Contact between RPE and choroidal endothelial cells (CECs) is an important step preceding vision-threatening neovascular AMD, a leading cause of legal blindness worldwide. However, no animal model or clinical study has focused on the effects of this contact. The physiologically relevant in vitro coculture model allows study of the effects of RPE and CEC interactions and contact on signaling pathways within each cell type and on CEC transmigration across the RPE. The authors show that VEGF189, a splice variant of VEGF, is upregulated by stressors found in AMD and by contact with CECs. This upregulation triggers activation of Rac1 mediated through VEGFR2 to increase CEC migration across the RPE.
Abstract
Purpose.
To determine the role of vascular endothelial growth factor 189 (VEGF189) in choroidal endothelial cell (CEC) migration across the retinal pigment epithelium (RPE) and to explore the molecular mechanisms involved.
Methods.
Using real-time PCR, the expression of VEGF splice variants VEGF121, VEGF165, and VEGF189 was determined in human RPE from donor eyes, cultured human RPE in contact with CECs exposed to hydrogen peroxide (H2O2) or hypoxia, and RPE/choroid specimens from mice treated with laser to induce choroidal neovascularization (CNV). Activation of VEGF receptors (VEGFRs), phosphoinositol 3-kinase (PI-3K) or Rac1 was measured in CECs cocultured in contact with RPE exposed to peroxide or silenced for VEGF189 expression. Migration of CECs across the RPE was determined using fluorescence microscopy.
Results.
VEGF189 expression was increased in human RPE from aged compared with young donor eyes and from mouse RPE/choroids after laser to induce CNV. VEGF189 was also upregulated in human RPE challenged with peroxide, hypoxia, or cultured in contact with CECs. CEC migration across RPE was greater after RPE exposure to peroxide to induce VEGF189; VEGFR2 and Rac1 activities were also increased in these CECs. When CECs were cocultured with RPE silenced for VEGF189, VEGFR2 and Rac1 activities in CECs were significantly reduced, as was CEC migration across the RPE. Inhibition of Rac1 activity significantly inhibited CEC transmigration without affecting PI-3K activity.
Conclusions.
RPE-derived cell-associated VEGF189 facilitates CEC transmigration by Rac1 activation independently of PI-3K signaling and may have importance in the development of neovascular AMD.
Age-related macular degeneration (AMD) is a leading cause of nonreversible blindness worldwide.1 Vision loss most often occurs in advanced forms, which are atrophic AMD (geographic atrophy) and neovascular AMD. In atrophic AMD, there is loss of the photoreceptors, retinal pigment epithelium (RPE), and choriocapillaris in the outer retina, whereas in neovascular AMD, blood vessels from the choroid grow into Bruch's membrane, the subretinal space, and neurosensory retina. Neovascular AMD accounts for 80% of the severe central vision loss (legal blindness) in AMD.
The steps involved in the development of neovascular AMD are complex and incompletely understood. From clinicopathologic studies, it appears that >50% of vision-threatening neovascular AMD occurs when choroidal endothelial cells (CECs) are induced to migrate toward the RPE and make contact with the RPE and its extracellular matrix. After contact with RPE, CECs can migrate across the RPE into the neurosensory retina, where choroidal neovascularization (CNV) develops.2,3 The normal outer neurosensory retina lacks blood vessels, and the new blood vessels that develop often leak and bleed, causing vision loss. Thus, the migration of CECs across the RPE and the development of CNV in the neurosensory retina are important events leading to severe vision loss from neovascular AMD.
The RPE barrier, which is composed of a monolayer of polarized epithelial cells linked by tight junctions, is important in several processes necessary for fine visual acuity.4 There is evidence that under normal conditions the RPE barrier compartmentalizes angiogenic agonists predominantly by secreting them basally, whereas inhibitors are secreted apically.5 In aging eyes, it has been postulated that the RPE becomes less able to handle its metabolic load6,7 and stressors such as light, hypoxia,8 and inflammation,9 leading to RPE barrier compromise.10,11 In addition, these stressors have also been shown to result in the increased expression of angiogenic factors.12 We previously reported that RPE-CEC contact led to reduced RPE barrier properties10 and facilitated CEC migration across the RPE, induced by vascular endothelial growth factor (VEGF).13
VEGF-A (hereafter referred to as VEGF) is one angiogenic factor produced by RPE. Five splice variants or isoforms of VEGF in humans and three in mice are alternatively spliced from a single gene, and each has different biological functions and bioavailability.14–16 The most studied human splice variants (mouse analogs in parentheses) are VEGF189 (VEGF188), which is predominantly cell associated, VEGF121 (VEGF120), which is soluble, and VEGF165 (VEGF164),17 which has intermediate properties. Experimental studies using genetically modified mice indicated that VEGF signaling was important in the formation of CNV in AMD.16,18–21 Clinical experience reveals that inhibition of all splice variants of VEGF with a humanized monoclonal antibody against VEGF led to improved visual acuity in approximately 40% of cases.22 However, concern is raised about the safety of using agents to block all VEGF functions because VEGF is also a survival factor for CEC and RPE.23,24 Given that VEGF has beneficial effects, it would be desirable to develop a strategy to inhibit only its pathologic functions.1
We studied the role of cell-associated VEGF188/189 (term includes the mouse and human analogs) in neovascular AMD. Specifically, we hypothesized that RPE-derived VEGF189 would be upregulated in response to certain stressors or early events that occur in advanced AMD and that this splice variant would facilitate CEC migration across RPE, a critical step in the development of vision-threatening neovascular AMD. To test this hypothesis, we used a coculture model that mimicked the physiologic positions of RPE and CECs and controlled for contact between the two cell types. We determined the expression of VEGF189 in young and old human donor RPE specimens and in mouse RPE after treatment with laser to induce CNV. We investigated the molecular mechanisms and the role of VEGF189 in CEC transmigration. Our results reveal that RPE-derived VEGF189 plays a critical role in facilitating CEC migration across the RPE by activating VEGFR2 and Rac1 in CECs in a pathway that appears independent of the phosphoinositol 3-kinase (PI-3K) signaling pathway.
