This work describes the role of tight junction signaling pathways in the proliferation of uveal melanoma.
Abstract
Purpose.
To investigate the role of tight junction (TJ)–associated signaling pathways in the proliferation of uveal melanoma.
Methods.
Human uveal melanoma cell lines overexpressing the TJ molecule blood vessel epicardial substance (Bves) were generated. The effects of Bves overexpression on TJ protein expression, cell proliferation, and cell cycle distribution were quantified. In addition, localization and transcription activity of the TJ-associated protein ZO-1–associated nucleic acid binding protein (ZONAB) were evaluated using immunofluorescence and bioluminescence reporter assays to study the involvement of Bves signaling in cell proliferation-associated pathways.
Results.
Bves overexpression in uveal melanoma cell lines resulted in increased expression of the TJ proteins occludin and ZO-1, reduced cell proliferation, and increased sequestration of ZONAB at TJs and reduced ZONAB transcriptional activity.
Conclusions.
TJ proteins are present in uveal melanoma, and TJ-associated signaling pathways modulate cell signaling pathways relevant to proliferation in uveal melanoma.
Uveal melanoma is the most common primary intraocular malignancy in adults.1 Several histopathologic features of these tumors are indicative of disease prognosis, including epithelioid cell morphology2 and the presence of vascular looping patterns.3,4 Additionally, molecular profiling of tumors has revealed genes associated with uveal melanoma growth, invasion, and metastasis, such as the downregulation of cell communication and development genes5,6 and the expression of matrix metalloproteinases.7 Further elucidation of the molecular participants in the pathogenesis of uveal melanoma would facilitate the development of targeted diagnostic approaches and therapeutic interventions.
Tight junctions (TJs) are major components of cell junctional assemblies, which play an important role in controlling the passage of solutes across epithelial barriers, such as the cornea and gastric mucosa.8 In addition to maintaining barrier function in epithelia, TJs serve as a cell signaling apparatus for multiple cell types throughout the body, contributing to the regulation of vesicular trafficking,9 cell proliferation,10 and cell motility.11 Alterations of TJ expression and function have been associated with the development of cancer through mechanisms such as the loss of contact inhibition of cell growth.12 Recently, we reported on the localization and function of a cell adhesion molecule, Bves (blood vessel epicardial substance), in corneal epithelial cells and the trabecular meshwork.13–16 Bves is an approximately 360-amino acid protein expressed in many cell types from early stages of embryonic development to adulthood. It contains an extracellular amino terminus, three transmembrane domains, and an intracellular carboxyl terminus.17 The C-terminal domain interacts with the TJ-associated protein zonula occludens 1 (ZO-1), a TJ-associated protein, through direct and indirect mechanisms.14 Alterations in Bves expression in several cell types are associated not only with profound effects on TJ formation but also on cell morphology, motility, and proliferation.14,17–21 These observations suggest that Bves may have roles that extend beyond the regulation of barrier formation in epithelia.
In cancer, the TJ complex may serve a critical role in facilitating communication between the cancer cell and the tumor microenvironment and in spatially and temporally orchestrating the mechanisms underlying tumor initiation and progression.22 Although the localization and function of TJs have been partially characterized in several cancer types, including cutaneous melanoma,22 their role in uveal melanoma is unclear. In this study, we investigated the role of Bves in the proliferation of uveal melanoma. Three human uveal melanoma cell lines stably transfected to overexpress Bves were analyzed using two- and three-dimensional cell proliferation assays and cell cycle distribution assays. Additionally, cells were measured for expression of TJ proteins and for the localization and activity of the TJ-associated transcription factor ZO-1–associated nucleic acid binding protein (ZONAB).23,24 ZONAB is a Y-box transcription factor that modulates cell proliferation through its interaction with the SH3 domain of ZO-1 at TJs, through its association with cell division kinase 4 (CDK4), and through its upregulation of cell cycle entry proteins.23 Specifically, in proliferating cells, ZONAB is observed primarily in the nucleus, where it promotes proliferation through binding of CDK4 and upregulation of proliferating cell nuclear antigen (PCNA) and cyclin D1, resulting in cell cycle entry.25 However, in slowly or nonproliferating cells, nuclear levels of ZONAB are reduced. The ZONAB protein is primarily localized in the cytoplasm, where it is sequestered from the nuclear compartment through its interaction with ZO-1.24 Because Bves is associated with ZO-1 at TJs,14 the association of Bves with ZO-1/ZONAB signaling in uveal melanoma was investigated.
