseline and >18 months. (A, B) Plots of differences in abnormal points for the interocular plot for the final visit (mean, 21 months; colored dots) versus the baseline visit (black dots). (C, D) Plots of the difference in abnormal points for the monocular plot for the final visit (mean, 21 months; colored dots) versus the baseline visit (black dots).
Figure 7 shows the analysis for OCT quadrant RNFL average thickness values. Values were decreased for 11 eyes for these measurements (20%). Four of these eyes did not show significant changes on HVF, FDT, or mfVEP tests.
Stereophotographs are often taken as the gold standard. Nine of 55 eyes (16.4%) showed progression on stereophotography, and three showed no changes on either the functional measures or OCT.
In summary, 18.2%, 23.6%, 16.4%, 20%, and 16.4% of eyes showed evidence of progression on HVF, FDT, mfVEP, OCT, and stereophotography, respectively. Twelve eyes (60%) with DH and 16 eyes (45.7%) with pseudoexfoliation glaucoma had results that were consistent with progression on at least one measure. It should be noted that all eyes that showed evidence of progression (i.e., that met our criterion) also showed trends consistent with deterioration at the 12-month follow-up visit.
“Significant Improvement” as a Proxy for Specificity
Only one eye showed “significant improvement,” our proxy for specificity of the procedure, in the MD value for HVF, whereas for the FDT test three eyes showed it in MD values and one eye showed it in PSD value. For the mfVEP test, five eyes showed a decrease in the number of abnormal points (i.e., improvement). For the OCT, only one eye showed significant improvement in RNFL thickness values. The number and percentage of eyes showing progression compared with those showing improvement for HVF, FDT, OCT, and mfVEP are summarized in Table 1.
Table 1.
Progression versus Improvement
| Progression n (%) | Improvement n (%) | |
|---|---|---|
| HVF MD | 10 (18.2) | 1 (1.8) |
| PSD | 3 (5.5) | 0 |
| FDT MD | 13 (23.6) | 3 (5.5) |
| PSD | 0 | 1 (1.8) |
| OCT | 11 (20) | 1 (1.8) |
| mfVEP | 9 (16.4) | 5 (9.1) |
Agreement among Tests
No eye showed progression on all measures. In fact, agreement among tests was not good (Table 2). Even when we limited the analysis to one structural measure (OCT) and two functional measures (HVF and FDT) only two eyes showed progression on all three measures. The best agreement was between the two functional tests (HVF and FDT) and between one of the functional tests, FDT, and OCT, in which five eyes showed progression on both measures. Finally, although nine eyes showed progression on stereophotographs, only five of these eyes showed progression on one or more of the other tests.
Table 2.
Number of Eyes Showing Progression on Different Tests
| HVF | FDT | mfVEP | OCT | Stereophotographs | |
|---|---|---|---|---|---|
| HVF | 10 | 6 | 2 | 2 | 2 |
| FDT | — | 13 | 2 | 5 | 3 |
| mfVEP | — | — | 9 | 2 | 0 |
| OCT | — | — | — | 11 | 4 |
| Stereophotographs | — | — | — | — | 9 |
Discussion
To determine whether an eye showed “significant” progression, we used an approach based on Bland-Altman's method for examining repeatability. Test results at the final visit (mean, 21 months) were compared with those obtained at baseline. The 95% limits of agreement for the baseline measurements were a proxy for specificity. Eyes showing progression (deterioration) were defined as those associated with data points that fell outside the 95% baseline limits of agreement (Figs. 4–7, dashed green lines). Using this definition, we found evidence of progression that occurred over a relatively short period. In our data set, 12 eyes (60%) with DH and 16 eyes (45.7%) with exfoliation glaucoma showed evidence of progression on at least one of the tests. Results for eyes with DH were consistent with those of Siegner and Netland,22 who demonstrated that eyes with DH show significantly greater progression of visual field defects and changes of the optic nerve head, with a mean time interval to progression of 16.8 and 23.8 months.