Methods
Animals
Adult transgenic mice bred to express only the cell-associated VEGF188 splice variant on a C57Bl/6 background were used to isolate murine RPE (mRPE188/188).19 Appropriate controls were age-matched wild-type mice of the same genetic background (mRPE-WT). RPE and CECs were isolated and grown in culture, as described. All animal experiments were approved by the Institutional Animal Care and Use Committees of the University of North Carolina and the Schepens Eye Research Institute in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and adhering to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Human RPE RNA Extraction
Posterior eyecups removed from human eyes within 24 hours of death were obtained from the North Carolina Eye Bank, and RPE were immediately collected for RNA extraction. All studies involving human eyes were done adhering to the tenets of the Declaration of Helsinki for research involving human tissue.
Cell Culture
Human fetal RPE (hfRPE) were isolated from donor eyes (Advanced Bioscience Resources, Alameda, CA) of 15- to 18-week-old fetuses, following a previously published protocol25; passages 1 to 3 hfRPE were used in experiments. Human CECs were isolated from donor eyes from the North Carolina Eye Bank, Inc. (Winston-Salem, NC). Primary CECs were isolated as previously described.13 Passages 2 through 5 cells were used in experiments. ARPE-19 cells were obtained from ATCC (Rockville, MD) and grown in Dulbecco's modified Eagle's medium/F12 (Invitrogen) (DMEM/F-12) plus 10% FBS and penicillin-streptomycin. Cells below passage 18 were used for experiments. Primary mouse RPE (mRPE) was isolated using a modified protocol, as previously described by Gibbs et al.26 Passages 3 to 5 cells were used in experiments. Cells were confirmed as RPE and not endothelial cells or fibroblasts by positive pan-cytokeratin staining (Abcam, Cambridge, MA).
Coculture Studies
For biochemical assays, CECs were grown on inserts (Transwell; Corning, Corning, NY) inserts with 1-μm diameter pores that were too small to allow cell migration but still allowed CEC cell processes to make contact with the basal aspects of the RPE grown on the underside of the inserts.27 In some experiments, hfRPE was incubated with H2O2. In others, ARPE-19 cells were transfected with VEGF189 siRNA, as described below. Twenty-four hours after contact and indicated treatments, CECs were collected from the tops of the inserts (Transwell; Corning) and were processed for PI-3K and Rac1 activity assays and VEGFR2 phosphorylation, as described in the following sections. The total protein was determined using the BCA protein assay (Thermo Scientific, Pittsburgh, PA), and equivalent amounts were used for each assay. PI-3K and Rac1 activity assays were performed as previously described using immunoprecipitation to probe phospho-Akt1 and GST-PBD pull-down to detect active Rac1.27 Phospho-VEGFR2 was measured, as previously described,28 by immunoprecipitation to detect phospho-VEGFR2. Western blot analysis was developed with enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ) and analyzed using gel analysis software (Un-Scan-It; Silk Scientific, Orem, UT).
Transmigration Assays
Transmigration was measured as previously described.13 ARPE-19 or hfRPE cells were plated as for coculture assays on inserts (Transwell; Corning) with 8-μm pores. Before plating, CECs were fluorescently labeled with cell-labeling solution (Vybrant Dio, V22886; Molecular Probes, Invitrogen, Carlsbad, CA) in accordance with the manufacturer's protocol. Labeled CECs were then plated into each insert. Forty-eight to 72 hours after plating, migrated CECs were counted using fluorescence microscopy.
VEGF189 Knockdown
VEGF189 gene silencing was performed in ARPE-19 cells using custom-designed small interfering RNA (siRNA; AAAUCAGUUCGAGGAAAGGTT (sense) and CCUUUCCUCGAACUGAUUUTT (antisense) purchased from Ambion (Austin, TX). Nontargeting control siRNA was used as a negative control (4390843; Applied Biosystem, Foster City, CA). ARPE-19 cells were transfected with the siRNA duplex (JetPRIME; Polyplus Transfection, Illkirch, France) and then were plated onto the undersides of inserts (Transwell; Corning). Twenty-four hours later, ARPE-19 cells were plated onto the inserts (Transwell; Corning) for coculture and transmigration assays.
Inhibition of Rac1 Activity
Rac1 inhibition was achieved by expression of Rac binding domain of Rac1 effector POSH (GFP-POSH-RBD) in CECs, as described previously,27 and were compared with control GFP alone. Briefly, GFP-POSH-RBD or GFP was transfected into CECs using DNA transfection (JetPRIME; Polyplus Transfection) before CECs were plated onto inserts.