Materials and Methods
Cell Lines
OM 431, OMM 1, and OMM 2.3 human uveal melanoma cell lines were a gift of Jerry Y. Niederkorn (University of Texas Southwestern Medical Center, Dallas, TX). OM431 cells were derived from a choroidal melanoma.26 OMM 1 cells were derived from subcutaneous metastasis of a uveal melanoma.27 OMM 2.3 cells were derived from a liver metastasis of a uveal melanoma.28,29 Cells were maintained at culture conditions of 37°C and 5% CO2, in complete DMEM supplemented with 10% FBS (Invitrogen Corporation, Carlsbad, CA). All cell lines were verified for human origin and for the presence of characteristic melanoma antigens with the use of commercially available antibodies (#ab14203; Abcam, Cambridge, MA). An isotype-matched control IgG was used as a negative control. Short tandem repeat (STR) analysis of the three cell lines was performed at the Johns Hopkins University Fragment Analysis Facility with a profiling kit (Powerplex 1.2; Promega, Madison, WI). STR profiles are shown in Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5746/-/DCSupplemental, and confirm that cell lines were not cross-contaminated with one another before analysis, in accordance with guidelines suggested by the uveal melanoma research community.30
Cells were transfected with full-length Bves cDNA with a FLAG tag, as previously described,14 or an empty vector control incorporating the same cytomegalovirus promoter using a transfection reagent (FuGENE 6; Roche Applied Science, Penzberg, Germany) according to the manufacturer's instructions. Cells were then maintained in medium containing selective antibiotic (200 μg/mL G418; MP Biomedical, Irvine, CA) for 2 weeks before experiments were conducted.
Immunofluorescence and Western Blot Analysis
Cell cultures were grown to either 50% or 90% confluence, then fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Inc., Hatfield, PA) in PBS, pH 7.2, for 10 minutes at room temperature. Cells were permeabilized in 0.1% Triton X-100 (Sigma-Aldrich Corp., St. Louis, MO) in PBS for 5 minutes at room temperature, rinsed three times in PBS, and blocked for 30 minutes in PBS with 10% goat serum (Sigma-Aldrich Corp.). Primary antibody staining was conducted overnight at 4°C with the following antibodies at 1:200 dilution: Bves 846 antisera,13 anti-ZO-1, anti-FLAG (M2 clone; Sigma-Aldrich), anti-Ki67 (Vector Laboratories, Burlingame, CA), and anti-ZONAB (Zymed-Invitrogen Corp., Carlsbad, CA), with DAPI used for a nuclear counterstain. Cells were imaged using an inverted fluorescence microscope (TE2000U Eclipse; Nikon, Tokyo, Japan) with imaging software (Image Pro Plus 5.1; Media Cybernetics, Silver Spring, MD). Distinct fluorescence channels were imaged sequentially using spectrally matched bandpass excitation and emission filters to avoid spectral overlap in colocalization analysis. Isotype-matched nonspecific primary antibodies were used as negative controls.
Cells were assayed for expression of Bves, occludin, and ZO-1 using immunoblotting techniques as previously described.13 Relative band densitometry was performed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).31 Ki67+ cells were quantified using imaging software (Image Pro Plus; Media Cybernetics), and the ratio of Ki67+ cells to total cells was used to calculate the Ki67 index.
Cell Proliferation Assays
Uveal melanoma cell lines were assayed by the XTT/PMS cell proliferation test as directed by the manufacturer (Sigma-Aldrich Corporation) at 24, 48, and 72 hours after seeding (initial density, 104 cells/cm2). Absorbance was read at 450 nm after subtracting a reference absorbance at 690 nm on a plate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA). Colony-formation assays were performed on basement membrane matrix (Matrigel; Sigma-Aldrich) as previously published,32 using a seeding density of 104 cells per well of a 24 well microplate.
