Abstract
Background
At term, cervical ripening in coordination with uterine contractions becomes a prerequisite for a normal vaginal delivery. Currently, cervical ripening is considered to occur independently from uterine contractions. Many evidences suggest that cervical ripening resembles an inflammatory process. Comparatively little attention has been paid to the increased flexibility of the pelvic symphysis that occurs in many species to enable safe delivery. The aim of this study was to investigate whether the guinea-pig interpubic joint relaxation process observed during late pregnancy and parturition resembles an inflammatory process.
Methods
Samples of pubic symphysis were taken from pregnant guinea-pigs sacrificed along gestation, parturition and postpartum. Serial sections of paraffin-embedded tissues were used to measure the interpubic distance on digitalized images, stained with Giemsa to quantify leukocyte infiltration and to describe the vascular area changes, or studied by the picrosirius-polarization method to evaluate collagen remodeling. P4 and E2 serum levels were measured by a sequential immunometric assay.
Results
Data showed that the pubic relaxation is associated with an increase in collagen remodeling. In addition, a positive correlation between E2 serum levels and the increase in the interpubic distance was found. On the other hand, a leukocyte infiltration in the interpubic tissue around parturition was described, with the presence of almost all inflammatory cells types. At the same time, histological images show an increase in vascular area (angiogenesis). Eosinophils reached their highest level immediately before parturition; whereas for the neutrophilic and mononuclear infiltration higher values were recorded one day after parturition. Correlation analysis showed that eosinophils and mononuclear cells were positively correlated with E2 levels, but only eosinophilic infiltration was associated with collagen remodeling. Additionally, we observed typical histological images of dissolution of the connective tissue matrix around eosinophils.
Conclusion
The present study shows that a timely regulated influx of infiltrating leukocytes is associated with an extensive collagen remodeling process that allows the pubic separation for a normal delivery in guinea-pig. Thus, the findings in this study support the hypothesis that the guinea-pig pubic symphyseal relaxation at parturition resembles an inflammatory process.
Background
A well established prerequisite for a normal parturition is sufficient cervical ripening in coordination with uterine contractions [1,2]. Comparatively little attention has been paid to the modifications of the pelvic girdle that occurs in many species to enable safe delivery. In most mammals, these modifications involve sexual dimorphism of the bony pelvis that causes the female to have a sufficiently large birth canal as well as increased flexibility of the sacroiliac and/or pelvic symphysis during late pregnancy. An additional adaptation is transformation of pelvic joint cartilage to an elastic interpubic ligament, allowing considerable separation of the pubic bones. This transformation is remarkable in several species, including guinea-pigs, mice, bats and humans [3].
In adult female guinea-pigs, the interpubic joint is a nonsynovial joint of the cartilaginous type that is movable and connected to the pubic bones by fibrous (symphysis) cartilage [4]. This fibrocartilage consists largely of compact collagen fibers embedded in an amorphous matrix that contains proteoglycans [5,6]. Relaxation of this articulation occur during the end of pregnancy showing proliferation of the connective tissue cells, increases in the vascularity, weight, and length of the interpubic ligament [7-9].
It has been clearly stated that, at parturition, a finely tuned hormonal controlled stroma remodeling take place in the uterine cervix, involving leukocyte infiltration, production of proinflammatory cytokines, angiogenesis, swelling, fibroblastic cell plasticity and collagen metabolism [10-17]. These mechanisms resemble an inflammatory process and have been also demonstrated in placenta and ovary [18,19]. Recently, it has been pointed the importance of tissue microenvironments in the generation and the maintenance of inflammatory responses, together with functional contributions from both haematopoietic and stromal cells. In reproductive tissues a significant role of the stroma has been established.
Based on the aforementioned evidences, we suggest that an inflammatory response could be involved in the guinea-pig interpubic joint relaxation process during late pregnancy and parturition. The present study was conducted to establish the temporal pattern of leukocyte infiltration in the pubic symphysis along gestation, parturition and postpartum, and to investigate multiple correlations of this leukocyte infiltration with collagen remodeling, pubic separation and hormonal steroid levels.
Methods
Animals
The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals issued by the USA National Academy of Science. Primiparous female guinea-pigs (12–15 weeks old) of the American Short Hair strain, bred at the Department of Human Physiology (Santa Fe, Argentina) were used. Animals were maintained in a controlled environment (22 ± 2°C; lights on from 06:00–20:00 h), and they had free access to pellet laboratory chow, tap water and a supplement of green hay and ascorbic acid added at the rate of 400 mg/l in dechlorinated tap water. Females were examined daily for vaginal opening for at least two consecutive cycles before being assigned to the experimental groups. The day when the vagina was fully open was designated as day 0 of the estrous cycle. The females were kept in presence of males of proven fertility for mating. Pregnant guinea-pigs of known mating date were obtained by placing estrous females with a buck and checking for plugs the following morning (day of plug = day 1 of pregnancy = D1). In our colony delivery occurs on D65 ± 2. Interesting to note that pregnant females at D63 show an interpubic distance of 15 mm (Figure 1a); according with previous observations this separation indicates that pups will be born within 48 h [20]. Therefore, D63 is considered as immediately before parturition.
