This study provides the first proof that late adenoassociated virus (AAV)–mediated RPE65 expression is capable of restoring normal structure and function in the remaining cones of the older rd12 mouse, which is an animal model of human Leber congenital amaurosis type 2.
Abstract
Purpose.
RPE65 function is necessary in the retinal pigment epithelium (RPE) to generate chromophore for all opsins. Its absence results in vision loss and rapid cone degeneration. Recent Leber congenital amaurosis type 2 (LCA with RPE65 mutations) phase I clinical trials demonstrated restoration of vision on RPE65 gene transfer into RPE cells overlying cones. In the rd12 mouse, a naturally occurring model of RPE65-LCA early cone degeneration was observed; however, some peripheral M-cones remained. A prior study showed that AAV-mediated RPE65 expression can prevent early cone degeneration. The present study was conducted to test whether the remaining cones in older rd12 mice can be rescued.
Methods.
Subretinal treatment with the scAAV5-smCBA-hRPE65 vector was initiated at postnatal day (P)14 and P90. After 2 months, electroretinograms were recorded, and cone morphology was analyzed by using cone-specific peanut agglutinin and cone opsin–specific antibodies.
Results.
Cone degeneration started centrally and spread ventrally, with cells losing cone-opsin staining before that for the PNA-lectin–positive cone sheath. Gene therapy starting at P14 resulted in almost wild-type M- and S-cone function and morphology. Delaying gene-replacement rescued the remaining M-cones, and most important, more M-cone opsin–positive cells were identified than were present at the onset of gene therapy, suggesting that opsin expression could be reinitiated in cells with cone sheaths.
Conclusions.
The results support and extend those of the previous study that gene therapy can stop early cone degeneration, and, more important, they provide proof that delayed treatment can restore the function and morphology of the remaining cones. These results have important implications for the ongoing LCA2 clinical trials.
Leber congenital amaurosis (LCA) is a group of hereditary retinal diseases causing early-onset blindness or severe visual impairment.1–5 Clinical manifestations have been observed in LCA patients as early as 6 weeks after birth, resulting in severe vision loss or impairment by the age of 6 months or 1 year.1,2,5,6 Although the LCA family of diseases has similar clinical findings, it is classified into different clinical subtypes according to the differences in gene mutations.1–3,5,6 To date, mutations in 14 genes have been identified in LCA patients.2 One of the identified genes is RPE65, which is expressed specifically and abundantly in the retinal pigment epithelium (RPE). Mutations in RPE65 are associated with LCA type 2.1,2,7
RPE65 is an isomerohydrolase in the classic visual cycle, which is the enzymatic pathway that regenerates the rod and cone chromophore 11-cis retinal after it is bleached during light absorption.8 Similar to rhodopsin in rods, which is composed of the apoprotein opsin and the chromophore 11-cis retinal, cone pigment is composed of cone opsin and 11-cis retinal.9,10 Hence, loss of RPE65 function results in loss of all photoreceptor function.9,11,12
Experimental therapeutic strategies for RPE65 mutations include pharmacologic intervention,13,14 cell transplantation,15 and gene delivery, which are currently being tested in Rpe65 knockout (Rpe65−/−) mice,16 Rpe65 transgenic mice engineered to carry specific mutations,12 naturally occurring mice with an Rpe65 mutation (rd12),17 and dogs with naturally occurring Rpe65 mutations.18 Early experiments focused on restoration of rod function, since the remaining ERG in Rpe65−/− mice was reported to be due to rod function only.19 However, subsequent experiments revealed that cone function could be documented in young animals before the onset of cone degeneration.10 In 2001, Acland et al.18 showed for the first time that AAV-RPE65-mediated gene therapy can successfully restore visual function in the dog model.
Subsequently, we showed that early treatment starting at P14 restores both normal rod and cone functions in rd12 mice,17 whereas late treatment starting at P90 (Pang JJ et al., unpublished results, 2006) or P3520 restores rod but not cone function. Znoiko et al.21 have shown that cone degeneration starts as early as 2 weeks of age in Rpe65−/− mice, and our own study revealed that cone degeneration starts at approximately P14 in rd12 mice, resulting in the disappearance of most cones by week 5, except for a few located in the peripheral dorsal and temporal quadrants.20 Hence, one explanation for the failed cone rescue is that we missed the therapeutic window for gene therapy starting after P35.20 However, it is also plausible that our injections did not transfect the RPE underlying the remaining cones located in a small area of the peripheral retina. In previous P35 and P90 studies, it was determined that the retinal detachment after injections covered more than 50% of the retina, but it is unknown whether the remaining cones located in the dorsal and temporal quadrants of the retina had been transfected after the subretinal injections.
In the present study, we used a self-complementary (adeno-associated virus) AAV5 vector to obtain rapid and strong therapeutic gene expression.22 In addition, we selected only those treated rd12 mice that exhibited more than 95% retinal detachment after subretinal injections for further evaluation. This strategy ensured transfection of the peripheral RPE and allowed us to test whether late treatment can restore the structure and function of the remaining peripheral cones. Results from this study will be important for the current ongoing LCA2 clinical trials that have been focused on restoring rod function in both children and young adults, although they also have various degrees of cone degeneration.23–25
Methods
Animals
C57BL/6J mice and the congenic inbred strain of rd12 (Rpe65rd12, or B6(A)-Rpe65rd12/J) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All mice were bred and maintained in the Animal Facilities of Wenzhou Medical College. The animals were maintained in a 12-hour light–12-hour dark cycle with an ambient light intensity of 18 lux and with free access to food and water. All experiments were approved by the Wenzhou Medical College's Institutional Review Board and were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
scAAV5-smCBA-hRPE65 Vector Preparation
Pseudotyped AAV5-capsid, self-complementary AAV vectors (scAAV) were used in this and our previous studies, as they have been shown to be more efficient vectors for transduction of RPE than standard, single stranded AAV vectors.22 The scAAV5-smCBA-hRPE65 vector contains the small, hybrid CMV-chicken β-actin (smCBA) promoter which has been shown to have identical transduction and tropism characteristics to full chimeric CMV-chicken β-actin (CBA) promoter when targeted at the mouse retina.26 The corresponding vector plasmid for scAAV5-smCBA-hRPE65 was constructed by replacing the humanized green fluorescent protein (GFP) cDNA of sc-trs-smCBA-hGFP,27 with the human RPE65 cDNA via a NotI digest. This vector contains flanking AAV serotype 2, inverted terminal repeats (ITRs); one ITR has the modifications required for packaging as a self-complementary AAV vector.27 Vectors were manufactured and purified by previously described methods.28 The vector titer was determined by real-time PCR, and final aliquots were resuspended in balanced salt solution (Alcon Laboratories, Fort Worth, TX) containing 0.014% Tween 20.