Cell Cycle Analysis
Cell cycle distribution of cells was quantified using a propidium iodide-based method on synchronized cell populations as previously described.33 At least 20,000 cells were analyzed per sample on a flow cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ), and distributions were measured using the Watson pragmatic algorithm within a software suite (FlowJo 7; Treestar Software, Ashland, OR).
To evaluate the effect of transfection on apoptosis and necrosis, a flow cytometric assay was used according to the manufacturer's instructions (LIVE/DEAD Annexin V/Propidium Iodide kit, Invitrogen).
Dual Luciferase ZONAB Reporter Assay
A dual luciferase reporter assay used to quantify ZONAB activity was a gift of Karl Matter (University College London, London, UK) and was used as previously reported.24 In this assay, an ErbB2 promoter containing a ZONAB-binding site is used to regulate firefly luciferase expression. As a control, a mutated promoter of similar length, which is incapable of binding ZONAB, was used to regulate Renilla luciferase expression. Both plasmids were cotransfected into OMM2.3 cells using transfection reagent (FuGENE 6; Roche) according to the manufacturer's instructions. Forty-eight hours after transfection, cell lysates were measured ratiometrically for dual luciferase activity using the Promega dual luciferase reporter assay kit according to the manufacturer's instructions on a dual-injection microplate luminometer (Lumimark Plus; Bio-Rad Inc., Hercules, CA).
Results were compared using one-way ANOVA (Sigmaplot 11; Systat) with Dunnett's posttest. P < 0.05 was considered significant.
Results
Expression and Localization of Bves in Uveal Melanoma Cell Lines
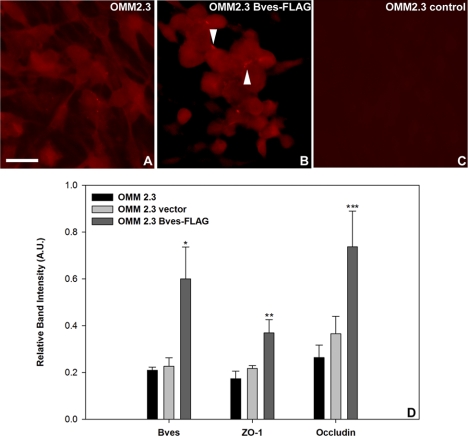
Bves overexpression in uveal melanoma was observed to alter trafficking of the protein and the formation of cell-cell contacts. Immunofluorescence analysis indicated that in wild-type uveal melanoma cells, Bves was cytoplasmically distributed regardless of cell density, and few cell-cell contact sites were observed (Fig. 1A). However, in cells transfected with a Bves-FLAG construct, increased localization of Bves toward sites of cell-cell contact was observed (Fig. 1B, arrowhead). Minimal immunoreactivity to an isotype-matched nonspecific control antibody was observed (Fig. 1C). Bves-FLAG, when expressed in melanoma cell lines, was trafficked to sites of cell-cell contact similarly to endogenous Bves. Results were consistent across all uveal melanoma cell lines tested. These data are consistent with previous localization studies of Bves in other ocular cell types, including trabecular meshwork13 and corneal epithelium.15,16
Figure 1.
Immunofluorescence localization of Bves and densitometric analysis of tight junction protein immunoblots in OMM2.3 cells. (A) Bves protein (red) is localized in the cytoplasm of wild-type OMM2.3 cells. (B) In OMM2.3 cells that overexpress Bves, increased localization of Bves is observed at sites of cell-cell contact (arrowheads). (C) Immunoreactivity to an isotype-matched negative control antibody was minimal. (D) Quantification of immunoblots indicates that occludin and ZO-1 proteins are upregulated in OMM2.3 cells overexpressing Bves. Data are reported as mean ± SEM (n = 4; *P = 0.018, **P = 0.027, ***P = 0.04). Results are representative of those observed for other wild-type and Bves-transfected uveal melanoma cell types. Scale bar, 20 μm.