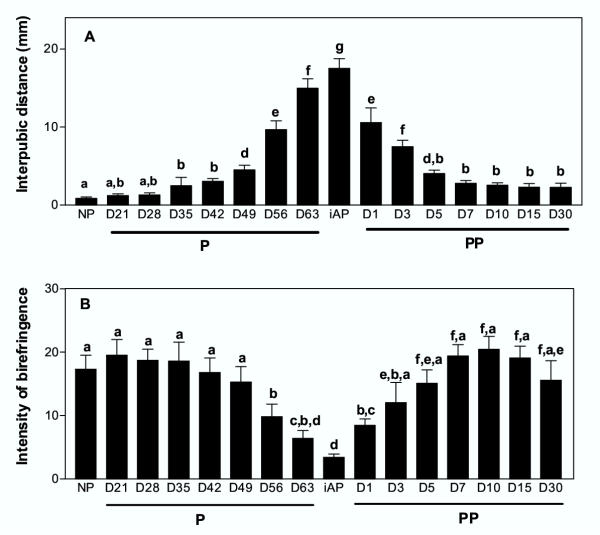
Figure 1.
Guinea-pig interpubic symphysis distance (A) and collagen remodeling in the interpubic ligament (B) were simultaneously measured in nonpregnant animals (NP), along pregnancy (P), immediately after parturition (iAP) and at postpartum (PP). Collagen remodeling was measured by the picrosirius-polarization method as changes in the intensity of birefringence. Note that the lowest intensity of birefringence evidences the highest collagen remodeling and coincides with the maximum value of ligament length. Bars represent the mean symphysis distance (± SEM) of five animals/group.
Sample collection and tissue processing
Animals were sacrificed by CO2 inhalation. Trunk blood and interpubic tissue were obtained from: a) nonpregnant (NP) females at estrous; b) along pregnancy at D21, D28, D35, D42, D49, D56, D63; c) immediately after parturition (iAP) (no longer than 1.5 h after they had already delivered all pups); and d) at D1, D3, D5, D7, D10, D15, D30 of postpartum (PP). Nonpregnant animals served as controls. Five animals per group were sacrificed, and the interpubic joint was cleaned from any fat, muscle and fascia and was fixed by immersion in 10% buffered formalin for 6 h, then decalcified with EDTA as described previously [9]. Samples were dehydrated in a series of ethanol solutions and embedded in paraffin (interpubic joints were guided in the blocks, to obtain transversal sections). Serial 5-μm thick sections were mounted on 3-aminopropyl triethoxysilane-coated slides; dewaxed and rehydrated, and then assigned to any of the following staining procedures: haematoxylin-eosin, picrosirius-haematoxylin, and Giemsa.
Measurement of P4 and estradiol (E2) levels
Serum samples from NP, D42, D56, D63, iAP and D1PP were used. P4 and E2 were measured directly in guinea-pig serum by a sequential immunometric assay using kits (IMMULITE Progesterone and IMMULITE Estradiol; Diagnostic Products Corporation, Los Angeles, CA) and results were recorded with the IMMULITE Analyzer [21]. Quality control of the assays was performed according to the manufacturer's instructions, as well as previous published results [21]. The intra-assay variance was calculated as the mean coefficient of variation of sextuplicates of a serum pool containing 10 ng/ml of P4 or 40 pg/ml of E2. They were 7.8 % and 12.4 %, respectively. The serum pool was run in all assays and the inter-assay coefficient of variation was calculated from such sextuplicates determinations. They were 8.2% for P4 and 11.0 % for E2.
Morphometry
Interpubic distance. The distance between the medial margins of hypertrophic cartilages was measured on digitalized images [9]. Briefly, images were recorded by a Sony ExwaveHAD color video camera attached to an Olympus BH2 microscope (illumination: 12-V halogen lamp, 100 W, equipped with a stabilized light source) using 4× D-plan objective lens. The microscope was set up properly for Koehler illumination and calibration of the measurement system was setup with a reference slide before any measurement was started. Images were recorded in a 24-bit true color TIFF (Tag Image File Format), a widely used format for storing image data; the resolution of the images was set to 640 × 480 pixels. At this magnification, each pixel of the image corresponds to 2.60 μm, and each field in the monitor represents a tissue area of 2.07 mm2. The interpubic distance was determined using an Image Pro-Plus 4.1.0.1® system (Media Cybernetics, Silver Spring, MA, USA).
Organization of Collagen Fibers. Collagen remodeling was evaluated by measuring the intensity of birefringence with the picrosirius-polarization method, as previously described [22,9]. Briefly, picrosirius-stained sections were analyzed by polarization microscopy. In this method, the intensity of collagen birefringence evaluated by polarization microscopy is inversely related to collagen remodeling, since only oriented collagen molecules present a bright birefringence. Normally, collagen fibers form thick bundles of densely packed and regularly arranged fibers, and they appear as brightly birefringent structures throughout the whole microscopic field. When collagen fibers are not dense and/or not regularly arranged, they are weakly birefringent.