Real-Time Quantitative PCR
Total RNA was extracted (RNeasy Mini Kit; Qiagen Valencia, CA). Assays were performed using the real-time PCR system (7500; Applied Biosystems). Briefly, 1 μg total RNA was reverse-transcribed into cDNA using a cDNA kit (High Capacity; Applied Biosystem) according to the manufacturer's protocol. Each reaction (TaqMan, 16 μL; Applied Biosystems) contained 20 ng cDNA, 8 μL mix (TaqMan PCR MasterMix; Applied Biosystems), and 1 μM forward primer, 1 μM reverse primer, and 1 μM probe. All the samples were analyzed for human β-actin expression in parallel as an internal control. Gene expression was normalized to the expression level of β-actin. Primers and probes were as follows: human VEGF121, 5′-CAT AGG AGA GAT GAG CTT CC-3′ (forward), 5′-CCT CGG CTT GTC ACA TTT TTC T-3′ (reverse), FAM- CA GCA CAA CAA ATG TGA ATG CAG ACC A-TAMRA (probe); for human VEGF165, 5′-CAT AGG AGA GAT GAG CTT CC-3′ (forward), 5′-AAG GCC CAC AGG GAT TTT CT-3′ (reverse), FAM-CA GCA CAA CAA ATG TGA ATG CAG ACC A-TAMRA (probe); for human VEGF189, 5′-CCA AAG AAA GAT AGA GCA AGA C-3′ (forward), 5′-AGG ACT TAT ACC GGG ATT TCT-3′ (reverse), FAM-TG CCC CTT TCC CTT TCC TCG AAC TG-TAMRA (probe).
Laser-Injury CNV Model
The laser-induced choroidal neovascularization (CNV) model was carried out as previously described.29 Three to six spots of laser photocoagulation (532 nm, 200 mW, 100 ms, 75 μm; OcuLight GL, Iridex, CA) were applied around the optic nerve. Seven days after laser injury, mice were euthanatized and posterior eyecups were harvested and kept in tissue storage reagent (RNAlater; Ambion) at −80°C for RNA extraction. The mRNA of VEGF splice variants was analyzed by RT-PCR.
Statistical Analysis
Significant differences between groups were determined by either one-way or two-way ANOVA. P < 0.05 was considered statistically significant.
Results
VEGF Splice Variants Expression in Human and Murine RPE
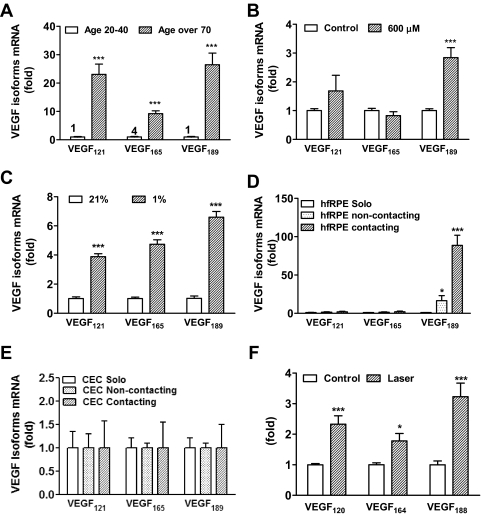
To determine the effect of RPE stressors relevant to human neovascular AMD, such as aging, hypoxia, and H2O2, we first measured the expression of VEGF splice variants VEGF121, VEGF165, and VEGF189 in aged (>70 years) and young (20–40 years) human RPE obtained from eye bank eyes. As shown in Figure 1A, in the RPE of young donors, the expression of VEGF165 (cycle threshold [CT] value, 30) was higher than that of VEGF121 and VEGF189 (CT value, 32). The expression of all VEGF splice variants was upregulated in RPE from older donor eyes compared with RPE from younger donors with an increase of approximately 26-fold for VEGF189, 23-fold for VEGF121, and 9-fold for VEGF165. Next, hfRPE was challenged with hypoxia or oxidative stress. When hfRPE was incubated with different doses of H2O2 (at a final concentration of 50 μM-600 μM) for 16 hours, a selective increase in the expression of VEGF189 occurred with 600 μM H2O2 (Fig. 1B). Incubation with 1% oxygen resulted in the upregulation of all splice variants, with the increase in VEGF189 expression over room air slightly greater than that of the other splice variants (Fig. 1C).
Figure 1.
Expression of VEGF isoforms in human and mouse RPE. The expression of VEGF isoforms was measured by RT- PCR in (A) human RPE from young (>20 years old) and old (>70 years old) donors. ***P < 0.0001 vs. young (n = 3); the number on the top of the bar means mRNA expression level of VEGF splice variants for real-time PCR. (B) hfRPE treated with H2O2 (600 μM) for 16 hours. ***P < 0.0001 vs. control (n = 6). (C) hfRPE incubated under hypoxic conditions 1% O2. ***P < 0.0001 vs. 21% O2 (n = 3). (D) hfRPE solo, noncontact, and contact with CECs. *P < 0.05, ***P < 0.0001 vs. solo (n = 6). (E) CECs solo, noncontact, and contact with hfRPE (n = 3). (F) Mouse RPE 7 days after laser injury CNV. *P < 0.05 and ***P < 0.0001 vs. control (n = 6). All data are shown as mean ± SEM.