Subretinal Injections
Subretinal injections were performed using previously described methods with modifications.17,22,26 Briefly, an aperture within the pupil area was made through the nasal cornea with a 30.5-gauge disposable needle. A 33-gauge, unbeveled, blunt needle mounted on a 5-μL syringe (Hamilton Co., Reno, NV) was introduced through the corneal opening, avoiding the lens and penetrating the neuroretina to reach the subretinal space. One microliter of vector suspension (1 × 1013 genome containing particles/mL) with 1% fluorescein was slowly, subretinally injected into the right eye of each rd12 mouse. Injections were always performed in the right eye, leaving the uninjected left eye as a control. The injected retinal area was visualized by fluorescein-positive subretinal blebs demarking the retinal detachment. All procedures were made under direct observation aided by an operating microscope at 2.5 × 10 magnification. Surgical complications included iris–cornea adhesion, iris or retinal hemorrhage, and damage to the lens, which caused cataract formation. After the injection procedure, 1% atropine eye drops and 0.3% tobramycin-dexamethasone eye ointment (Alcon Laboratories) were given three times each day for 3 days. We kept for further evaluation only those injected mice that had more than 95% retinal detachment after subretinal injection and with minimal complications. The untreated contralateral rd12 eyes and the age-matched normal C57BL/6J eyes were used as the control. Approximately 250 mice were used.
Electroretinograms
Two months after the vector injection, ERGs of both treated and untreated eyes were recorded; and ERGs of age-matched, uninjected C57BL/6J mice were used as the normal control. Full-field ERGs were recorded with a custom-built Ganzfeld dome connected to a computer-based system (Q450SC UV; Roland, Wiesbaden, Germany). White, green (505 ± 6 nm), and UV LEDs (363 ± 6 nm) were used as the stimulation light sources for recording the total cone, photopic flicker, and M- or S-cone (UV-sensitive cone) ERGs, respectively. Maximum light intensity for the different stimulus conditions varied and hence cannot be compared for quantitative analysis (white LEDs, 1.96cd/m2; green LEDs, 0.75 cd/m2; UV LEDs, 3 mWs/m2; and flicker, 2.0 cd/m2). All testing was performed in a climate-controlled, electrically isolated dark room under dim red light illumination. Mice were dark-adapted overnight and anesthetized with ketamine (72 mg/kg) and xylazine (4 mg/kg). Body temperature was maintained by placing the animals on a 37°C warming pad. Corneas were anesthetized with a drop of 0.5% proparacaine hydrochloride, and pupils dilated with 1% atropine and 2.5% phenylephrine hydrochloride. A small amount of 2.5% methylcellulose gel was applied to the eye, and a special Ag/AgCl wire loop electrode was placed over the cornea to record the ERGs. Needle reference and ground electrodes were inserted into the cheek and tail, respectively. Recordings were started after 10 minutes of light adaptation at 30 cd/m2 (background light). To record photopic-cone, M-cone, and S-/UV-cone ERG responses, we amplified the signals 1000-fold, filtered between 1 and 100 Hz, with white background light (30 cd/m2). For flicker-ERGs, a stimulus frequency of 20 Hz was used, and the signals were amplified 1000-fold and filtered between 1 and 300 Hz.
Immunocytochemistry
After ERG examination, both eyes of treated rd12 and normal C57BL/6J mice were enucleated, and retinal whole mounts or radial sections were prepared by using previously described methods with some modifications.22,29,30 Briefly, after enucleation, both eyes were marked on the 12 o'clock point (dorsal) of the limbus with a cauterizing iron and fixed in fresh 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) overnight at 4°C. After fixation, the cornea, lens, and vitreous were removed to generate eye cups. Retina whole mount preparations were generated by first removing the optic nerve bud and then carefully separating the neuroretina from the eye cup. Frozen sections (12 μm thick) were prepared from eye cups cryoprotected in 30% sucrose and embedded in OCT.22
Humans have three different types of cone, L (longwave- or red-sensitive), M (medium wave- or green-sensitive), and S (shortwave- or blue-sensitive), whereas there are only two sensitivity maxima in mice, representing M-cones containing a green-sensitive pigment with an absorption maximum near 511 nm, and S-cones with an ultraviolet (UV)-sensitive pigment with peak absorption near 359 nm.31 Previous study has shown that monoclonal antibodies specific for human M- and S-cone opsins could identify the two cell populations in the mouse.32 Two methods were used to identify cone photoreceptors. Peanut agglutinin (PNA), which binds the interphotoreceptor matrix sheath surrounding the cone outer segments, was used to identify the overall cone distribution pattern, whereas anti-M- or S-cone opsin antibodies were used to identify specific M- or S-cone distribution patterns. After the specimens were washed with 0.1 M PBS, immunocytochemistry was performed by first incubating the retinas with 10% normal goat serum (NGS) blocking solution for 1 hour, and then staining with rabbit anti-M or anti-S opsin (1:200; Temecula, Chemicon, CA) or FITC-conjugated peanut agglutinin (1:200; Vector Laboratories, Burlingame, CA) overnight at 4°C. After the exposure to primary antibodies, the specimens were washed in 0.1 M PBS, incubated in anti-rabbit IgG conjugated to Cy3 fluorochrome (Beyotime, Huhan, China) overnight at 4°C and excess secondary antibody was removed by washing three-times (5 minutes each). To mount them for photography, the retinas were flattened with four relaxing cuts. The retina whole mounts and sections were photographed with a fluorescence microscope (Axio Imager Z1; Carl Zeiss Meditec, Oberkochen, Germany) equipped with a 100-W mercury light source and FITC or Cy3 filters. Cells labeled for PNA-lectin or cone opsins were photographed and manually counted in retina whole mounts in the area around the optic nerve and at a distance of 1000 μm from the optic nerve in the dorsal/temporal, ventral/temporal, ventral/nasal, and dorsal/nasal quadrants of the retina, each within one field at 40× magnification (38,420 μm2).
Statistical Analysis
Five mice per group were analyzed for statistical purposes; comparing both the number of PNA- and cone opsin–positive cells, as well as the b-wave amplitudes and time to peak of photopic cone ERGs. Mann–Whitney U test, and Dunnett multiple comparison test were used. Data are presented as the mean ± SD. P < 0.05 was considered statistically significant.