Thus, measurements of the intensity of birefringence were performed in specimens from the experimental groups. For each animal, the light intensity of the whole symphyseal stroma (more than 50 fields) from one section of each specimen was measured, using 40× D-plan objective lens. At this magnification, each pixel of the image corresponds to 0.26 μm and each field in the monitor represented a tissue area of 0.02 mm2. Using Auto-Pro macro language, an automated standard sequence operation was created to measure average optical intensity (OI).
Infiltrating cells
In order to identify the infiltrating leukocytes, sections were stained for 60 min in 1:10 Giemsa stain (Aldrich, Milwaukee, USA). The point counting procedure [23] was used to obtain the volume fraction (Vv) of the leukocytes that invaded the symphyseal tissue. Leukocytes were estimated in the chosen fields using a glass disc with a squared grid inserted in a focusing eyepiece and an ×100 immersion objective. The fraction of points occurring within the structure of leukocytes was determined and then compared to the total number of points lying within the interpubic tissue. The Vv of the objects (neutrophils, eosinophils and mononuclear leukocytes) was calculated as described by Luque et al. [22].
Statistical Analysis
The ANOVA test and Bonferroni posttest were used to analyze the differences between groups. Correlation analyses were performed using Pearson analysis [24].
Results
Changes in the length of interpubic ligament are associated with both E2 serum levels and collagen remodeling
During pregnancy, the length of the ligament increased from 0.86 mm in nonpregnant controls to ~18–20 mm on the day of parturition. On postpartum day 5 (D5PP), the ligament had significantly decreased up to 4 mm and from D7PP onwards, no significant changes were observed (Figure 1A). The intensity of birefringence, as an indication of collagen remodeling, was simultaneously measured in the interpubic tissue. At mid-pregnancy (D21 to D49) a high birefringence was scanned as an indication of the presence of a dense and highly organized collagen framework (Figure 1B and 2A-B). Characteristic features of collagen remodeling were exhibited by the interpubic tissue starting on D56, when a sharp decrease in the intensity of birefringence was detected. The process was highly significant around parturition (D63, iAP) (Figure 1B), reflecting a widespread reduction in density and orientation of collagen fiber bundles (Figure 2C,2D). At postpartum, collagen birefringence increased steadily reaching at D5PP values similar to the nonpregnant controls (Figure 1B).
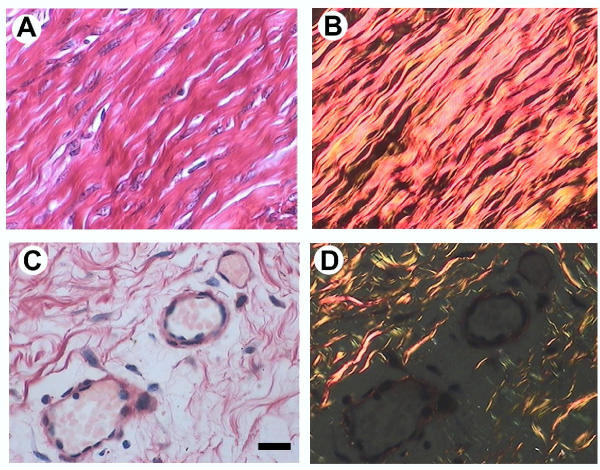
Figure 2.
Photomicrographs of sections showing central region of the guinea-pig pubic symphysis obtained at D21 of pregnancy (A, B) and at parturition (C, D). Sections were studied by the picrosirius-polarization method (a specific procedure for the detection of oriented collagenous structures in tissue sections). Densely packed bundles of interlaced collagen fibers are observed in the interpubic ligament with conventional illumination (A) or under polarizing optics (B). All brightly birefringent structures, which shine against a dark background, are of a collagenous nature. At parturition, irregularly disposed thin and thick collagenous fibers are observed in sections when studied under conventional illumination (C); polarization microscopy of the same field shows a high degree of collagen remodeling (D). Note the presence of high number of blood vessels at parturition (C). Scale bar = 25 μm.
Histological analysis of the female non-relaxed interpubic joint at the beginning of pregnancy (D21), shows the central nucleus of the joint formed of coarse, closely packed, strongly birefringent bundles of collagen fibers (Figures 2A,2B). In intrapartum animals, the pubic bones were separated and the connective tissue of the symphyseal ligament showed a marked hyperplasia, which corresponds with fragmentary and irregularly separated collagenous fibers with a marked loss of birefringence (Figure 2C,2D). This reduction in collagen birefringence evidences that the relaxation attained by the interpubic joint at parturition is attributable, at least in part; to an active collagen remodeling process that facilitates a transformation from hard and unyielding to soft, swollen and flexible structure. Another remarkable histological observation in the interpubic ligament from D49 until parturition is the growth of new blood vessels (i.e.: angiogenesis) (Figure 2C).