The effect of RPE-CEC contact on the expression of VEGF splice variants was analyzed in hfRPE-CEC cocultures. As shown in Figure 1D, contact with CECs led to a much greater expression of VEGF189 in RPE compared with noncontacting coculture or solo RPE culture (P = 0.0028), but there was little change in VEGF121 and VEGF165 expression in these conditions. There was no significant change in expression of VEGF splice variants in CECs (Fig. 1E). To determine the expression of VEGF189 in CNV in vivo, VEGF splice variants were measured in isolated mouse RPE/choroids from wild-type mice treated with laser. All three splice variants were induced in retinas that had been treated with laser injury compared with nonlasered controls (Fig. 1F). There was a 3-fold increase in the mRNA expression of VEGF188 (P = 5.5 × 10−6).
VEGF189 Effect on CEC Migration across RPE
As shown in Figures 1B and 1D, preferential upregulation of VEGF189 occurs when RPE is treated with H2O2 or grown in contact with CECs. Thus, we next sought to determine whether this specific upregulation of cell-associated VEGF in RPE would facilitate CEC migration across the RPE. To test this possibility, RPE and CECs were plated for transmigration assays. hfRPE was plated on the underside of inserts (Transwell; Corning) and allowed to grow for 3 days before the addition of CECs and an additional 2 days of incubation. Sixteen hours before the termination of the experiment, H2O2 (to a final concentration of 600 μM) was added to the well containing the RPE. VEGF189 mRNA was upregulated in hfRPE treated with H2O2 (Fig. 1B). The expression of VEGF splice variants in solo CECs treated with H2O2 was unaffected compared with control (Fig. 2A). H2O2 treatment led to a 2-fold increase in CEC migration across the hfRPE (Fig. 2B; P = 0.0014). Transmigration assays were also performed using murine VEGF188/188 RPE (mRPE188/188) and human CECs. Migration of CEC across RPE was higher with RPE that produced only VEGF188 (mRPE188/188) compared with RPE that expressed all VEGF splice variants (mRPE WT) (Fig. 2C; P = 0.011).
Figure 2.
RPE-derived VEGF189/188 facilitates CEC migration across RPE. (A) Expression of VEGF splice variants in solo CECs treated with or without H2O2 (n = 3); CEC transmigration was measured in cocultures of RPE and CEC. (B) CEC transmigration was measured when the cocultured hfRPE was incubated in the presence or absence of 600 μM H2O2 for 16 hours H2O2 treatment of hfRPE increased CEC transmigration. **P < 0.001 vs. control (n = 6). (C) CEC migration across the filter when cultured alone (solo) or with mRPE-WT or mRPE188/188. CEC transmigration was highest when cocultured with mRPE188/188. *P < 0.05 and ***P < 0.0001 vs. solo and ###P < 0.0001 vs. mRPE (n = 6). All data are described as mean ± SEM.
To confirm the effect of RPE-derived cell-associated VEGF189 on CEC migration across RPE, we depleted endogenous VEGF189 in ARPE-19 cells using siRNA. Two different siRNA sequences were designed to target VEGF189, and a random sequence nontargeting siRNA was used as a negative control. To test the efficiency of VEGF189 knockdown, the expression of VEGF splice variants was measured in ARPE-19 cells by real-time PCR 48 hours after transfection with siRNA. As shown in Figure 3A, both siRNA sequences against VEGF189 knocked down the expression of VEGF189 without affecting the expression level of VEGF121 and VEGF165. Given that siRNA sequence A led to a slightly greater knockdown, this siRNA sequence was used in transmigration assays. Twenty-four hours after siRNA transfection, ARPE-19 cells were plated onto the underside of the inserts (Transwell; Corning), and 4 hours later CECs were plated into the inserts. After 48 hours, CEC migration across the VEGF189-silenced ARPE-19 was decreased approximately 40% compared with controls (6960.78 ± 2248.45/cm2 transmigrated CECs across VEGF189-silenced RPE vs. 10,261.51 ± 1187.32/cm2 transmigrated CECs across control RPE) (Fig. 3B; P = 0.0098).
Figure 3.
Knockdown of VEGF189 in RPE decreases CEC transmigration. (A) mRNA levels of VEGF isoforms in ARPE-19 cells transfected with VEGF189 siRNA were measured by RT-PCR. Nontargeting siRNA was used as a negative control. Both VEGF189 siRNA sequences A and B specifically reduced VEGF189 while not affecting expression of the other isoforms. ***P < 0.0001 vs. control (n = 6). (B) CEC transmigration assay was performed using ARPE-19 cell monolayer that had been depleted of VEGF189 by siRNA. CEC transmigration was decreased when cocultured with ARPE-19 with reduced VEGF189. *P < 0.05 vs. control siRNA (n = 6). All data are described as mean ± SEM.