Results
Time Course of Cone Degeneration in the rd12 Retina
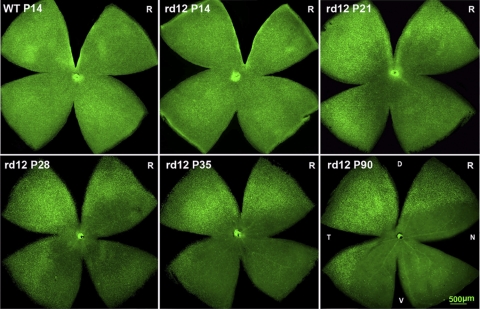
Early and rapid cone degeneration, particular in the ventral retina, has been reported in the genetically engineered Rpe65−/− mouse.10,16,33 Similar observations have been found in the rd12 mouse retina,20 although a careful temporal analysis is has not been conducted. To determine the process and pattern of the overall cone degeneration in rd12 mice, we analyzed retinal wholemounts of rd12 and C57BL/6J mice, between P14 and P90, stained with the cone-specific marker, PNA-lectin, which binds to the interphotoreceptor matrix sheath surrounding the cone outer segments.34 Please note that PNA-lectin and M- or S-cone opsin positive cone cell counts obtained in the five fields across the retina—DT (dorsal temporal), VT (ventral temporal), C (central), VN (ventral nasal) and DN (dorsal nasal)—did not vary in the C57BL/6J retina between P74 and P150 (data not shown); hence we present and use only the P90 data as the normal adult control. At P14, the cone distribution pattern in the rd12 retina appeared similar to that of the C57BL/6J retina when viewed at lower magnification, although the total cone number was slightly decreased in the rd12 retina (average number, 482 ± 26 cells/field), when compared with age-matched C57BL/6J mice (503 ± 13 cells/field, P < 0.05, Fig. 1; Table 1). By P21, the number of PNA-positive cones in the rd12 retina was decreased dramatically (280 ± 24), especially in the C and VN quadrants of the retina (Fig. 1; Table 1). By P35, further losses of cones in the C and VN quadrants of the retina were documented (Table 1). Similar to the Rpe65−/− mouse,21 cone degeneration in the DT quadrant was relatively mild in rd12 mice (Table 1). By 3 months-of-age, while all cones in the C and VN areas had degenerated, some PNA-positive cones remained in the DT quadrant of the retina (Fig. 1; Table 1). Some PNA-positive cones remained in the DT quadrant of the rd12 retina for up to 16 months (data not shown).
Figure 1.
Cone degeneration pattern in rd12 retinal wholemounts (PNA-lectin staining). Cone distribution was almost normal in the P14 rd12 retina when compared to the wild type control. From P14 to P90, the density of PNA-positive cones decreased dramatically in the central, ventral and nasal quadrants of the rd12 retina. R, right eye; L, left eye; WT, wild type C57BL/6J mouse; P, postnatal day; D, dorsal; V, ventral; T, temporal; N, nasal.
Table 1.
PNA-Positive Cell Counts in One Field of Five Different Locations in Retina Whole Mounts of Treated and Untreated rd12 Mice
| Location | WT Mice |
Progress of Degeneration in rd12 Mice |
Treated and Untreated after 2 Months of Injection in rd12 Mice |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P14 | P90 | P14 | P21 | P28 | P35 | P90 | P14Inj + 2M | P14Uninj + 2M | P90Inj + 2M | P90Uninj + 2M | |
| DT | 511 ± 11 | 506 ± 9 | 488 ± 30 | 430 ± 26 | 369 ± 17 | 306 ± 23 | 252 ± 19 | 391 ± 19 | 270 ± 20*† | 226 ± 19 | 187 ± 15*‡ |
| VT | 497 ± 10 | 490 ± 12 | 482 ± 19 | 390 ± 14 | 206 ± 18 | 97 ± 9 | 15 ± 4 | 376 ± 18 | 13 ± 3*† | 10 ± 5 | 7 ± 2*‡ |
| C | 495 ± 15 | 493 ± 10 | 476 ± 26 | 51 ± 11 | 20 ± 5*§ | 1.8 ± 0.8 | 0.8 ± 0.8 | 369 ± 20 | 1.0 ±0.7*† | 1.0 ± 0.7 | 0.5 ± 0.5**‡ |
| VN | 504 ± 13 | 498 ± 11 | 479 ± 21 | 122 ± 38 | 70 ± 8*‖ | 54 ± 12 | 1.4 ± 0.5 | 372 ± 10 | 4 ± 1*† | 1.6 ± 0.8 | 0.8 ± 0.8**‡ |
| DN | 508 ± 17 | 500 ± 14 | 485 ± 34 | 405 ± 28 | 246 ± 16 | 206 ± 27 | 140 ± 21 | 385 ± 16 | 151 ± 13*† | 102 ± 10 | 82 ± 10*‡ |
| Average | 503 ± 13 | 497 ± 11 | 482 ± 26*¶ | 280 ± 24 | 223 ± 13 | 133 ± 14 | 82 ± 9 | 379 ± 17 | 88 ± 8 | 68 ± 7 | 55 ± 6 |
Inj, injected; Uninj, uninjected. Mean ± SD, n = 5/cohort.
P < 0.05;
P > 0.05.
Comparing same area of P14 treated and untreated rd12 eyes.
Comparing same area of P90 treated and untreated rd12 eyes.
Comparing rd12 between P21 and P28 in the central area.
Comparing rd12 between P21 and P28 in VN field.
Comparing rd12 and WT at P14.
Cone Morphology Was Rescued by Gene Replacement Therapy
Previous experiments with 11-cis retinal injections in Rpe65−/− mice have suggested that morphology and function of true cones or cone-like cells on the Nrl−/− background can be preserved when injections are started early and applied frequently,35,36 but not if injections were initiated late or applied infrequently.37 In the present study, we investigated two treatment strategies: initiation at P14, before the major cone degeneration, and at P90, a time point when only some dorsal and temporal cones remain.
Rescue Effect after P14 Treatment
It was of interest to determine what percentage of cones could be rescued if gene therapy was initiated at P14, a time point when little cone cell death has occurred in the rd12 retina, although there are structural abnormalities in the mutant cones (described later).