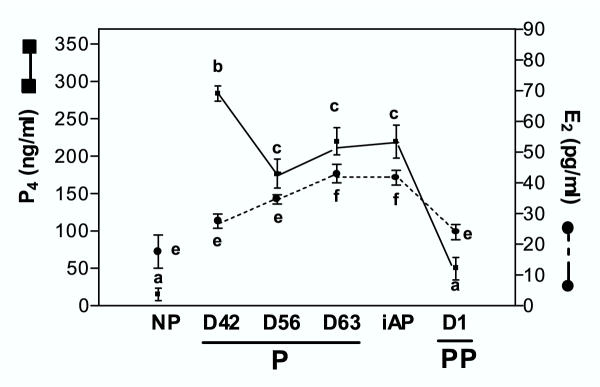
Serum levels of P4 and E2 were measured from mid-pregnancy to postpartum (Figure 3). E2 reached their maximum values at peripartum (D63, iAP), coincident with the highest symphyseal ligament length and collagen remodeling values. P4 levels were high during pregnancy and at parturition, with a sharp decrease on D1PP. When a correlation analysis between E2 or P4 and the interpubic distance was done, a positive temporal association was observed only with E2 serum levels (r = 0.6678; p < 0.01).
Figure 3.
Serum levels of P4 and E2 in nonpregnant (NP), along pregnancy (P), immediately after parturition (iAP), and at day 1 of postpartum (PP). Sequential immunometric assay was performed according to Material and Methods. Means with different letters differ significantly (p < 0.05).
Leukocytes infiltration in the interpubic ligament around parturition resembles an inflammatory response
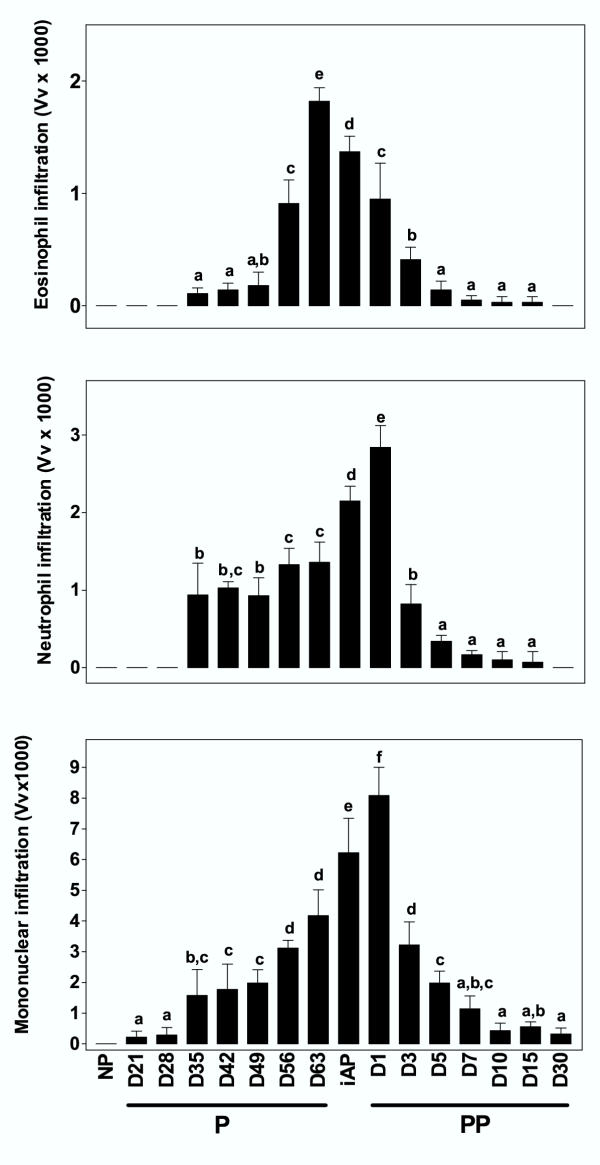
A leukocytes infiltration in the interpubic tissue was found from D35 of pregnancy until D5PP. Around parturition this infiltration is highly intense and almost all inflammatory cells types were observed (Figure 4). The leukocyte infiltration corresponded to neutrophils, eosinophils and mononuclear cells; being mononuclear leukocytes more abundant (Figure 4). Neutrophils and mononuclear cells showed greater density values at D1PP (Vv = 2.840 ± 0.280 and Vv = 8.080 ± 0.920, respectively), after that migration was reduced gradually. Eosinophils infiltration began at D35 (Vv = 0.110 ± 0.050) and reached maximum values immediately before parturition (D63) (Vv = 1.820 ± 0.120). Correlation analysis from mid-pregnancy (D42) to parturition (iAP) between infiltrating cells and E2 levels showed that eosinophils and mononuclear leukocytes are positively correlated with E2 levels (r = 0.9037, p < 0.0001 and r = 0.5877, p < 0.05, respectively). In addition, along pregnancy and parturition, eosinophilic infiltration was exclusively negatively correlated with the intensity of birefringence (r = -0.5488, p < 0.01). In agreement with this correlation, we have observed typical histological images of dissolution of the connective tissue matrix around eosinophilic leukocytes (Figure 5).