VEGF189 Effect on Phosphorylated VEGFR2 in CECs
To test our hypothesis that RPE-derived VEGF189 by H2O2 and in contact with CECs triggers signaling to facilitate CEC migration through binding VEGFRs in CECs, we first confirmed that VEGFR1 and VEGFR2 were expressed in CECs using real-time PCR (Fig. 4A) showing a near 10-fold greater expression in VEGFR2 mRNA than in VEGFR1 mRNA. We then measured the activation of both VEGFRs in CECs that had been grown in contact with RPE. hfRPE was grown in contact with CECs for 24 hours, and, during the last 16 hours of incubation, hfRPE, was incubated with H2O2 to induce the expression of VEGF189 (Fig. 1B). After 16 hours of incubation, CECs were collected and analyzed for phosphorylated VEGFR1 and VEGFR2. Tyrosine phosphorylation of VEGFR2 in CECs was significantly increased when hfRPE was treated with 600 μM H2O2 (Fig. 4B). VEGFR1 phosphorylation was unchanged (Fig. 4C). As a control, to rule out the possibility that the increase in VEGFR2 phosphorylation in CECs occurred through a direct effect of H2O2 on CEC VEGFR2, we measured the phosphorylation of VEGFR1 and VEGFR2 in solo-cultured CECs treated with H2O2. There was no significant change in VEGFR1 or VEGFR2 phosphorylation in solo CECs treated with H2O2 compared with control (Fig. 4D).
Figure 4.
CEC contact with hfRPE leads to VEGFR2 increased phosphorylation in CECs. (A) Expression of VEGFR1 and VEGFR2 in CECs (n = 3). Immunoprecipitation of phospho-VEGFR2 and VEGFR1 in CECs. CECs were grown in contact with hfRPE. During the last 16 hours of contact, hfRPE was incubated with 600 μM H2O2. H2O2 treatment of RPE increased the phosphorylation of VEGFR2 in CECs (B) but did not affect VEGFR1 phosphorylation (C; representative blot shown). The bar graph on the right shows phospho-VEGFR2 band density normalized to total VEGFR2. **P < 0.001 vs. control (PBS) (n = 3). (D) Representative blots showing that the phosphorylation of VEGFR1 and VEGFR2 was unchanged in solo CECs treated with H2O2. CECs grown in contact with ARPE-19 cells were transfected with VEGF189 siRNA. After 24 hours of contact, CECs were collected for the detection of phospho-VEGFR2/total VEGFR2 and phospho-VEGFR1/total VEGFR1 by immunoprecipitation and Western blot analysis. VEGFR2 phosphorylation was decreased (E), but VEGFR1 phosphorylation was unchanged in CECs cocultured in contact with RPE with reduced VEGF189 (F). The bar graph on the right shows phospho-VEGFR2 band density normalized to total VEGFR2. *P < 0.05 vs. control siRNA (n = 3) and a representative blot of phospho-VEGFR1.
To determine whether the activation of VEGFR2 and VEGFR1 in CECs was due to H2O2-induced upregulation of RPE-associated VEGF189, VEGF189 was depleted by siRNA in ARPE-19 cells, and cells plated onto inserts 24 hours after transfection. CECs were plated into the inserts 4 hours later. After 24 hours of coculture, phosphorylation of VEGFR2 in CECs was decreased in cocultures in which ARPE-19 had been silenced for VEGF189 (Fig. 4E), supporting the hypothesis that RPE-derived VEGF189 activated VEGFR2 in CECs. However, VEGFR1 phosphorylation was unchanged under the same conditions (Fig. 4F).
VEGF189 Effect on CEC Transmigration and Activity of Rac1
Results indicated that oxidative stressor H2O2 upregulated VEGF189 in the RPE, facilitating CEC migration across RPE by activating VEGFR2 in CECs. We previously reported that PI-3K and Rac1 were activated in CECs grown in contact with RPE, and these signaling pathways were important in CEC migration across the RPE.27 Therefore, we determined whether Rac1 and PI-3K signaling were triggered by RPE cell-associated VEGF189 and whether they were necessary for CEC transmigration. Coculture assays were performed, and the activities of Rac1 and PI-3K were determined in CECs. Rac1 was activated in CECs grown in contact with mRPE188/188 compared with wild-type mRPE (Fig. 5A). Coculture of CECs with hfRPE preincubated with H2O2 also led to the activation of Rac1 in CECs (Fig. 5B), but not to an increase in Akt1 phosphorylation (Fig. 5C). There was no difference in Rac1 activation in solo CECs treated with H2O2 compared with control (Fig. 5D), reducing the possibility that H2O2 treatment might have directly activated Rac1 in CECs.
Figure 5.
Coculture of CECs with RPE expressing elevated VEGF189/188 leads to increased Rac1 activity. Rac1 activity assay was measured in CECs grown in contact with (A) mRPE-WT and mRPE188. The bar graph on the right shows active-Rac1 band density normalized to total Rac1. *P < 0.05 vs. mRPEWT (n = 3). (B) hfRPE treated with 600 μM H2O2 for 16 hours. After 24 hours of contact, CECs were collected for the detection of active Rac1 and total Rac1 by GST-PBD pull-down and Western blot analysis, as described. Rac1 activity was increased with both treatments. The bar graph on the right shows active-Rac1 band density normalized to total Rac1. **P < 0.001 vs. control (PBS) (n = 3). (C) PI-3K activity using phosphorylation of Akt-1 as a readout was measured in CECs grown in contact with hfRPE treated with H2O2 as in B. CECs were then collected for the detection of phospho-Akt and total Akt by immunoprecipitation and Western blot analysis as described in Experimental Procedures. Upregulation of VEGF189 by H2O2 treatment of RPE does not affect phosphorylation of Akt-1 in CECs. The bar graph on the right shows phospho-Akt (Ser473) band density normalized to total Akt1. P > 0.05 vs. control (PBS) (n = 3). (D) Representative blot showing that Rac1 activity was unchanged in solo CECs treated with H2O2.