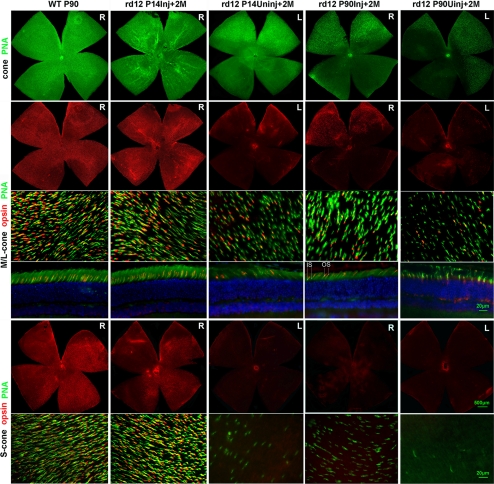
Two months after P14 injections, retinal wholemounts stained with PNA-lectin revealed a similar distribution pattern for cones in the rd12 and the normal C57BL/6J retinas (Fig. 2). Cell counts demonstrated that the treated rd12 retinas contained 76% (379/497) of the wild type levels of PNA-positive cones (Fig. 2; Table 1), while only a few cones remained in the dorsal and temporal quadrants of the contralateral untreated eyes (Fig. 2).
Figure 2.
Cone survival in treated rd12 (PNA and cone-opsin staining) eyes. Two months after P14 treatment, staining for PNA-lectin and M- and S-cone opsins revealed similar distribution patterns in treated rd12 retinas when compared with those from C57BL/6J eyes. In P90 treated rd12 eyes, the PNA-lectin and M-cone opsin–positive cones located in the dorsal and temporal quadrants of the retinas, although decreased in number, still showed a distribution pattern similar to that in the normal C57BL/6J eyes; no S-cone opsin–positive cells were identified. In the contralateral P90+2M uninjected left eyes, some PNA and a few abnormal M-cone opsin–positive cells were still present in the limited regions of dorsal and temporal retinal quadrants. In radial sections prepared from the P90+2M uninjected eye cup, most of the M-cone opsin staining was observed in cone inner segments, cell bodies, and cone pedicles (outer plexiform layer). Green: cone specific PNA staining; red: M- or S-cone opsin staining; blue: DAPI (4′,6-diamidino-2-phenylindole) stained nuclei. R, right eye; L, left eye; P, postnatal day; Inj., injected; Uninj., uninjected; M, months.
To examine whether the preservation of PNA-positive cells is cone opsin–specific, retina wholemounts were stained with cone opsin–specific antibodies. Similar to previous findings,38 we found that M-cones were distributed throughout the retinal whole mount (Fig. 2, second row), whereas S-cone opsin–positive cones were distributed in a dorsoventral gradient, with the highest number of cells in the nasal and ventral quadrants and fewer cells in the dorsal temporal quadrant (Fig. 2, fifth row). In treated rd12 eyes (Fig. 2), the average number of M-cone opsin–positive cones counted in the five fields across the retina was 76% (Table 2) of that present in the wild type control; the number of S-cone opsin–positive cones was 68% (Table 3) of the normal number (Fig. 2; Tables 2, 3). These counts were similar to those obtained with PNA-lectin staining, suggesting that both types of cones are equally amenable to protection by early treatment.
Table 2.
M/L-Cone Opsin-Positive Cell Counts in One Field of Five Different Locations in Retina Wholemounts of Treated and Untreated rd12 Mice
| Location | WT Mice |
rd12 Mice |
||||
|---|---|---|---|---|---|---|
| P90 | P90 | P14Inj + 2M | P14Uninj + 2M | P90Inj + 2M | P90Uninj + 2M | |
| DT | 399 ± 36 | 48 ± 15 | 308 ± 26 | 66 ± 20*† | 123 ± 19 | 28 ± 5*‡ |
| VT | 393 ± 24 | 0 | 299 ± 30 | 0*† | 1.4 ± 0.5 | 0*‡ |
| C | 389 ± 40 | 0 | 295 ± 35 | 0*† | 0.8 ± 0.2 | 0*‡ |
| VN | 397 ± 30 | 0 | 297 ± 28 | 0*† | 0.6 ± 0.5 | 0*‡ |
| DN | 400 ± 26 | 17 ± 6 | 305 ± 20 | 23 ± 13*† | 78 ± 15 | 4 ± 3*‡ |
| Average | 396 ± 31 | 13 ± 4 | 301 ± 28 | 18 ± 7 | 41 ± 7 | 6 ± 2 |
Mean ± SD, n = 5/cohort. Inj, injected; Uninj, uninjected.
P < 0.05.
Comparing same area of P14 treated and untreated rd12 eyes.
Comparing same area of P90 treated and untreated rd12 eyes.
Table 3.
S-cone Opsin–Positive Cell Counts in One Field of Five Different Locations in Retina Wholemounts of Treated and Untreated rd12 Mice
| Location | WT Mice |
rd12 Mice |
||||
|---|---|---|---|---|---|---|
| P90 | P90 | P14Inj + 2M | P14Uninj + 2M | P90Inj + 2M | P90Uninj + 2M | |
| DT | 34 ± 12 | 0 | 30 ± 9 | 0*† | 0 | 0 |
| VT | 116 ± 26 | 0 | 87 ± 21 | 0*† | 0.4 ± 0.5 | 0 |
| C | 300 ± 39 | 0 | 198 ± 23 | 0*† | 0 | 0 |
| VN | 384 ± 54 | 0 | 250 ± 30 | 0*† | 0 | 0 |
| DN | 320 ± 20 | 0 | 220 ± 18 | 0*† | 0 | 0 |
| Average | 231 ± 30 | 0 | 157 ± 20 | 0 | 0 | 0 |
Mean ± SD; n = 5/cohort. Inj, injected; Uninj, uninjected.
P < 0.05.
Comparing the same area of P14 treated and untreated rd12 eyes.
The high-magnification images of the retina whole mounts from the dorsal temporal quadrant, double-stained with PNA-lectin and M- (Fig. 2, third row) or S-cone (Fig. 2; sixth row) opsin, confirmed our findings using PNA-lectin. Cones in the treated rd12 retina had a similar distribution pattern when compared with that of the wild-type C57BL/6J retina. It was also confirmed that the remaining PNA-positive cones in the dorsal and temporal quadrants of the untreated rd12 eyes were M- but not S-cones (Fig. 2, second and fifth rows), and those remaining M-cone opsin–positive cones showed abnormal morphology, especially a reduction in outer-segment length (Fig. 2).