Figure 4.
Temporal changes in leukocytes infiltration in the guinea-pig interpubic joint along pregnancy (P), immediately after parturition (iAP), and postpartum (PP). Bars represent mean values of volume density (area fraction occupied by leukocytes; Vv, ± SD) of five animals/group. Highest Vv values of eosinophils were recorded before parturition (D63), whereas neutrophils and mononuclear cells reached their highest values one day after parturition (D1). Infiltration was absent in nonpregnant (NP) tissue samples. Means with different letters differ significantly (p < 0.05).
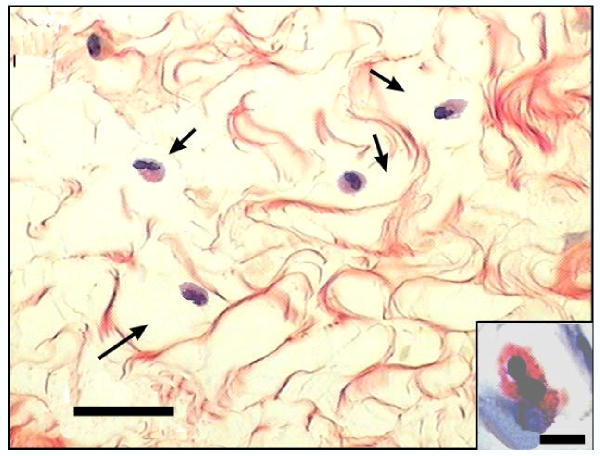
Figure 5.
Photomicrograph of a section from the interpubic ligament obtained at parturition. Picrosirius-haematoxylin staining allowed the identification of eosinophils and collagen fibers. The empty halos (arrow) show collagen degradation surrounding eosinophils. This feature is shown at higher magnification (insert). Low magnification scale bar = 20 μm, inset scale bar = 5 μm.
Discussion
The present study supports our contention that the guinea-pig pubic symphyseal relaxation at parturition resembles an inflammatory process. The data demonstrate that a timely regulated influx of infiltrating leukocytes is associated with an extensive collagen remodeling process that allows the pubic separation for a normal delivery in guinea-pig. These findings show how these processes work closely together.
Relaxation of the pelvic joint during pregnancy has been recognized for many years in guinea-pigs [25]. Previous reports have demonstrated the existence of an extensive collagenolytic process in association with interpubic relaxation [26,8,9]. Using morphometry for quantifying changes in the interpubic distance together with collagen remodeling in the symphyseal ligament, we have obtained results that are in agreement with previous reports. The pubic relaxation is temporarily associated with a marked reduction in the intensity of birefringence, reflecting a widespread reduction in density and orientation of collagen fiber bundles, together with an increase in interpubic length.
The current study indicates that the pubic symphyseal relaxation at parturition resembles an inflammatory process. Results showed that an infiltration of leukocytes into the pubic symphysis occurs in guinea-pig. Eosinophils reached their highest level immediately before parturition, whereas greater levels of neutrophilic and mononuclear infiltration were recorded one day after parturition. This infiltration pattern suggests that eosinophils could perform its major effect before parturition, whereas mononuclear and neutrophilic cells might play a role after parturition. Similarly, cervical dilation during parturition has been characterized as a widespread collagenolysis that follows a heavy polymorphonuclear leukocyte invasion of the uterine cervix [10,11]. The cells that infiltrate human, guinea-pig and sheep cervical tissue at term are neutrophils [10,21,27-29] whereas eosinophils are responsible for the infiltration of the rat cervix [11,22,30]. In the pubic symphysis at term, eosinophils and mononuclear cells were the main infiltrating ones. Proinflammatory cytokines together with an increase in the vascular area have been described in the uterine cervix at term [15,16,31], supporting the inflammatory response. In this study an increased angiogenesis was observed in the pubic symphysis from mid-pregnancy (D49) to parturition.
The significance of leukocyte infiltration in these tissues at term has not been clearly defined. It was observed in the uterine cervix that the polymorphonuclear leukocytes degranulate and this event coincides with the widespread collagenolysis observed in the extracellular matrix [10,11]. In addition, there is vast evidence that both collagenase and proteolytic enzymes do increase at term in humans and rats [32,33]. Previous studies have shown that eosinophils and neutrophils contain collagenase and other enzymes capable of digesting extracellular matrix proteins [34-36]. Therefore, it has been suggested that polymorphonuclear leukocytes are involved, at least in part, in the loosening of the collagenous framework that follows the leukocyte invasion of the uterine cervix. For the pubic symphysis, our results are in agreement with this hypothesis, since collagen remodeling is positively correlated with eosinophils infiltration. Moreover, we have observed typical histological images of dissolution of the connective tissue matrix around eosinophilic leukocytes. Nevertheless, the hypothesis that eosinophils are involved in enzymatic degradation and depolymerization of the pubic symphysis collagen needs to be tested in future experiments. Another possible role for the leukocytes invasion of the interpubic ligament in late pregnancy might be the formation of intercommunicating channels through the extracellular matrix. These channels would allow a rapid diffusion of hormones or collagenase-activating factors that may act at term on pre-existing collagenases (such as collagen-bound collagenases), since it has been shown that the migration of neutrophils increases the permeability of certain tissues [37].