The percentage of eyes showing progression was greatest for FDT MD (23.6%), followed by OCT (20%), HVF MD (18.2%), mfVEP (16.4%), and stereophotography (16.4%). These differences in detection of progression were not significant (Cochran's Q test; n = 33). Further, the inferred false-positive rates, based on the percentage of eyes showing improvement, were low and similar for all tests except the mfVEP.
In addition, the agreement among these tests and between the tests and stereophotography regarding which eyes showed progression was very poor. Poor agreement between functional and structural measurements has also been reported by Strouthidis et al.23 They compared Heidelberg Retina Tomograph optic disc rim area progression with visual field progression in 198 subjects with ocular hypertension over a period of 7 years. Although they found that visual field progression occurred at least as frequently as rim area progression, agreement between the two measurements was poor.23 A similar structure-function dissociation was reported in a group of subjects with glaucomatous visual field loss who were followed longitudinally.24
Although poor agreement might have been, in part, attributed to measurement error, it is undoubtedly true that these tests do not measure the same thing, as demonstrated for the mfVEP and HVF.25 For example, local defects will not be detected with MD or PSD measures of HVF or FDT, whereas they may appear on the mfVEP plot. In addition, a structural measure such as the OCT may or may not be more sensitive than a functional test, depending on the underlying relationship between structure and function and the relative variability of each test.26 Although these arguments, and the data in the present study, illustrate the importance of following patients with a combination of functional and structural tests, they also highlight the need to better understand how to combine the results of these tests and to better understand the relationship among tests.
There were weaknesses in the design of this study that undoubtedly contributed to the poor agreement among tests. First, stereophotographs are not a good standard for assessing progression. For example, in a recent study,21 even when three glaucoma specialists agreed, there was a 40% false-positive rate. Another drawback of this study was the relatively short time period of 2 years for follow-up. For example, Chauhan et al.27 made recommendations regarding the frequency of examinations needed to detect various amounts and rates of visual field change. They estimated that it would take 3 years to detect a −6-dB loss (a large loss) on the HVF if the patients were tested twice per year, as in the present study, and if they had a moderate (average) amount of variability on HVF tests. They concluded that even with three tests per year, a −4-dB loss would be needed for detection in 2 years, again assuming a moderate amount of variability.27
Despite its limitations, the implication of this study is clear. More than one test should be used to follow up patients with glaucoma and to evaluate the effects of treatment in future clinical trials. However, as pointed out by Shah et al.,28 the clinician has to consider the sensitivity and specificity tradeoffs when combining tests. Sensitivity will increase and specificity will decrease because of the greater probability of results being outside the normal limits for the combination compared with each component test alone.
Footnotes
Supported in part by National Institutes of Health Grant EY02115, Corinne Graber Research Fund of The New York Glaucoma Research Institute, unrestricted funds from Research to Prevent Blindness, and a grant from Pfizer Inc.