In previous publications, it has been shown that 11-cis retinal is necessary for the maturation-dependent restriction of cone opsins to the cone outer segments.10,35–37,39 Cones in cross sections derived from dorsal and temporal quadrants of the treated rd12 eyes showed normal M-cone opsin localization in the cone outer segments (Fig. 2, fourth row). In the same area of the untreated eyes, whereas a small percentage of the M-cone opsin could still be observed in cone outer segments, most of the opsin was mislocalized to the inner segments, cone nuclei, axons, and cone pedicles in the outer plexiform layer (Fig. 2).
Rescue Effect after P90 Treatment
At the onset of P90 treatment, the overall PNA-lectin–positive cone number was reduced to 16% of wild-type levels (82/503); these remaining cones were M-cones (13/82) or cones without any discernible cone opsins present in the outer segment. M-cones were present in particular in the DT quadrant of the retina (48/399).
Two months after P90 injections, 83% (68/82) of the PNA-positive cones were maintained in treated rd12 eyes, although this represents only 14% (68/497) of the normal cone count. Only 45% (226/506) of the normal cones in the DT quadrant were maintained (Fig. 3, Table 1). The cones in the contralateral, untreated retinas (Fig. 2, first row) were almost absent, with the exception of some in the most dorsal and temporal quadrants.
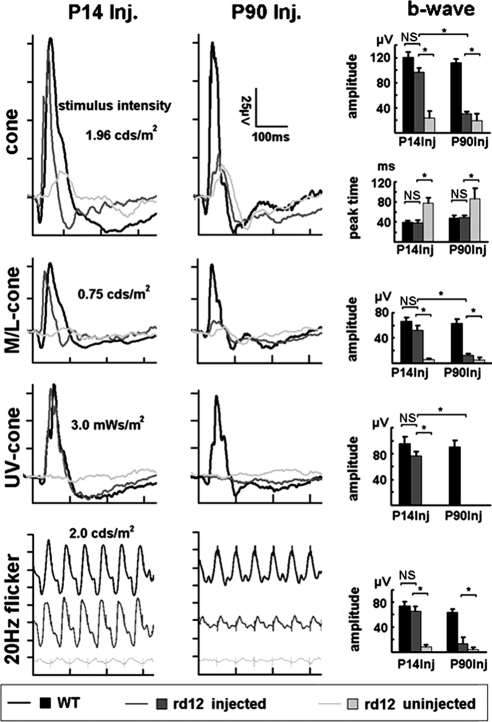
Figure 3.
Cone-related ERG responses in rd12 mice after gene therapy. After P14 treatment, the full cone, M-cone, UV-cone, and 20-Hz flicker-ERGs in treated rd12 eyes were similar in amplitude and kinetics to those elicited from age-matched C57BL/6J eyes. This result is in comparison to the severely reduced amplitudes and delayed kinetics of the ERGs recorded from the contralateral, uninjected eyes. After P90 treatment, responses to full cone, M-cone, and 20-Hz flicker ERGs were restored with intermediate amplitudes, but normal b-wave peak times; however, no UV-cone responses were detected in P90 treated eyes. In uninjected rd12 eyes, although weak responses to the full cone stimuli were detected, the peak time of their b-waves was significantly prolonged. The stimulus intensity for the full-cone, M-cone, UV-cone, and 20-Hz flicker-ERGs were 1.96 cd/m2, 0.75 cd/m2, 3 mWs/m2, and 2.0 cd/m2, respectively. WT, wild-type C57BL/6J mouse; P, Postnatal day; Inj., injected; Uninj., uninjected; M, months; *P < 0.05; NS, nonsignificant.
In P90 treated rd12 eyes (Fig. 2), a total of 10% of M-cones (41/396, Table 2), but no S-cones were identified (Fig. 3, Tables 2, 3). M-cones from the DT and DN quadrants benefited the most from the treatment. Although approximately threefold (123/48) more M-cones were present in the DT (Table 2) compared with that at the P90 level, only one-third of the M-cones in the DT quadrant retina remained (123/308; Table 2), compared with that in the P14 treated rd12 retina. However, on the flip side, the number of M-cone opsin–positive cells increased by 215% ([41–13]/13) after the P90 treatment (Table 2).
In untreated rd12 retinas, scattered PNA-lectin–positive cones were still observed in the dorsal and temporal quadrants (Fig. 3). Although M-cone opsin–positive cell counts were sharply reduced and exhibited short outer segments, all S-cones had disappeared. Compared with C57BL/6J, untreated rd12 retinas had only 1.5% (6/396) of total M-cones or 7% (28/399) of M-cones remaining in the DT quadrant (Fig. 3; Table 2).
Finally, as in the cross sections derived from the DT quadrant of treated P14 eyes, normal M-cone opsin localization in the cone outer segments was observed in the rd12 retinas treated at P90 (Fig. 2, fourth row).
Cone Function Was Rescued by Gene Replacement Therapy
To confirm that preservation of structure correlated with improvement of function, different strategies were used to record cone ERGs, including the photopic ERGs that do not distinguish between the activities of the two cone types, M- and S-cone–specific, single-flash ERGs and 20-Hz flicker ERGs.
Rescue Effect after P14 Treatment
Two months after P14 treatment (Fig. 3), the a-wave amplitudes of the photopic (full cone) ERGs were negligible in untreated (0.62 ± 0.86 μV; mean ± SD) eyes, but were significantly improved in treated rd12 eyes (9.12 ± 1.23 μV) to levels approximately two thirds of that in normal age-matched uninjected C57BL/6J control mice (14.30 ± 2.68 μV). The b-wave amplitudes were 96.7 ± 6.3 μV in treated and 23.88 ± 10.84 μV in untreated rd12 eyes. This increase in function corresponded to a restoration of approximately 80% of the cone function present in uninjected C57BL/6J eyes (120.7 ± 7.8 μV). Furthermore, b-wave kinetics, as determined by b-wave peak times or the implicit time of the photopic ERGs, were prolonged to 77.8 ± 10.6 ms in untreated eyes, but had similar delays in treated rd12 (38.4 ± 5.3 ms) and C57BL/6J eyes (43.4 ± 4.0 ms).