Effect of Bves Overexpression on Expression of Tight Junction Molecules
Melanoma cells stably transfected with Bves constructs exhibited an increase in Bves compared with wild-type cells (Fig. 1D). The negative control cells stably transfected with empty vector exhibited similar levels of Bves compared with wild-type cells. This increase in Bves levels was associated with significantly increased occludin and ZO-1 proteins compared with wild-type and empty vector transfected cells (Fig. 1D). Immunofluorescence analysis indicated that the TJ protein ZO-1 colocalized with Bves at sites of cell-cell contact (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5746/-/DCSupplemental), similar to previous studies in other cell types.15 These results indicate that Bves overexpression modulates cell-cell adhesion and TJ formation in uveal melanoma cells in vitro as seen in previous studies using various cell types, including trabecular meshwork cells.13
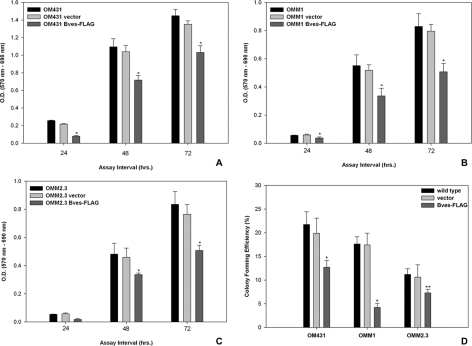
Effect of Bves Overexpression on Cell Proliferation and Cell Cycle Progression
Uveal melanoma cell lines overexpressing Bves exhibited significantly-lower cell proliferation rates than wild-type cell lines (Figs. 2A–C; P < 0.001). Vector control-transfected cells exhibited proliferative capacities that were similar to those of wild-type cells. When cells were grown on a basement membrane matrix (Matrigel; Sigma-Aldrich) substrate, three-dimensional proliferative capacities measured by colony formation efficiency correlated with trends observed for two-dimensional assays (Fig. 2D). Results were similar across all three cell lines, including those transfected with empty vector as a control. Quantification of Ki67+ cells using immunofluorescence microscopy exhibited results reflecting this trend (Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5746/-/DCSupplemental).
Figure 2.
Analysis of cell proliferation and three-dimensional colony-forming ability of uveal melanoma cell lines. (A–C) Cell lines transfected with Bves exhibit reduced cell proliferation compared with empty vector–transfected or wild-type–matched cell lines as measured by XTT assay. (D) Similarly, colony-forming capacity of Bves-transfected cell lines in basement membrane matrix is reduced. (A) OM431, (B) OMM1, (C) OMM2.3. Data are reported as mean ± SEM (n = 4; *P < 0.001, **P = 0.031).
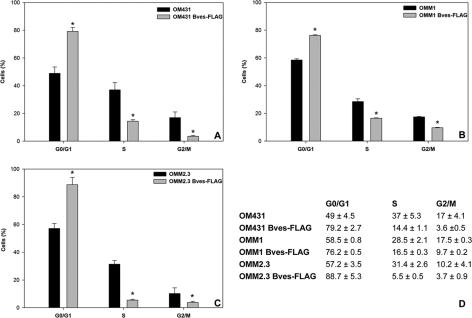
Because there were no differences in apoptotic or necrotic cell fraction attributable to transfection vehicle or overexpression of Bves as determined by flow cytometry (Supplementary Fig. S3, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5746/-/DCSupplemental), cell cycle distribution of uveal melanoma cell lines was quantified. Cell cycle analysis revealed that a greater fraction of Bves-FLAG–transfected cells were arrested in the G1 phase than wild-type cells (Fig. 3). However, a significantly larger fraction of wild-type melanoma cells was distributed in S and G2/M phases compared with Bves-overexpressing melanoma cells (Fig. 3D). These findings suggest that reduced proliferative capacity of Bves-overexpressing cells may be due to cell cycle arrest.
Figure 3.
Flow cytometric analysis of cell cycle progression in uveal melanoma cell lines. In all three cell lines tested, cell cycle distribution in S and G2/M phases were reduced for Bves-transfected cells (A–C). (A) OM431, (B) OMM1, (C) OMM2.3. (D) Table of cell cycle distribution percentages for each cell type. Data are reported as mean ± SEM (n = 3; *P < 0.001).
