Abstract
Objective:
Histologic studies show that the amygdala is affected by Alzheimer disease (AD) pathology, and its medial aspect is the most involved. We aimed to assess in vivo local structural differences in the amygdala of patients with AD using high-field MRI.
Methods:
A total of 19 patients with AD (mean age 76, SD 6 years, mean Mini-Mental State Examination score [MMSE] 13, SD 4) and 19 healthy elderly controls (age 74, SD 5, MMSE 29, SD 1) were enrolled. The radial atrophy mapping technique was used to reconstruct the 3-dimensional surface of the amygdala. Maps of surface tissue loss in patients with AD vs controls were computed and statistically tested with permutation tests thresholded at p < 0.05, to correct for multiple comparisons. A digital atlas of the amygdalar nuclei was used to infer which nuclei were involved.
Results:
Both amygdalar volumes were significantly smaller in patients with AD (right 1,508 mm3, SD 418; left 1,646, SD 419) than controls (right 2,129 mm3, SD 316; left 2,077, SD 376; p < 0.002). In the dorsomedial part, significant local tissue loss (20%–30%) was mapped in the medial and central nuclei. Ventrally, the lateral nucleus (La) and the basolateral ventral medial nucleus (BLVM) were also involved (20%–30% loss).
Conclusions:
We found in vivo local structural differences in the amygdala of patients with AD. The nuclei involved have known connections to the hippocampus (BLVM, La) and olfactory system (medial nucleus) and with cholinergic pathways (central nucleus). This pattern is consistent with the known pathophysiology of neural systems affected by AD.
Declarative memory is the main cognitive function affected by Alzheimer disease (AD). While the role of the hippocampus and hippocampal pathology in AD are widely known, the role of the amygdala is much less studied. The amygdala plays a key role in enhancing explicit memory both for pleasant and unpleasant emotional stimuli, by modulating of the encoding and consolidation processes.1 It also has abundant neural connections with the hippocampus.2 The amygdala is also among the brain structures where tau deposition occurs in the earliest stages of Alzheimer pathology,3,4 with more marked tau and Aβ deposition in the medial than lateral nuclei.5
Amygdalar volumetric studies using MRI techniques repeatedly find differences in patients with AD relative to healthy controls.6–8 However, none has focused on mapping local structural changes in the amygdala, although the amygdala is known to be made of a heterogeneous group of nuclei. These nuclei have inputs from and outputs to different brain regions subserving a number of specific functions.
As so few studies focus on the involvement of amygdalar nuclei in AD, in vivo studies on local structural differences may help to elucidate their contribution to the symptoms of the disease.
The objective of this study was to assess in vivo the local structural differences of the amygdala in patients with AD compared to controls using the radial atrophy mapping technique on high-field (3 T) MRI.
METHODS
Study subjects and assessment.
Subjects were 19 patients with moderate to severe probable AD, diagnosed according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria,9 and 19 elderly healthy volunteers. Patients and controls were recruited in the context of a pharmacologic fMRI study of memantine.10,11 Patients were selected from those seen at the IRCCS Centro S. Giovanni di Dio Fatebenefratelli, in Brescia, Italy. Patients with Clinical Dementia Rating of 2 or greater were included. Exclusion criteria were as follows: history of TIA or stroke, head trauma, alcohol or substance abuse, corticosteroid therapy, recent weight loss, or a modified Hachinski ischemic scale score greater than 4.12 Standardized history taking, behavioral and functional assessment, and physical and neurologic examination were carried out for all participants. The original case report form of the clinical assessment is available at http://www.centroalzheimer.it/public/Protocollo_MEM_T0.doc (in Italian). Moreover, a comprehensive neuropsychological battery appropriate for patients' cognitive impairment severity was administered with cognitive tests for nonverbal reasoning (Raven Colored Progressive Matrices), language comprehension (Token Test), verbal fluency (Letter and Semantic Fluency), short- and long-term memory (Digit and Spatial Span; Story Recall; Rey-Osterrieth Complex Figure Recall), constructional abilities (Rey-Osterrieth Complex Figure Copy), attention, and executive functions (Trail Making Test).13 Global cognitive function was assessed with the Mini-Mental State Examination (MMSE).14
Standard protocol approvals, registration, and patient consents.