Because of the lower light intensity of the green and UV LEDs, only b-wave amplitudes and kinetics could be analyzed for the cone-specific ERGs. Similar to the full cone ERG, the b-wave amplitudes of the M-cone ERG were almost absent in untreated rd12 eyes (6.13 ± 1.62 μV), but were significantly increased in the treated rd12 eyes (51.92 ± 8.23 μV). Again, this corresponded to a restoration of almost 80% of normal M-cone function (66.74 ± 5.71 μV). The b-wave peak time of the M-cone ERG was 74.2 ± 7.3 ms in untreated rd12, but accelerated to normal levels in treated rd12 eyes (38.4 ± 5.1 ms; 42.2 ± 3.7 ms in C57BL/6J; P > 0.05). No UV-cone ERGs could be recorded in untreated rd12 mice, while approximately 80% of the UV/S cone function was rescued after P14 treatment (76.75 ± 6.12 μV in treated rd12 compared with 95.13 ± 11.55 μV in C57BL/6J eyes).
As a final measure of cone function, we assessed ERG amplitudes in response to 20-Hz flicker. Those amplitudes were 7.9 ± 4.2 μV in untreated rd12, 65.5 ± 7.6 μV in treated rd12, and 74.0 ± 7.1 μV in C57BL/6J eyes. With this measure, 89% of wild-type cone function was documented in treated rd12 eyes, which was statistically indistinguishable from that of C57BL/6J mice, but significantly different from noninjected rd12 eyes (P < 0.05).
Rescue Effect after P90 Treatment
The a-wave amplitude of the photopic ERG had declined to 0.23 ± 0.64 μV in the untreated rd12 eyes, but significantly improved to 3.26 ± 1.44 μV in P90-treated rd12 eyes; it was 11.91 ± 1.87 μV in age-matched C57BL/6J eyes (Fig. 3). Likewise, b-wave amplitudes declined to 19.48 ± 11.13 μV in untreated rd12 eyes, whereas treatment led to improved b-wave amplitudes in treated rd12 eyes (30.71 ± 3.28 μV), which represented 26% of normal cone amplitudes (112.35 ± 6.24 μV in C57BL/6J eyes). Despite only a partial recovery of amplitude, b-wave kinetics completely recovered in treated rd12 eyes (48.64 ± 4.45 ms) when compared to age-matched C57BL/6J mice (48.00 ± 5.43 ms), whereas untreated rd12 eyes exhibited peak times almost doubled in length (86.63 ± 21.61 ms, P < 0.05).
As with full cone ERGs, the b-wave amplitudes of M-cone ERGs were almost negligible (5.50 ± 3.05 μV) in untreated rd12 eyes, whereas P90 treatment restored approximately 20% of M-cone function in rd12 eyes (12.35 ± 2.19 μV), when compared with that in C57BL/6J eyes (62.78 ± 6.93 μV). Likewise, the b-wave peak-times of M-cone ERGs were completely normal in treated rd12 eyes (47.9 ± 1.6 ms), when compared to those in C57BL/6J control mice (48.5 ± 3.6 ms); whereas kinetics were significantly delayed in untreated rd12 eyes (85.9 ± 8.6 ms, P < 0.05). As b-wave amplitudes of the S/UV-cone ERGs were not detectable in P74 (P14 + 2 months) untreated rd12 mice, it was not surprising that P90 treatment did not recover any S/UV-cone function. Finally, improvement of cone function with P90 treatment could also be documented by responses to 20-Hz flicker ERGs, which was 4.7 ± 2.8 μV in untreated rd12, 13.5 ± 9.8 μV in P90-treated rd12, and 63.7 ± 5.7 μV in C57BL/6J eyes. This technique revealed a restoration of 21% of cone function.
Discussion
The main results of the present study are (1) rd12 cones develop in almost normal numbers and degenerate rapidly in the ventral and central quadrants of the retina at around 2 weeks of age; (2) during degeneration, staining for cone opsin is lost before the loss of the PNA-lectin–positive cone sheath; (3) RPE65 gene replacement therapy using AAV viral vectors and starting before extensive cone degeneration at P14 resulted in almost wild-type levels of cone function and structure; (4) gene replacement therapy at P90 when central and ventral cones had disappeared rescued a significant number of the remaining M-cones in the dorsal and temporal quadrants of the retina, and the delayed ERG kinetics and mislocalized M-cone opsins were both corrected; and (5) more M-cone opsin–positive cells were identified 2 months after P90 treatment, suggesting that cone opsin expression is reinitiated in cells with PNA-lectin–positive cone sheaths. These results have important implications for the current LCA2 clinical trials, especially for older patients.
With PNA-lectin staining, our data demonstrated that the rd12 retina had almost a normal number of cones by eye opening (P14). Similar to Rpe65−/− mice,16,21 cone degeneration progresses very quickly between P14 and P21, spreading from the central to the ventral and nasal quadrants in the rd12 retina (Fig. 1). By P90, only some M-cones in the dorsal and temporal quadrants remained. Of note, similar patterns of cone survival have also been seen in other mouse models of retinal degeneration, such as the rd1 mouse40 and mouse models of color blindness with cone photoreceptor function loss (Pang JJ, unpublished results, 2008).
In the absence of RPE65 in the rd12 retina, no 11-cis retinal is generated, and hence only unliganded cone opsins are present in the untreated rd12 retina. It has been shown that in different models of chromophore deprivation (Rpe65−/− or Lrat−/− mice), the unliganded cone opsins could not be transported to the cone outer segment, but rather mislocalized to the inner segments, cell body, axon and pedicle of cones, indirectly causing shortening of the cone outer segments.10,35–37,39 Although both rod and cone photoreceptors depend on the RPE65 isomerase for function in LCA2 or its mouse models,7,9,41 cone structure and hence survival appears to be more susceptible to chromophore deprivation. Continuous activation of the phototransduction cascade by the opsin apoprotein (unliganded opsin) is a cause of rod degeneration in another mouse model of Leber congenital amaurosis42; however, it is unclear whether cone cell death in the rd12 mice is caused by the same mechanism.
Although it has been observed in different chromophore-deficient models,16,21,35,39 it is not clear why the central, ventral, and nasal quadrants exhibit faster cone degeneration than the dorsal and temporal quadrants of the retina. It is of interest to note that the ventral, nasal, and central quadrants harbor UV-cone opsin–sensitive cones, whereas the surviving cones are pure M-cones. Although there is evidence of an additional retinoid cycle in Müller cells in several different species, including the mouse,43 it is unclear how such an alternative pathway might support cone survival in the dorsal and temporal retina.40 In patients with RPE65 mutations, cone photoreceptors are lost in a wide area of the central retina during the first decade of life and, as in the mouse model, the defects are greater in the inferior than in the superior region. On the other hand, the patients' macular cone photoreceptors can survive for decades, although some age-related cone degeneration has been reported.41,44 Of interest, the remaining cones in the dorsal and temporal parts of old rd12 mice and the cones in the human macular area share similarities; that is, they are M- and M/L-cones in mouse and human, respectively, and they appear to occupy the most important part of the retina, the dorsal part, which is required for foraging in the mouse, and the macula, which offers high visual acuity in the human. Since only the most important area of cones remained both in older rd12 mice and in LCA2 patients, this phenomenon might also be a result of evolution.