Association of Bves with ZONAB Transcription Activity
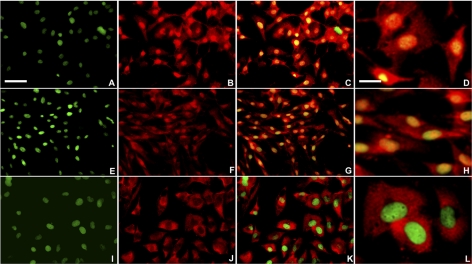
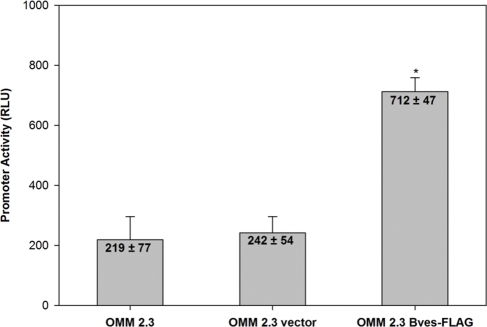
Based on immunofluorescence analysis of wild-type and empty vector-transfected cells, ZONAB was associated primarily with the nucleus or nuclear membrane, irrespective of cell density (Figs. 4A–F), whereas in Bves-overexpressing uveal melanoma cells, ZONAB was primarily localized within the cytoplasm and was excluded from the nuclear region, suggestive of reduced ZONAB-mediated transcriptional activity (Figs. 4G–I). These observations are consistent with the understanding that Bves overexpression reduces cell proliferation by increasing TJ formation and accumulation of ZO-1 at TJs, where sequestration of ZONAB inhibits the upregulation of proliferative factors. To confirm this finding, a reporter assay was used to detect ZONAB nuclear activity.24 In this assay, ZONAB interaction with the ErbB2 promoter results in transcriptional repression firefly luciferase. Thus, high luminescence compared with baseline levels is indicative of lower nuclear ZONAB activity. Consistent with immunofluorescence analysis of ZONAB, it was observed that Bves overexpression is associated with significantly reduced nuclear ZONAB activity (Fig. 5). These data are consistent with the hypothesis that Bves exerts control on cell proliferation through its participation in the ZO-1/ZONAB signaling pathway.
Figure 4.
Localization of ZONAB in the OMM2.3 uveal melanoma cell line. Wild-type (A–D), empty vector control (E–H), or Bves-overexpressing cell lines (I–L) were analyzed for ZONAB nuclear association. Red: ZONAB; green: nucleus; yellow: nuclear colocalization of ZONAB. (D, H, L) High-magnification insets of overlay. Bves-overexpressing cells exhibit reduced colocalization of ZONAB with the nuclear compartment, as indicated by green nuclear fluorescence. Scale bars: 50 μm (A), 10 μm (D).
Figure 5.
Dual luciferase ZONAB reporter assay. A luminometer was used to quantify ZONAB nuclear activity in wild-type, empty vector control, or Bves-overexpressing cell lines according to the dual luciferase reporter assay described in Methods. The relatively higher luciferase activity in Bves-overexpressing cells is indicative of reduced transcriptional repression by ZONAB in these cells, and therefore, reduced nuclear activity of ZONAB. Data are reported as mean ± SEM (n = 4; *P < 0.001).
Discussion
Our findings suggest that the TJ protein Bves reduces proliferative activity in uveal melanoma cells through its regulatory effects on TJ formation, which, in turn, modulate ZONAB transcriptional activity. In uveal melanoma cell lines overexpressing Bves, but not controls, an increase in TJ protein expression was observed (Fig. 1), as were reduced cell proliferation in two and three dimensions (Fig. 2) and reduced G0/G1 to S cell cycle phase transition (Fig. 3). Both ZONAB nuclear/cytoplasmic localization and transcriptional activity were shifted in response to overexpression of Bves in uveal melanoma cell lines. Specifically, overexpression of Bves resulted in a reduction of nuclear ZONAB (Fig. 4) and a reduction of ZONAB-associated transcriptional activity, as measured by dual luciferase reporter assay (Fig. 5).