The participant or his or her primary caregiver provided written informed consent, after discussion of the risks and benefits of participating. No compensation was provided. The study was approved by the local ethics committee.
MRI acquisition.
Three-dimensional high-resolution T1-weighted MRI scans were acquired on a 3.0-T Siemens Allegra scanner at the Neuroradiology Unit of the Ospedale Maggiore Borgo Trento, Verona, Italy, with a standard head coil. Scans were acquired with a gradient echo 3-dimensional technique with the following acquisition protocol: repetition time = 2,300 msec, echo time = 3.93 msec, inversion time = 1,100 msec, flip angle = 12°, gap = 50%, voxel = 1 × 1 × 1 mm, acquisition matrix = 256 × 256, slice thickness = 1 mm, total number of slices = 160, acquisition time 8′37″.
Image processing.
Images were reoriented along the AC-PC line, all voxels below the cerebellum were removed with MRIcro (http://www.sph.sc.edu/comd/rorden/mricro.html), and the origin of the spatial coordinates was manually set to the anterior commissure. Images were normalized with the Statistical Parametric Mapping (SPM2) software (www.fil.ion.ucl.ac.uk/spm) to a customized template created from the scans of all patients and all controls with a linear (12 parameter) transformation to preserve local shape differences in anatomy across subjects, so that they could be quantified in standardized space.
The amygdalae were manually segmented on the reoriented and normalized images by a single tracer (E.C.), blind to diagnosis and blind to the aim of the study, on contiguous coronal 1.0-mm-thick sections following a standardized and validated protocol15 and using an interactive software program developed at the Laboratory of NeuroImaging of the University of California at Los Angeles (http://www.loni.ucla.edu/ICBM/ICBM_ResSoftware.html#seg3).
The amygdala is located in the superior medial part of the temporal lobe, partly superior and anterior to the hippocampus. In the coronal plane, the starting point for amygdala segmentation was at the level where it is separated from the entorhinal cortex by the intrarhinal sulcus, or tentorial indentation, which forms a marked indent at the site of the inferior border of the amygdala. The amygdalostriatal transition area located between the lateral amygdalar nucleus and the ventral putamen was taken as the posterior-lateral border. The posterior end of the amygdala was defined as the point where gray matter started to appear superior to the alveus and laterally to the hippocampus. If the alveus was not visible, the inferior horn of the lateral ventricle was used as a border.15 Normalized amygdalar volumes were obtained from the tracings on spatially normalized images, and retained for statistical analyses. Intraclass correlation coefficients for the amygdala were 0.87 for intrarater and 0.83 for interrater reliability.
Radial atrophy mapping.
Three-dimensional parametric surface mesh models were generated from the manually segmented amygdala tracings.16 Each amygdala contour was separated into dorsal and ventral components and reparametrized by normalizing the spatial frequency of surface points between and within slices. A medial curve was automatically defined as the 3-dimensional curve traced out by the centroid of the amygdalar boundary in each image slice. For each boundary point, the radial 3-dimensional distance from the surface points to the medial curve of the amygdala was measured automatically and defined as the radial size. Shorter radial distances were used as an index of amygdala contraction.16 This procedure allows us to perform statistical comparisons between groups at corresponding surface locations.