Disclosure: D. Xin, None; V.C. Greenstein, None; R. Ritch, None; J.M. Liebmann, None; C.G. De Moraes, None; D.C. Hood, None
References
- 1. Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–748 [PubMed] [Google Scholar]
- 2. Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2213–2220 [PubMed] [Google Scholar]
- 3. Garway-Heath DF, Viswanathan A, Westcott M, Kamal D, Fitzke FW, Hitchings RA. Perimetry Update. The Hague: Kugler; 1999:381–389 [Google Scholar]
- 4. Swanson WH, Felius J, Pan F. Perimetric defects and ganglion cell damage: interpreting linear relations using a two-stage neural model. Invest Ophthalmol Vis Sci. 2004;45:466–472 [DOI] [PubMed] [Google Scholar]
- 5. Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315 [DOI] [PubMed] [Google Scholar]
- 6. Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972 [DOI] [PubMed] [Google Scholar]
- 7. Bjerrum J. Om en Tilfojelse til den Saedvanlige Synsfelfundersogelse samt om Synfelet ved Glaukom. Nord Ophthalmol Tskr (Copenh) 1889;2:141–185 [Google Scholar]
- 8. Bjerrum J. Om Glaukomets Kliniske Afgraensning. Nord Ophthalmol Tskr (Copenh) 1892;5:129–138 [Google Scholar]
- 9. Drance SM, Fairclough M, Butler DM, Kottler MS. The importance of disc hemorrhage in the prognosis of chronic open angle glaucoma. Arch Ophthalmol. 1977;95:226–228 [DOI] [PubMed] [Google Scholar]
- 10. Rasker MT, van den Enden A, Bakker D, Hoyng PF. Deterioration of visual fields in patients with glaucoma with and without optic disc hemorrhages. Arch Ophthalmol. 1997;115:1257–1262 [DOI] [PubMed] [Google Scholar]
- 11. Hood DC, Greenstein VC. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res. 2003;22:201–251 [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, Hood DC, Chen CS, Hong JE. A signal-to-noise analysis of multifocal VEP responses: an objective definition for poor records. Doc Ophthalmol. 2002;104:287–302 [DOI] [PubMed] [Google Scholar]
- 13. Hood DC, Zhang X, Hong JE, Chen CS. Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol. 2002;104:303–320 [DOI] [PubMed] [Google Scholar]
- 14. Fortune B, Zhang X, Hood DC, Demirel S, Johnson CA. Normative ranges and specificity of the multifocal VEP. Doc Ophthalmol. 2004;109:87–100 [DOI] [PubMed] [Google Scholar]
- 15. Hood DC, Zhang X, Winn BJ. Detecting glaucomatous damage with multifocal visual evoked potentials: how can a monocular test work? J Glaucoma. 2003;12:3–15 [DOI] [PubMed] [Google Scholar]
- 16. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–310 [PubMed] [Google Scholar]
- 17. Raasch TW, Bailey IL, Bullimore MA. Repeatability of visual acuity measurement. Optom Vis Sci. 1998;75:342–348 [DOI] [PubMed] [Google Scholar]
- 18. Brown B, Lovie Kitchin J. Repeated visual acuity measurement: establishing the patient's own criterion for change. Optom Vis Sci. 1993;70:45–53 [DOI] [PubMed] [Google Scholar]
- 19. Vanden Bosch ME, Wall M. Visual acuity scored by the letter-by letter or probit methods has lower retest variability than the line assignment method. Eye. 1997;1:411–417 [DOI] [PubMed] [Google Scholar]
- 20. Arditi A, Cagenello R. On the statistical reliability of letter-chart visual acuity measurements. Invest Ophthalmol Vis Sci. 1993;34:120–129 [PubMed] [Google Scholar]
- 21. Jampel HD, Friedman D, Quigley H, et al. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am J Ophthalmol. 2009;147:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siegner SW, Netland PA. Optic disc hemorrhages and progression of glaucoma. Ophthalmology. 1996;103:1014–1024 [DOI] [PubMed] [Google Scholar]
- 23. Strouthidis NG, Scott A, Peter NM, Garway-Heath DF. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity, and agreement. Invest Ophthalmol Vis Sci. 2006;47:2904–2910 [DOI] [PubMed] [Google Scholar]
- 24. Artes PH, Chauhan BC. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res. 2005;24:333–354 [DOI] [PubMed] [Google Scholar]
- 25. Hood DC, Thienprasiddhi P, Greenstein VC, et al. Detecting early to mild glaucomatous damage: a comparison of the multifocal VEP and automated perimetry. Invest Ophthalmol Vis Sci. 2004;45:492–498 [DOI] [PubMed] [Google Scholar]
- 26. Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah NN, Bowd C, Medeiros FA, et al. Combining structural and functional testing for detection of glaucoma. Ophthalmology. 2006;113:1593–1602 [DOI] [PubMed] [Google Scholar]