The motivation underlying our study of the role of TJs in uveal melanoma stems from several reports suggesting that aberrant TJ signaling promotes cancer formation. Immunohistochemical studies demonstrated that the expression of TJ molecules ZO-1 and occludin are reduced or absent in several cancers and are associated with poor prognosis.34–37 Reduced expression of ZO-1, occludin, and claudin-4 was correlated with poorly defined differentiation, higher metastatic frequency, and lower survival rates in gastric cancer.38 The downregulation of TJ proteins in cancer has been linked to the hypothesis that TJs are suppressors of cancer formation and progression.39 Although the role of TJs in the progression of uveal melanoma has not been extensively investigated, we hypothesized that increasing Bves expression in uveal melanoma reduces the aggressiveness of cancer cells by enhancement of cell-cell contact and TJ-associated signaling mechanisms that inhibit proliferation.
We found that Bves reduces cell proliferation in uveal melanoma by modulating the nucleo-junctional localization of the protein ZONAB, according to a mechanism previously reported.40 Specifically, Bves interacts with ZO-1 to form TJs, which in turn serve as a docking site for cell signaling proteins that drive cell polarization, morphogenesis, differentiation, and proliferation. One of the signaling proteins that bind to ZO-1 at TJs is ZONAB, a Y-box transcription factor that, when in the nucleus, drives transcription of PCNA and cyclin D1 genes for the promotion of cell proliferation.23–25,41–44 These genes are involved in regulating cell cycle progression and may be involved in the alteration in cell cycle distribution observed on overexpression of Bves in uveal melanoma cells. The regulatory effect of Bves on TJ proteins (Fig. 1D) consequently increases extranuclear sequestration of ZONAB by ZO-1 (Fig. 4), thus inhibiting its accumulation in the nucleus, which is required for reduced proliferation.23 By increasing the expression of Bves in uveal melanoma cells, the ZO-1/ZONAB interaction, initially recognized for its role in contact-inhibited cell proliferation in epithelial cells,23 is activated. It is important to note, however, that the exact mechanism of ZONAB sequestration by TJs remains to be elucidated. Specifically, on overexpression of Bves in uveal melanoma cells, ZONAB appears to be associated with the nuclear region, but it is unclear whether ZONAB is in the nucleus or is merely bound to the nuclear membrane. To confirm the exact localization of ZONAB in cells overexpressing Bves, further ultrastructural analysis of ZONAB in cells is needed using confocal or electron microscopic techniques.
These studies establish a role of TJs in the proliferation of uveal melanoma. Ongoing studies in the laboratory are focused on the characterization of Bves function in uveal melanocytes and the role of Bves in cancer transformation of these cells. We hypothesize that Bves is responsible for maintenance of the melanocyte phenotype by establishing contact between the cell and microenvironment. Aberrant Bves signaling may be disrupted on cancer transformation in these cells. Restoration of Bves reduces proliferation rates in both primary and metastatic cell lines through a ZO-1/ZONAB signaling axis, suggesting that throughout disease progression, Bves signaling can partly reverse aggressiveness. On the other hand, Bves, as an adhesion molecule, may play a role in facilitating metastatic cell seeding in distant organs; therefore, the complete role of Bves in melanoma must be elucidated to understand stage-specific functions of the protein. More studies are warranted to completely reveal the role of ZONAB in cancer initiation and progression. In normal cell types, ZONAB regulates cell proliferation in a density-dependent manner.23,45 However, nuclear association of ZONAB in uveal melanoma cells occurred irrespective of cell density, suggesting that ZONAB trafficking and function are aberrant in cancer.
Current work is focused on the analysis of Bves expression in primary and metastatic uveal melanoma clinical specimens to investigate a potential diagnostic or therapeutic value for Bves. The interaction of Bves with other signaling pathways may reveal additional distinct roles for this protein in tumor cell migration, extravasation, and survival.
Supplementary Material
Footnotes
Supported in part by National Eye Institute Grants EY017185 (MSC) and P30 EY008126 (Core Grant in Vision Research), the Knights Templar Eye Foundation (AJ), a Research to Prevent Blindness Robert E. McCormick Scholar Award (MSC), an RPB unrestricted research grant to the Vanderbilt Eye Institute, and The Leslie and Judy Smith Discovery Grant.