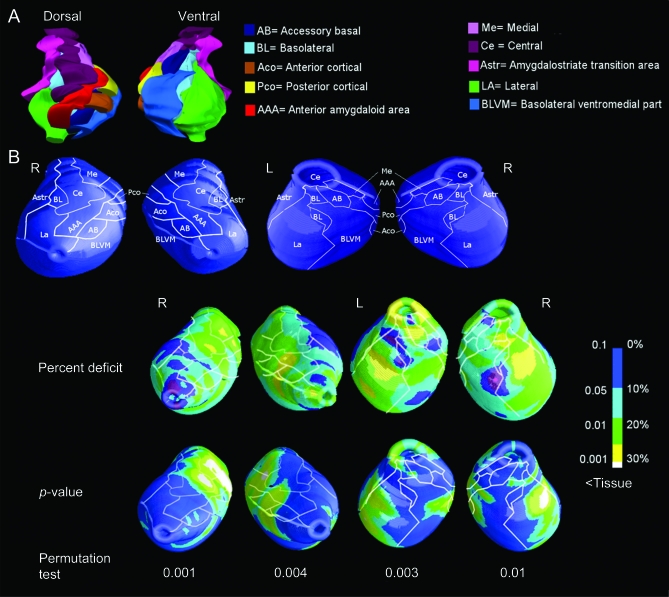
The reconstruction of the anatomic amygdalar nuclei was based on the localization of the right amygdalar nuclei in histologic sections from a human atlas17 (figure 1A). The contour of each nucleus was outlined in a digital 3-dimensional template and their boundaries were projected onto the surface of the 3-dimensional mesh model obtained with the radial atrophy mapping technique (figure 1B).
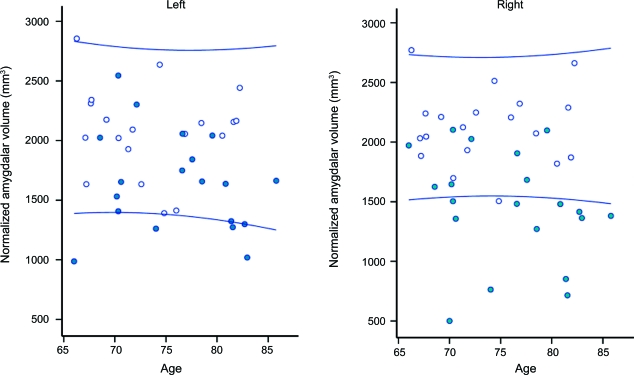
Figure 1. Effect of aging and Alzheimer disease (AD) on total amygdalar volumes.
The figure shows amygdalar volume distribution in 19 patients with AD (closed circles) and 19 older healthy controls (open circles). The lines denote 95% confidence bounds of the control distribution.
Statistical analysis.
The group of patients was compared to the controls. The percent difference in mean radial distances between patient and control groups (and the associated p value describing the significance of group differences) were plotted onto the model surface at each point of the amygdala using a color code to produce statistical maps.
Corrected p values were computed for the overall maps of the left and right amygdala using a permutation testing approach. Permutation methods measure the distribution of features in statistical maps that would be observed by accident if the subjects were randomly assigned to groups.16 The proportion of the amygdala surface with p values lower than 0.05 was stored and compared to the distribution of this suprathreshold area that arose by chance when the diagnostic group labeling of the data was repeatedly scrambled. By comparing the suprathreshold area to that seen by chance in null data, this provides an appropriate overall p value for the observed effects that is corrected for multiple spatial comparisons. Due to the significant difference in education level between cases and controls, Pearson correlation analysis between amygdalar morphology and educational level was performed in the control group in order to evaluate whether any effect of education on amygdalar morphology interfered with the effect of diagnosis.
Differences in sociodemographic, clinical, and neuropsychological features between groups were assessed using an analysis of variance for continuous variables and Fisher exact test for dichotomous variables. Amygdalar volumetric differences between groups were assessed by a one-way analysis of covariance, using sex and education as covariates in the model. The sensitivity of amygdalar volume to detect AD cases was computed based on a fixed specificity of 95%. All statistical analyses were performed using SPSS software version 12.0.