On the other hand, E2 treatment of intact or ovariectomized guinea-pigs caused a significant increase in interpubic length [38-40]. In addition, relaxin promoted an extensive dissolution and disorientation effect on symphyseal collagen when administered to estrogen-pretreated animals [5,26,39,40]. In this study, E2 correlated with interpubic distance and eosinophils infiltration, suggesting that changes in the symphyseal stroma and eosinophils invasion are hormonally regulated processes. It would be interesting to asses whether relaxin effects on collagen remodeling of the interpubic joint is achieved through the promotion of eosinophilic invasion and degranulation.
Biological effects of hormones depend not only on serum levels but on receptors concentration and signaling pathways in target organs as well. Results of experimental and clinical studies with P4 and its antagonist indicate that parturition is composed of two major steps: a relatively long conditioning (preparatory) phase, followed by a short secondary phase (active labor) [2,41]. In the cervix, the preparatory phase involves a change in the composition of the connective tissue and the invasion by inflammatory cells [2]. The conditioning phase can be induced with antiprogestins in all species studied so far, including humans and other primates [41]. Thus, it has been hypothesized that withdrawal of P4 inhibition is the major mechanism in the initiation of conditioning phase [2]. In species without a fall in P4 serum levels prior to parturition (guinea-pigs, non-human primates and humans), it has been proposed that a functional withdrawal of P4 due to decreased activity in the target organs, e.g. at receptor or post-receptor level, represents the major mechanism in the initiation of this preparatory phase and therefore the onset of parturition [2]. Recently, we have demonstrated that before parturition, diminished responsiveness of the cervix to P4 might be caused by a decrease in progesterone receptor (PR) levels and this may be the mechanism of functional P4 withdrawal in the uterine cervix at term [21]. In this study results showed that, preparatory changes similar to those observed in the cervix (i.e.: collagen remodeling and leukocyte infiltration) also take place in pubic symphysis at term, simultaneously with high serum levels of P4. This parallelism between cervical ripening and symphyseal relaxation at term suggests that pubic symphyseal relaxation could be hormonally controlled by some mechanism of functional P4 withdrawal such as decreased expression of PR at the end of gestation. Our laboratory is carrying out experiments to clarify this issue.
Conclusions
In summary, our results provide support for the hypothesis that an inflammatory process and trafficking of immune cells may play an important role in the remodeling of the interpubic joint before and after parturition, and suggest that changes in the symphyseal ligament and eosinophils invasion are hormonally regulated processes. Many questions remain to be answered: Is local Pg withdrawal a prerequisite for leukocyte invasion at pubic symphysis?, Is a preterm birth associated with inflammatory reaction and cytokine release in pubic symphysis?. Critical aspects of these processes need to be addressed by studies on immune cell trafficking and their hormonal regulation during the period that immediately precede birth. An understanding of the regulation of symphyseal relaxation at term would be of great benefit to the diagnosis, management, and outcome of parturition under physiological and pathophysiological conditions.
Author's contributions
HR & HHO carried out the study and drafted the manuscript. JGR performed the statistical analysis. JGR & MMT participated in the design of the study. EHL conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript
Acknowledgments
Acknowledgements
This manuscript is dedicated to the memory of Professor Gregorio S. Montes (1952–2002). We are grateful to Mr. Juan Grant and Mr. Juan C. Villarreal for animal care, to Drs. Miguel Nanni and Silvia Sandoz (Paraná, Argentina) for steroids assay assistance, and to Patricia E. Rodríguez (Paraná, Argentina) for grammatical revision of the manuscript. This study was supported by grants from the Argentine National Council for Science and Technology (CONICET) (PIP 528/98), Universidad Nacional del Litoral (CAI+D 027–195) and the Argentine National Agency for the Promotion of Science and Technology (ANPCyT) (PICT-99 N° 5–7001). H.H.O. is Fellow and J.G.R. & E.H.L. are Career Investigator of the CONICET.
Contributor Information
Horacio A Rodríguez, Email: harodrig@fbcb.unl.edu.ar.
Hugo H Ortega, Email: hhortega@fcv.unl.edu.ar.
Jorge G Ramos, Email: gramos@fbcb.unl.edu.ar.
Mónica Muñoz-de-Toro, Email: monicamt@fbcb.unl.edu.ar.
Enrique H Luque, Email: eluque@fbcb.unl.edu.ar.