To further examine the effect of RPE-derived VEGF189 in the activation of CEC Rac1 activation, Rac1 activity assays were performed in CECs grown in coculture with ARPE-19 transfected with siRNA against VEGF189. As shown in Figure 6A, Rac1 activity in CECs was decreased when grown in contact with coculture with ARPE-19 silenced for VEGF189 compared with control siRNA. Akt1 phosphorylation in CECs was not affected by knockdown of VEGF189 in ARPE-19 cells (Fig. 6B), consistent with results shown in Figure 5C. To determine whether Rac1 activation was necessary for CEC transmigration stimulated by RPE-derived VEGF189, we used a construct containing the Rac-binding domain of Rac1 effector POSH (GFP-POSH-RBD), which we previously found inhibits Rac1 activity in CECs.27 The construct and a control GFP construct were transfected into CECs before CEC plating. The transmigration assay was performed 48 hours after hfRPE-CEC coculture. During the last 16 hours of hfRPE-CEC coculture, hfRPE were challenged with 600 μM H2O2. As shown in Figure 6C, and consistent with the results shown in Figure 2B, CEC transmigration increased significantly when hfRPE were incubated with H2O2 (P = 0.04), but this effect was inhibited when Rac1 activity was decreased in CECs by transfection with GFP-POSH. These results provide support that VEGF189 facilitates CEC migration across the RPE, mediated by VEGFR2-induced activation of Rac1 in CECs.
Figure 6.
RPE-derived VEGF189 stimulates CEC transmigration mediated by Rac1 activation. Activities of Rac1 (A) and PI-3K (B) were measured in CECs grown in contact with ARPE-19 cells with reduced VEGF189. Twenty-four hours after contact, CECs were collected for the detection of active Rac1 and phospho-Akt by GST pull-down and immunoprecipitation. Whole cell lysates were used to detect total Rac1 and Akt by Western blot analysis. Knockdown of VEGF189 decreased Rac1 activity compared with control siRNA, whereas p-Akt remained unchanged. The bar graph on the right shows active-Rac1 or phospho-Akt band density normalized to total Rac1 or total Akt1. *P < 0.05 vs. control siRNA (n = 3). (C) CEC transmigration assay was performed using hfRPE and CECs in which Rac1 activity had been inhibited by transfection with GFP-POSH-RBD. Twenty-four hours after transfection, CECs were added, and transmigration was measured after 48 hours. During the last 16 hours of contacting coculture, hfRPE was incubated with 600 μM H2O2. CECs in which Rac1 activity was inhibited by expressing GFP-POSH did not exhibit increased transmigration when cocultured with H2O2-treated hfRPE compared with cells expressing GFP alone. ***P < 0.0001 vs. GFP alone (n = 6).
Discussion
VEGF-A is the most widely studied ligand of the VEGF family, and it plays an important role in the formation of CNV by several mechanisms,30,31 including the release of matrix metalloproteinases,32 endothelial cell survival,33 CEC migration and proliferation,34–36 increased permeability,37 and integrin turnover with endothelial migration.38
In healthy young mouse RPE/choroidal specimens, VEGF164 and VEGF120 are the dominant splice variants, whereas VEGF188 is virtually undetectable.39 Our data from human eye samples showed that VEGF165 was also the dominant splice variant in young adult RPE. However, in contrast to that found with VEGF165, the change in mRNA expression levels of VEGF189 and VEGF121 were significantly increased in RPE from aged compared with young donor eyes. The observation that VEGF189 was increased in RPE from aged donors suggested that VEGF189 may play an important role in AMD. To mimic the stress relevant to human neovascular AMD in vitro, hfRPE was cultured under hypoxic conditions or after exposure to H2O2. Notably, we observed that the expression of VEGF189 was preferentially upregulated in response to this stress. The upregulation of VEGF188 was also seen in RPE/choroids from mice that developed CNV after laser injury. These lines of evidence provide strong support that RPE-derived VEGF189, though minimally expressed in youth, is upregulated by stressors postulated to play a role in AMD, including hypoxia, oxidative stress, and contact between RPE and CECs before the development of neurosensory retinal CNV.
We next tested the hypothesis that RPE-derived VEGF189 plays an important role in CEC transmigration. Contact between RPE and CECs is an important step preceding the development of vision-threatening neovascular AMD.2,3 Given that there are no animal models or clinical studies that allow direct examination of the effects of contact between RPE and CECs, we used an in vitro coculture model to study the effects of RPE and CEC interactions13 and contact on signaling pathways within each cell type and on CEC migration across the RPE. Results obtained with this model demonstrated that the expression of VEGF189, compared with that of other VEGF splice variants, was preferentially upregulated in RPE grown in contact with CECs and that it contributed to CEC migration across the RPE. That there was only a 40% reduction in transmigration after VEGF189 knockdown indicates either that the small remaining amount of VEGF189 resistant to knockdown is enough to trigger signaling in CECs or that other factors that are also partially cell associated, including VEGF165, may be acting in parallel. We also showed that the RPE-derived VEGF189 binds to and activates VEGFR2 on CECs to trigger downstream signaling events facilitating CEC transmigration.