In animal models of RPE65 deficiency, rods undergo a slow progressive degeneration,9,45,46 which is why restoration of rod vision appears to be possible over a broad range of ages.13,17,18,22,47 Cones, on the other hand, degenerate more quickly and at an earlier age.21 Previous reports have shown that cone rescue is possible if gene delivery is provided no later than P14 to Rpe65−/− and rd12 mice.16,17,33,48 Our current data confirmed and extended those findings. However, P14-treated rd12 eyes stained with M- or S-cone opsin (Fig. 2) revealed that only 76% and 68% of the expected cone population could be recovered, suggesting that degeneration may have been triggered before P14 in rd12 mice, especially in the S-cones, although subretinal injection-related damage also contributes to these incomplete rescues.17,26
The preservation or rescue of these remaining M-cones deserves further analysis. First, in our initial study (Pang JJ, unpublished results, 2006), we did not find cone rescue after P90 treatment in rd12 mice due to the technical difficulties associated with mouse subretinal injections and a poor understanding of the cone degeneration pattern in rd12 mice. In our present study, we selected only those treated rd12 mice that have almost 100% retinal detachment after subretinal injection, hence almost 100% of the RPE was transfected with the RPE65-carrying virus. Therefore, we rescued most of the remaining M-cones both morphologically and functionally after P90 treatment. Complete coverage was achieved by improving our transcornea subretinal injection technique, which allowed us to achieve 100% retinal detachment with minimal injection-related damage.17,22,26 As the earliest age for successful trans-corneal subretinal injection in mouse is the time of eye opening (P14),26 earlier treatments were not attempted.
Second, 2 months after P90 treatment, 83% of PNA-lectin-positive cones in the DT quadrant were preserved; whereas the average number of M-cone opsin–positive cones increased by 215%. In other words, M-cone opsin–positive cones made up only 16% of the PNA-lectin–positive population (13/82) at P90, but this fraction increased to 50% (41/82) after gene therapy. Since these cell counts were made in flat mounts visualized with a regular fluorescence microscope, in which mainly outer segments could be analyzed and not the entire cell, we cannot distinguish between the “ghosts” devoid of any cone opsin and the “ghosts” that still express cone opsin, but traffic it to the cell body, axon, and pedicle, rather than the inner and outer segment. Nevertheless, the dramatic increase in M-cone opsin in cone outer segments, a prerequisite for the assembly of normal cone function,10,36,39 is a proof of concept that outer segments can be rebuilt in the mouse. We thus, for the first time, provided evidence that the remaining abnormal M-cones in older rd12 mice can be restored to normal physiological conditions after late treatments at P90.
One of the most prominent features of LCA is the diminished ERG at an early age.2 Specific ERG techniques are valuable in evaluating the function of retinal cone cells. In this study, full cone, M- or S-cone, and flicker-ERGs were used to assess cone function in both treated and untreated rd12 eyes. Our results showed that no S-cone, but only M-cone ERG responses could be detected in untreated 5-month-old rd12 mice, similar to the responses observed in LCA2 patients41 (Fig. 3). These remaining ERG responses had significantly delayed kinetics, suggesting impaired synaptic transmission.49 Whether the mislocalized cone opsin contributes to this synaptic delay needs further investigation. However, that eliminating the mislocalization of the M-cone opsin in P90-treated rd12 retinas (Fig. 2) restored photopic cone and M-cone ERG responses with normal b-wave kinetics clearly indicates that no permanent damage was inflicted on the cone pedicles by the mislocalized cone opsin at this age.
Thus, we conclude that human AAV-mediated, early P14 gene therapy could rescue both function and morphology of M- and UV-cones in rd12 mice. Function of the remaining M-cones could also be rescued after late P90 treatment. Our results and the recent clinical trial24 suggest that there may be a longer window for intervention in human LCA2 patients.
Acknowledgments
The authors thank Luanna Bartholomew (MUSC) for critical review.
Footnotes
Supported in part by Grant 2009ZX09503 from the Major Projects of National Science and Technology of China; Grant 2007AA021004 from the Key Projects of National High-Tech R&D Program (863 Program) of China; retinal gene therapy study grants from the Eye Hospital, School of Ophthalmology and Optometry, Wenzhou Medical College, Wenzhou, China; and NIH Grants EY018331 (JP) and EY14793 (MUSC Vision Core). BR is a Research to Prevent Blindness Olga Keith Weiss Scholar.