References
- Challis JRG, Lye SJ. Parturition. In: Knobil E, Neill JD, editor. The physiology of reproduction. 2. Vol. 2. New York: Raven Press; 1994. pp. 985–1032. [Google Scholar]
- Garfield RE, Saade G, Buhimschi C, Buhimschi I, Shi L, Shi S-Q, Chwalisz K. Control and assessment of the uterus and cervix during pregnancy and labour. Hum Reprod Update. 1998;4:673–695. doi: 10.1093/humupd/4.5.673. [DOI] [PubMed] [Google Scholar]
- Sherwood OD. Relaxin. In: Knobil E, Neill JD, editor. The physiology of reproduction. 2. Vol. 1. New York: Raven Press; 1994. pp. 861–1110. [Google Scholar]
- Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Clin Orthop. 1986;203:261–272. [PubMed] [Google Scholar]
- Chihal HJ, Espey LL. Utilization of the relaxed symphysis pubis of guinea pigs for clues to the mechanism of ovulation. Endocrinology. 1973;93:1441–1445. doi: 10.1210/endo-93-6-1441. [DOI] [PubMed] [Google Scholar]
- Steinetz BG, O'Byrne EM, Butler MC, Hickman LB. Hormonal regulation of the connective tissue of the symphysis pubis. In: Bigazzi M, Greenwood FC, Gasparri F, editor. Biology of Relaxin and its Role in the Human. Amsterdam: Excerpta Medica; 1983. pp. 71–92. [Google Scholar]
- Ruth EB. Metamorphosis of the pubic symphysis III. Histological changes in the symphysis of the pregnant guinea-pig. Anat Rec. 1937;67:409–421. [Google Scholar]
- Wahl LM, Blandau RJ, Page RC. Effect of hormones on collagen metabolism and collagenase activity in the pubic symphysis ligament of the guinea-pig. Endocrinology. 1977;100:571–579. doi: 10.1210/endo-100-2-571. [DOI] [PubMed] [Google Scholar]
- Ortega HH, Muñoz de Toro M, Luque EH, Montes GS. Morphological characteristics of the interpubic joint (symphysis pubica) of rats, guinea pigs and mice in different physiological situations. A comparative study. Cell Tissues Organs. 2003;173:105–114. doi: 10.1159/000068947. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Zugaib M, Montes GS, Toledo OMS, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–281. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- Luque EH, Montes GS. Progesterone promotes a massive infiltration of the rat uterine cervix by the eosinophilic polymorphonuclear leukocytes. Anat Rec. 1989;223:257–265. doi: 10.1002/ar.1092230304. [DOI] [PubMed] [Google Scholar]
- Kelly RW. Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocr Rev. 1994;15:684–706. doi: 10.1210/er.15.5.684. [DOI] [PubMed] [Google Scholar]
- Luque EH, Muñoz de Toro M, Ramos JG, Rodríguez HA, Sherwood OD. Role of relaxin and estrogen in the control of eosinophilic invasion and collagen remodeling in rat cervical tissue at term. Biol Reprod. 1998;59:795–800. doi: 10.1095/biolreprod59.4.795. [DOI] [PubMed] [Google Scholar]
- Varayoud J, Ramos JG, Joazeiro PP, Montes GS, Muñoz de Toro MM, Luque EH. Characterization of fibroblastic cell plasticity in the lamina propia of the rat uterine cervix at term. Biol Reprod. 2001;65:375–383. doi: 10.1095/biolreprod65.2.375. [DOI] [PubMed] [Google Scholar]
- Young A, Thomson AJ, Ledingham MA, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66:445–449. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- Sensström MB, Ekman G, Westergren-Thorsson G, Malmström A, Byström B, Endrésen U, Mlambo N, Norman M, Stabi B, Brauner A. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6:375–381. doi: 10.1093/molehr/6.4.375. [DOI] [PubMed] [Google Scholar]
- Winkler M, Fischer DC, Ruck P, Marx T, Kaiserling E, Oberpichler A, Tschesche H, Rath W. Parturition at term: parallel increases in interleukin-8 and proteinase concentrations and neutrophil count in the lower uterine segment. Hum Reprod. 1999;14:1096–1100. doi: 10.1093/humrep/14.4.1096. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and Parturition – A Review. Placenta. 2003;Suppl A:S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- Gaytán F, Morales C, Bellido C, Tarradas E, Sánchez-Criado JE. Effects of indomethacin on ovarian leukocytes during the periovulatory period in the rat. Reprod Biol Endocrinol. 2003;1:26–36. doi: 10.1186/1477-7827-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewin AL, Hutz RJ. Guinea Pig Female. In: Knobil E, Neill JD, editor. Encyclopedia of Reproduction. Vol. 2. New York: Academic Press; 1999. pp. 583–588. [Google Scholar]