Rac1 is one of the small Rho family GTPases activated by guanine nucleotide exchange factors. In the GTP-bound activated state, Rac1 can modulate cell behavior through effector proteins. Rac1 is most often associated with cell motility and migration40–42 as a key regulator of actin polymerization and reorganization in cell-membrane protrusions during directed endothelial cell migration.43 Rac1 has been shown both to activate and to be activated by PI-3K. PI-3K, in turn, can be activated by multiple stimuli, such as integrins44 and receptor growth-factor binding,31 including VEGF.45,46 Furthermore, it is an important mediator of signal transduction downstream of a variety of cell surface receptors, including VEGFR2.47
One well-known function of PI-3K is the regulation of cell migration. Endothelial cell chemotaxis is dependent on PI-3K activation of intracellular signaling cascades.48 There are also other kinases, such as c-Jun N-terminal kinase (JNK),49 p38, extracellular signal-regulated protein kinase (ERK), and mitogen-activated protein kinases (MAPKs), that play important roles in the regulation of cell movement,50 and these pathways may also play a role in neovascular AMD. We previously identified a signaling pathway involving Rac1 and PI-3K that mediates CEC migration across an RPE monolayer in response to a VEGF gradient.27 Here, we studied the splice variant most greatly upregulated in RPE in aged eyes and in response to contact with CECs, stressors relevant to human neovascular AMD, to specifically determine its role in possible PI-3K–triggered Rac1 activation and endothelial migration. Our results show that VEGF-induced CEC migration involves Rac1 but not PI-3K. In light of our previous findings,27 these data suggest the existence of parallel pathways involving PI-3K through which splice variants other than VEGF189, such as VEGF165, might stimulate CEC transmigration. Our results do not preclude the role of other kinases, such as p38 MAPK or ERK, in CEC migration possibly involved downstream of Rac1.
Our findings suggest that stressors relevant to human neovascular AMD induce the expression of soluble and cell-associated VEGF splice variants in RPE. Increased levels of soluble VEGF splice variants, such as VEGF121 and VEGF165, provide a chemotactic gradient for migrating CECs, which, on making contact with the RPE, lead to RPE barrier compromise10 and further upregulation of VEGF189. VEGF2 phosphorylation in CECs leads to the activation of PI-3K and Rac1 and contributes to their transmigration.27 This study provides evidence that RPE-derived cell-associated VEGF188/189, which is upregulated by age and contact with CECs, may play an important role in the development of neurosensory retinal CNV in neovascular AMD.
Footnotes
Supported by National Institutes of Health Grants R01 EY017011 (MEH) and EY015435 (PAD); Macula Society, Retina Research Foundation, Mills and Margaret Cox Endowment (MEH); and National Institutes of Health Grants GM029860 (KB) and 3-R01-GM029860-28S (KB).
Disclosure: H. Wang, None; P. Geisen, None; E.S. Wittchen, None; B. King, None; K. Burridge, None; P.A. D'Amore, None; M.E. Hartnett, None
References
- 1. Penn JS, Madan A, Caldwell RB, et al. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartnett ME, Elsner AE. Characteristics of exudative age-related macular degeneration determined in vivo with confocal and indirect infrared imaging. Ophthalmology. 1996;103:58–71 [DOI] [PubMed] [Google Scholar]
- 3. Stevens TS, Bressler NM, Maguire MG, et al. Occult choroidal neovascularization in age-related macular degeneration: a natural history study. Arch Ophthalmol. 1997;115:345–350 [DOI] [PubMed] [Google Scholar]
- 4. Marmor MF. From sea lemons to c-waves. Cell Mol Neurobiol. 1983;3:285–295 [DOI] [PubMed] [Google Scholar]
- 5. Slomiany MG, Rosenzweig SA. IGF-1-induced VEGF and IGFBP-3 secretion correlates with increased HIF-1alpha expression and activity in retinal pigment epithelial cell line D407. Invest Ophthalmol Vis Sci. 2004;45:2838–2847 [DOI] [PubMed] [Google Scholar]
- 6. Green WR, Enger C. Age-related macular degeneration histopathologic studies: the 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535 [DOI] [PubMed] [Google Scholar]
- 7. Beatty S, Koh M, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2002;45:115–134 [DOI] [PubMed] [Google Scholar]
- 8. Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242:91–101 [DOI] [PubMed] [Google Scholar]
- 9. Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–126 [PubMed] [Google Scholar]
- 10. Hartnett ME, Lappas A, Darland D, et al. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 2003;77:593–599 [DOI] [PubMed] [Google Scholar]
- 11. Luna JD, Chan CC, Derevjanik NL, et al. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor, and interleukin-1-mediated breakdown. J Neurosci Res. 1997;49:268–280 [DOI] [PubMed] [Google Scholar]
- 12. Kuroki M, Voest E, Amano S, et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98:1667–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geisen P, McColm JR, Hartnett ME. Choroidal endothelial cells transmigrate across the retinal pigment epithelium but do not proliferate in response to soluble vascular endothelial growth factor. Exp Eye Res. 2006;82:608–619 [DOI] [PubMed] [Google Scholar]
- 14. Ishida S, Usui T, Yamashiro K, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Usui T, Ishida S, Yamashiro K, et al. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci. 2004;45:368–374 [DOI] [PubMed] [Google Scholar]