Disclosure: X. Li, None; W. Li, None; X. Dai, None; F. Kong, None; Q. Zheng, None; X. Zhou, None; F. Lü, None; B. Chang, None; B. Rohrer, None; W.W. Hauswirth None; J. Qu, None; J. Pang, None
References
- 1. Cremers FP, van den Hurk JA, den Hollander AI. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11:1169–1176 [DOI] [PubMed] [Google Scholar]
- 2. den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419 [DOI] [PubMed] [Google Scholar]
- 3. Fazzi E, Signorini SG, Scelsa B, Bova SM, Lanzi G. Leber's congenital amaurosis: an update. Eur J Paediatr Neurol. 2003;7:13–22 [DOI] [PubMed] [Google Scholar]
- 4. Fazzi E, Signorini SG, Uggetti C, Bianchi PE, Lanners J, Lanzi G. Towards improved clinical characterization of Leber congenital amaurosis: neurological and systemic findings. Am J Med Genet A. 2005;132A:13–19 [DOI] [PubMed] [Google Scholar]
- 5. Koenekoop RK. An overview of Leber congenital amaurosis: a model to understand human retinal development. Surv Ophthalmol. 2004;49:379–398 [DOI] [PubMed] [Google Scholar]
- 6. Harris EW. Leber's congenital amaurosis and RPE65. Int Ophthalmol Clin. 2001;41:73–82 [DOI] [PubMed] [Google Scholar]
- 7. Marlhens F, Bareil C, Griffoin JM, et al. Mutations in RPE65 cause Leber's congenital amaurosis. Nat Genet. 1997;17:139–141 [DOI] [PubMed] [Google Scholar]
- 8. Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351 [DOI] [PubMed] [Google Scholar]
- 10. Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46:3876–3882 [DOI] [PubMed] [Google Scholar]
- 11. Jacobson SG, Aleman TS, Cideciyan AV, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci U S A. 2005;102:6177–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samardzija M, Tanimoto N, Kostic C, et al. In conditions of limited chromophore supply rods entrap 11-cis-retinal leading to loss of cone function and cell death. Human Molecular Genetics. 2009;18:1266–1275 [DOI] [PubMed] [Google Scholar]
- 13. Van Hooser JP, Aleman TS, He YG, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci USA. 2000;97:8623–8628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rohrer B, Ablonczy Z, Znoiko S, Redmond M, Ma JX, Crouch R. Does constitutive phosphorylation protect against photoreceptor degeneration in Rpe65−/− mice? Adv Exp Med Biol. 2003;533:221–227 [DOI] [PubMed] [Google Scholar]
- 15. Gouras P, Kong J, Tsang SH. Retinal degeneration and RPE transplantation in Rpe65(−/−) mice. Invest Ophthalmol Vis Sci. 2002;43:3307–3311 [PubMed] [Google Scholar]
- 16. Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest Ophthalmol Vis Sci. 2006;47:1177–1184 [DOI] [PubMed] [Google Scholar]
- 17. Pang JJ, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13:565–572 [DOI] [PubMed] [Google Scholar]
- 18. Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95 [DOI] [PubMed] [Google Scholar]
- 19. Seeliger MW, Grimm C, Stahlberg F, et al. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet. 2001;29:70–74 [DOI] [PubMed] [Google Scholar]
- 20. Pang JJ, Boye SE, Lei B, et al. Self-complementary AAV-mediated gene therapy restores cone function and prevents cone degeneration in two models of Rpe65 deficiency. Gene Ther. 2010;17:815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci. 2005;46:1473–1479 [DOI] [PubMed] [Google Scholar]
- 22. Li W, Kong F, Li X, et al. Gene therapy following subretinal AAV5 vector delivery is not affected by a previous intravitreal AAV5 vector administration in the partner eye. Mol Vis. 2009;15:267–275 [PMC free article] [PubMed] [Google Scholar]
- 23. Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 24. Cideciyan AV, Hauswirth WW, Aleman TS, et al. Vision 1 year after gene therapy for Leber's congenital amaurosis. N Engl J Med. 2009;361:725–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pang JJ, Boye SL, Kumar A, et al. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest Ophthalmol Vis Sci. 2008;49:4278–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118 [DOI] [PubMed] [Google Scholar]
- 28. Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N. Production and purification of recombinant adeno-associated virus. Methods Enzymol. 2000;316:743–761 [DOI] [PubMed] [Google Scholar]
- 29. Pang J, Cheng M, Haire SE, Barker E, Planelles V, Blanks JC. Efficiency of lentiviral transduction during development in normal and rd mice. Mol Vis. 2006;12:756–767 [PubMed] [Google Scholar]
- 30. Pang JJ, Lauramore A, Deng WT, et al. Comparative analysis of in vivo and in vitro AAV vector transduction in the neonatal mouse retina: effects of serotype and site of administration. Vision Res. 2008;48:377–385 [DOI] [PubMed] [Google Scholar]
- 31. Jacobs GH, Neitz J, Deegan JF., 2nd Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature. 1991;353:655–656 [DOI] [PubMed] [Google Scholar]
- 32. Szel A, Rohlich P, Mieziewska K, Aguirre G, van Veen T. Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. J Comp Neurol. 1993;331:564–577 [DOI] [PubMed] [Google Scholar]
- 33. Bemelmans AP, Kostic C, Crippa SV, et al. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3:e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson LV, Hageman GS, Blanks JC. Interphotoreceptor matrix domains ensheath vertebrate cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1986;27:129–135 [PubMed] [Google Scholar]
- 35. Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK. Rpe65−/− and Lrat−/− mice: comparable models of Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2008;49:2384–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kunchithapautham K, Coughlin B, Crouch RK, Rohrer B. Cone outer segment morphology and cone function in the Rpe65−/− Nrl−/− mouse retina are amenable to retinoid replacement. Invest Ophthalmol Vis Sci. 2009;50:4858–4864 [DOI] [PubMed] [Google Scholar]
- 37. Feathers KL, Lyubarsky AL, Khan NW, et al. Nrl-knockout mice deficient in Rpe65 fail to synthesize 11-cis retinal and cone outer segments. Invest Ophthalmol Vis Sci. 2008;49:1126–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Applebury ML, Antoch MP, Baxter LC, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523 [DOI] [PubMed] [Google Scholar]
- 39. Zhang H, Fan J, Li S, et al. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28:4008–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia-Fernandez JM, Jimenez AJ, Foster RG. The persistence of cone photoreceptors within the dorsal retina of aged retinally degenerate mice (rd/rd): implications for circadian organization. Neurosci Lett. 1995;187:33–36 [DOI] [PubMed] [Google Scholar]
- 41. Jacobson SG, Aleman TS, Cideciyan AV, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci U S A. 2007;104:15123–15128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35:158–164 [DOI] [PubMed] [Google Scholar]
- 43. Muniz A, Betts BS, Trevino AR, et al. Evidence for two retinoid cycles in the cone-dominated chicken eye. Biochemistry. 2009;48:6854–6863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacobson SG, Aleman TS, Cideciyan AV, et al. Defining the residual vision in leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2009;50:2368–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Samardzija M, von Lintig J, Tanimoto N, et al. R91W mutation in Rpe65 leads to milder early-onset retinal dystrophy due to the generation of low levels of 11-cis-retinal. Hum Mol Genet. 2008;17:281–292 [DOI] [PubMed] [Google Scholar]
- 46. Pang JJ, Chang B, Hawes NL, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Mol Vis. 2005;11:152–162 [PubMed] [Google Scholar]
- 47. Rohrer B, Goletz P, Znoiko S, et al. Correlation of regenerable opsin with rod ERG signal in Rpe65−/− mice during development and aging. Invest Ophthalmol Vis Sci. 2003;44:310–315 [DOI] [PubMed] [Google Scholar]
- 48. Dejneka NS, Surace EM, Aleman TS, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9:182–188 [DOI] [PubMed] [Google Scholar]
- 49. Rohrer B. Gene dosage effect of the TrkB receptor on rod physiology and biochemistry in juvenile mouse retina. Mol Vis. 2001;7:288–296 [PubMed] [Google Scholar]