Disclosure: A. Jayagopal, None; J.-L. Yang, None; F.R. Haselton, None; M.S. Chang, None
References
- 1. Singh AD, Kivela T. The collaborative ocular melanoma study. Ophthalmol Clin North Am. 2005;18:129–142, ix [DOI] [PubMed] [Google Scholar]
- 2. Gamel JW, McLean IW. Quantitative analysis of the Callender classification of uveal melanoma cells. Arch Ophthalmol. 1977;95:686–691 [DOI] [PubMed] [Google Scholar]
- 3. Folberg R, Rummelt V, Parys-Van Ginderdeuren R, et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993;100:1389–1398 [DOI] [PubMed] [Google Scholar]
- 4. Folberg R, Pe'er J, Gruman LM, et al. The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: a matched case-control study. Hum Pathol. 1992;23:1298–1305 [DOI] [PubMed] [Google Scholar]
- 5. Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onken MD, Ehlers JP, Worley LA, Makita J, Yokota Y, Harbour JW. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res. 2006;66:4602–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai K, Conway RM, Crouch R, Jager MJ, Madigan MC. Expression and distribution of MMPs and TIMPs in human uveal melanoma. Exp Eye Res. 2008;86:936–941 [DOI] [PubMed] [Google Scholar]
- 8. Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–298 [DOI] [PubMed] [Google Scholar]
- 9. Kohler K, Louvard D, Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. J Cell Biol. 2004;165:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsukita S, Yamazaki Y, Katsuno T, Tamura A. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930–6938 [DOI] [PubMed] [Google Scholar]
- 11. Lee JF, Zeng Q, Ozaki H, et al. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281:29190–29200 [DOI] [PubMed] [Google Scholar]
- 12. Swisshelm K, Macek R, Kubbies M. Role of claudins in tumorigenesis. Adv Drug Deliv Rev. 2005;57:919–928 [DOI] [PubMed] [Google Scholar]
- 13. Russ PK, Kupperman AI, Presley SH, Haselton FR, Chang MS. Inhibition of RhoA signaling with increased Bves in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2010;51:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawaguchi M, Hager HA, Wada A, Koyama T, Chang MS, Bader DM. Identification of a novel intracellular interaction domain essential for Bves function. PLoS One. 2008;3:e2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osler ME, Chang MS, Bader DM. Bves modulates epithelial integrity through an interaction at the tight junction. J Cell Sci. 2005;118:4667–4678 [DOI] [PubMed] [Google Scholar]
- 16. Ripley AN, Chang MS, Bader DM. Bves is expressed in the epithelial components of the retina, lens, and cornea. Invest Ophthalmol Vis Sci. 2004;45:2475–2483 [DOI] [PubMed] [Google Scholar]
- 17. Knight RF, Bader DM, Backstrom JR. Membrane topology of Bves/Pop1A, a cell adhesion molecule that displays dynamic changes in cellular distribution during development. J Biol Chem. 2003;278:32872–32879 [DOI] [PubMed] [Google Scholar]
- 18. Smith TK, Hager HA, Francis R, Kilkenny DM, Lo CW, Bader DM. Bves directly interacts with GEFT, and controls cell shape and movement through regulation of Rac1/Cdc42 activity. Proc Natl Acad Sci U S A. 2008;105:8298–8303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Osler ME, Smith TK, Bader DM. Bves, a member of the Popeye domain-containing gene family. Dev Dyn. 2006;235:586–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wada AM, Reese DE, Bader DM. Bves: prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development. 2001;128:2085–2093 [DOI] [PubMed] [Google Scholar]
- 21. Reese DE, Zavaljevski M, Streiff NL, Bader D. bves: A novel gene expressed during coronary blood vessel development. Dev Biol. 1999;209:159–171 [DOI] [PubMed] [Google Scholar]
- 22. Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872–891 [DOI] [PubMed] [Google Scholar]