- Rodriguez HA, Kass L, Varayoud J, Ramos JG, Ortega H, Durando M, Muñoz de Toro M, Luque EH. Collagen remodelling in the guinea-pig uterine cervix at term is associated with a decrease in PR expression. Mol Hum Reprod. [DOI] [PubMed]
- Luque EH, Ramos JG, Rodriguez HA, Muñoz de Toro MM. Dissociation in the control of cervical eosinophilic infiltration and collagenolysis at the end of pregnancy or after pseudopregnancy in ovariectomized steroid-treated rats. Biol Reprod. 1996;55:1206–1212. doi: 10.1095/biolreprod55.6.1206. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Siegel S. Nonparametric statistics for the behavioral sciences. New York: Mc Graw-Hill; 1956. [Google Scholar]
- Thoms H. Relaxation of the symphysis pubis in pregnancy. J Am Med Assn. 1936;106:1364–1366. [Google Scholar]
- Hisaw FL, Zarrow MX. The phisiology of relaxin. Vitam Horm. 1950;8:151–178. doi: 10.1016/s0083-6729(08)60670-6. [DOI] [PubMed] [Google Scholar]
- Owiny JR, Gilbert RO, Wahl CH, Nathanielsz PW. Leukocytic invasion of the ovine cervix at parturition. J Soc Gynecol Invest. 1995;2:593–596. doi: 10.1016/1071-5576(95)00003-W. [DOI] [PubMed] [Google Scholar]
- Hegele-Hartung C, Chwalisz K, Beier HM, Elger W. Ripening of the uterine cervix of the guinea-pig after treatment with the progesterone antagonist onapristone (ZK 98,299): an electron microscopic study. Hum Reprod. 1989;4:369–377. doi: 10.1093/oxfordjournals.humrep.a136909. [DOI] [PubMed] [Google Scholar]
- Luque EH, Bassani MM, Ramos JG, Maffini M, Canal A, Kass L, Caldini E, Ferreira JMC, Jr, Muñoz de Toro M, Montes GS. Leukocyte infiltration and collagenolysis in cervical tissue from intrapartum sheep. J Vet Med A. 1997;44:501–510. doi: 10.1111/j.1439-0442.1997.tb01136.x. [DOI] [PubMed] [Google Scholar]
- Duchesne MJ, Badia E. Immunohistochemical localization of the eosinophil major basic protein in the uterus horn and cervix of the rat at term and after parturition. Cell Tissue Res. 1992;270:79–86. doi: 10.1007/BF00381882. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1β and tumor necrosis factor α in guinea-pigs. Hum Reprod. 1994;9:2173–2181. doi: 10.1093/oxfordjournals.humrep.a138413. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Ito A, Mori Y, Hirakawa S. Changes in the human uterine cervical collagenase with specific reference to cervical ripening. Biochem Med. 1979;22:332–337. doi: 10.1016/0006-2944(79)90020-6. [DOI] [PubMed] [Google Scholar]
- Rajabi MR, Dean DD, Beydoun SN, Woessner JF., Jr Elevated tissue levels of collagenase during dilatation of uterine cervix in human parturition. Am J Obstet Gynecol. 1988;159:971–976. doi: 10.1016/s0002-9378(88)80183-2. [DOI] [PubMed] [Google Scholar]
- Hibbs MS, Mainardi CL, Kang AH. Type-specific collagen degradation by eosinophils. Biochem J. 1982;207:621–624. doi: 10.1042/bj2070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truden JL, Boros DL. Collagenase, elastase, and nospecific protease production by vigorous or immunodulated liver granulomas and granuloma macrophages/eosinophils of S. mansoni-infected mice. Am J Pathol. 1985;121:166–175. [PMC free article] [PubMed] [Google Scholar]
- Osmers R, Rath W, Adelmann-Grill BC, Fittkow C, Kuloczik M, Szeverenyi M, Tschesche H, Kuhn W. Origin of cervical collagenase during parturition. Am J Obstet Gynecol. 1992;166:1455–1460. doi: 10.1016/0002-9378(92)91619-l. [DOI] [PubMed] [Google Scholar]
- Milks LC, Conyers GP, Cramer EB. The effect of neutrophil migration on epithelial permeability. J Cell Biol. 1986;103:2729–2738. doi: 10.1083/jcb.103.6.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrow MX. The role of steroid hormones in the relaxation of the symphysis pubis of the guinea pig. Endocrinology. 1948;42:129–140. doi: 10.1210/endo-42-2-129. [DOI] [PubMed] [Google Scholar]
- Talmage RV. The role of estrogen in the estrogen-relaxin relationship in symphyseal relaxation. Endocrinology. 1950;47:75–82. doi: 10.1210/endo-47-1-75. [DOI] [PubMed] [Google Scholar]
- Talmage RV, Garrett FA. Effects of repeated injections of the steroids and relaxin on the symphysis pubis of the guinea pig as studied by X-ray. Endocrinology. 1951;48:162–168. doi: 10.1210/endo-48-2-162. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Garfield RE. Antiprogestins in the induction of labour. Ann N Y Acad Sci. 1994;734:387–413. doi: 10.1111/j.1749-6632.1994.tb21770.x. [DOI] [PubMed] [Google Scholar]