- 16. Lee S, Jilani SM, Nikolova GV, et al. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;69:681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor: multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954 [PubMed] [Google Scholar]
- 18. Stalmans I, Ng YS, Rohan R, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carmeliet P, Ng YS, Nuyens D, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–501 [DOI] [PubMed] [Google Scholar]
- 20. Ng YS, Rohan R, Sunday ME, et al. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220:112–121 [DOI] [PubMed] [Google Scholar]
- 21. Ruhrberg C, Gerhardt H, Golding M, et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ciulla TA, Rosenfeld PJ. Antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2009;20:158–165 [DOI] [PubMed] [Google Scholar]
- 23. Li W, He Z, Li Y, Yanoff M. Vascular endothelial growth factor regulates both apoptosis and angiogenesis of choriocapillaris endothelial cells. Microvasc Res. 2000;59:286–289 [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann S, Masood R, Zhang Y, et al. Selective killing of RPE with a vascular endothelial growth factor chimeric toxin. Invest Ophthalmol Vis Sci. 2000;41:2389–2393 [PubMed] [Google Scholar]
- 25. Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium (hfRPE) exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gibbs D, Williams DS. Isolation and culture of primary mouse retinal pigmented epithelial cells. Adv Exp Med Biol. 2003;533:347–352 [DOI] [PubMed] [Google Scholar]
- 27. Peterson LJ, Wittchen ES, Geisen P, et al. Heterotypic RPE-choroidal endothelial cell contact increases choroidal endothelial cell transmigration via PI-3Kand Rac1. Exp Eye Res. 2007;84:737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Budd S, Byfield G, Martiniuk D, et al. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Exp Eye Res. 2009;89:718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nozaki M, Sakurai E, Raisler BJ, et al. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J Clin Invest. 2006;116:422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klagsbrun M, D'Amore PA. Regulators of angiogenesis. Annu Rev Physiol. 1991;53:217–239 [DOI] [PubMed] [Google Scholar]
- 31. Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674 [DOI] [PubMed] [Google Scholar]
- 32. Lamoreaux WJ, Fitzgerald MEC, Reiner A, et al. Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc Res. 1998;55:29–42 [DOI] [PubMed] [Google Scholar]
- 33. Vinci MC, Visentin B, Cusinato F, et al. Effect of vascular endothelial growth factor and epidermal growth factor on iatrogenic apoptosis in human endothelial cells. Biochem Pharmacol. 2004;67:277–284 [DOI] [PubMed] [Google Scholar]
- 34. Roeckl W, Hecht D, Sztajer H, et al. Differential binding characteristics and cellular inhibition by soluble VEGF receptors 1 and 2. Exp Cell Res. 1998;241:161–170 [DOI] [PubMed] [Google Scholar]
- 35. Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581 [DOI] [PubMed] [Google Scholar]
- 36. Ishibashi T, Hata Y, Yoshikawa H, et al. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch Clin Exper Ophthalmol. 1997;235:159–167 [DOI] [PubMed] [Google Scholar]
- 37. Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985 [DOI] [PubMed] [Google Scholar]
- 38. Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004;95:372–379 [DOI] [PubMed] [Google Scholar]
- 39. Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47:3135–3142 [DOI] [PubMed] [Google Scholar]
- 40. Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709 [DOI] [PubMed] [Google Scholar]
- 41. Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597 [DOI] [PubMed] [Google Scholar]
- 42. Itoh RE, Kurokawa K, Ohba Y, et al. Activation of Rac and Cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22:6582–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nobes C, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62 [DOI] [PubMed] [Google Scholar]
- 44. Keely P, Parise L, Juliano R. Integrins and GTPases in tumour cell growth, motility and invasion. Trends Cell Biol. 1998;8:101–106 [DOI] [PubMed] [Google Scholar]
- 45. Zhao M, Bai H, Wang E, et al. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117:397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flaxenburg JA, Melter M, Lapchak PH, et al. The CD40-induced signaling pathway in endothelial cells resulting in the overexpression of vascular endothelial growth factor involves ras and phosphatidylinositol 3-kinase. J Immunol. 2004;172:7503–7509 [DOI] [PubMed] [Google Scholar]
- 47. Gille H, Kowalski J, Yu L, et al. A repressor sequence in the juxtamembrane domain of Flt-1 (VEGFR-1) constitutively inhibits vascular endothelial growth factor-dependent phosphatidylinositol 3-kinase activation and endothelial cell migration. EMBO J. 2000;19:4064–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arefieva TI, Kuktina NB, Antonova OA, Krasnikova TL. MCP-1 stimulated chemotaxis of monocytic and endothelial cells is dependent on activation of different signaling cascades. Cytokine. 2005;31:439–446 [DOI] [PubMed] [Google Scholar]
- 49. Okada Y, Saika S, Shirai K, et al. JNK MAPK signaling contributes in vivo to injury-induced corneal epithelial migration. Ophthalmic Res. 2009;42:185–192 [DOI] [PubMed] [Google Scholar]
- 50. Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628 [DOI] [PubMed] [Google Scholar]