- 23. Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sourisseau T, Georgiadis A, Tsapara A, et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albert DM, Ruzzo MA, McLaughlin MA, Robinson NL, Craft JL, Epstein J. Establishment of cell lines of uveal melanoma: methodology and characteristics. Invest Ophthalmol Vis Sci. 1984;25:1284–1299 [PubMed] [Google Scholar]
- 27. Luyten GP, Naus NC, Mooy CM, et al. Establishment and characterization of primary and metastatic uveal melanoma cell lines. Int J Cancer. 1996;66:380–387 [DOI] [PubMed] [Google Scholar]
- 28. Chen PW, Murray TG, Uno T, Salgaller ML, Reddy R, Ksander BR. Expression of MAGE genes in ocular melanoma during progression from primary to metastatic disease. Clin Exp Metastasis. 1997;15:509–518 [DOI] [PubMed] [Google Scholar]
- 29. Verbik DJ, Murray TG, Tran JM, Ksander BR. Melanomas that develop within the eye inhibit lymphocyte proliferation. Int J Cancer. 1997;73:470–478 [DOI] [PubMed] [Google Scholar]
- 30. Folberg R, Kadkol SS, Frenkel S, et al. Authenticating cell lines in ophthalmic research laboratories. Invest Ophthalmol Vis Sci. 2008;49:4697–4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun W, Zhou S, Chang SS, McFate T, Verma A, Califano JA. Mitochondrial mutations contribute to HIF1alpha accumulation via increased reactive oxygen species and up-regulated pyruvate dehydrogenease kinase 2 in head and neck squamous cell carcinoma. Clin Cancer Res. 2009;15:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li G, Kim DH, Kim TD, et al. Protein-bound polysaccharide from Phellinus linteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett. 2004;216:175–181 [DOI] [PubMed] [Google Scholar]
- 33. Arai M, Sato H, Kobayashi H, et al. Selective inhibition of bleomycin-induced G2 cell cycle checkpoint by simaomicin alpha. Biochem Biophys Res Commun. 2004;317:817–822 [DOI] [PubMed] [Google Scholar]
- 34. Nemeth Z, Szasz AM, Somoracz A, et al. Zonula occludens-1, occludin, and E-cadherin protein expression in biliary tract cancers. Pathol Oncol Res. 2009;15:533–539 [DOI] [PubMed] [Google Scholar]
- 35. Orban E, Szabo E, Lotz G, et al. Different expression of occludin and ZO-1 in primary and metastatic liver tumors. Pathol Oncol Res. 2008;14:299–306 [DOI] [PubMed] [Google Scholar]
- 36. Hoover KB, Liao SY, Bryant PJ. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol. 1998;153:1767–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer. 2004;40:2717–2725 [DOI] [PubMed] [Google Scholar]
- 38. Ohtani S, Terashima M, Satoh J, et al. Expression of tight-junction-associated proteins in human gastric cancer: downregulation of claudin-4 correlates with tumor aggressiveness and survival. Gastric Cancer. 2009;12:43–51 [DOI] [PubMed] [Google Scholar]
- 39. Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–767 [DOI] [PubMed] [Google Scholar]
- 40. Matter K, Balda MS. Epithelial tight junctions, gene expression and nucleo-junctional interplay. J Cell Sci. 2007;120:1505–1511 [DOI] [PubMed] [Google Scholar]
- 41. Nie M, Aijaz S, Leefa Chong San IV, Balda MS, Matter K. The Y-box factor ZONAB/DbpA associates with GEF-H1/Lfc and mediates Rho-stimulated transcription. EMBO Rep. 2009;10:1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pannequin J, Delaunay N, Darido C, et al. Phosphatidylethanol accumulation promotes intestinal hyperplasia by inducing ZONAB-mediated cell density increase in response to chronic ethanol exposure. Mol Cancer Res. 2007;5:1147–1157 [DOI] [PubMed] [Google Scholar]
- 43. Kavanagh E, Buchert M, Tsapara A, et al. Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J Cell Sci. 2006;119:5098–5105 [DOI] [PubMed] [Google Scholar]
- 44. Tsapara A, Matter K, Balda MS. The heat-shock protein Apg-2 binds to the tight junction protein ZO-1 and regulates transcriptional activity of ZONAB. Mol Biol Cell. 2006;17:1322–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lima WR, Parreira KS, Devuyst O, et al. ZONAB promotes proliferation and represses differentiation of proximal tubule epithelial cells. J Am Soc Nephrol. 2010;21:478–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.