RESULTS
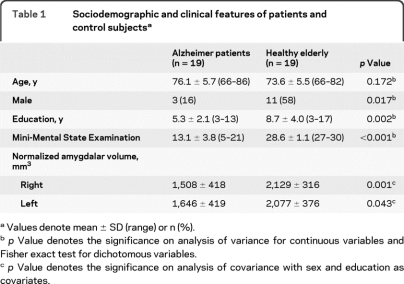
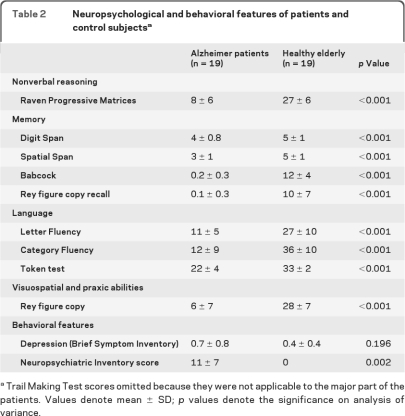
All patients were under AChEI treatment (donepezil, rivastigmine, or galantamine) and one out of the 19 patients was also treated with antidepressants (trazodone). None of the controls was taking any of those medications. Normalized amygdalar volumes displayed 21% (left) and 29% (right) lower volumes in patients with AD compared to healthy elderly controls (table 1). We found significant differences between groups in all neuropsychological domains. No difference was found in depressive symptoms (table 2).
Table 1.
Sociodemographic and clinical features of patients and control subjectsa
Values denote mean ± SD (range) or n (%).
p Value denotes the significance on analysis of variance for continuous variables and Fisher exact test for dichotomous variables.
p Value denotes the significance on analysis of covariance with sex and education as covariates.
Table 2.
Neuropsychological and behavioral features of patients and control subjectsa
Trail Making Test scores omitted because they were not applicable to the major part of the patients. Values denote mean ± SD; p values denote the significance on analysis of variance.
Figure 1 shows that at any age, amygdalar volumes tended to be lower in patients with AD than in controls. Separation was better to the right, where 12/19 patients with AD were below the 95% confidence limits of the control distribution (sensitivity and specificity of 63% and 95%), while overlap was more substantial for the left amygdala (sensitivity and specificity of 36% and 95%).
The surface-based modeling analysis showed bilateral tissue loss with a major involvement of the right amygdala, consistent with the volumetric findings. Tissue reduction was mapped mainly to the medial amygdalar aspect and the lateral nucleus (La) (figure 2). Dorsally, the medial nucleus (Me), which extends from the posterior-dorsal medial part to the anterior-dorsal medial part of the amygdala, showed 20% to 30% tissue loss, bilaterally. The central nucleus (Ce) displayed 20%–30% tissue loss mainly to the right. The anterior and posterior cortical nuclei and anterior amygdaloid area were also involved, predominantly to the right, with around 20% tissue loss (p < 0.01).
Figure 2. Local atrophy in patients with Alzheimer disease (AD).
(A) Three-dimensional reconstruction of the right human amygdala (left: dorsal view; right: ventral view) divided into its subnuclei, as traced out from the atlas.17 (B) Contour outline of the surface nuclei on the dorsal right and left and on the ventral right and left amygdalar surface mesh model from our sample. At the bottom of the figure, pattern of percent tissue loss and statistical significance of the amygdala in patients with AD vs healthy elderly subjects.
Ventrally, the La showed 20%–30% tissue loss bilaterally, but mainly in the right amygdala, and the basolateral ventromedial nucleus (BLVM) was also involved, showing a 20% tissue loss (p < 0.01). The permutation test was highly significant (figure 2, p < 0.01), indicating that the pattern of deficits in the maps was very unlikely to have occurred by chance. Educational level was not associated with amygdalar morphology in healthy elderly and AD (p > 0.05 on permutation test).
DISCUSSION
This study explored the pattern of amygdalar atrophy in AD. Comparing the volume of the amygdala between groups, using sex and education as covariates, we found that this structure shows significant atrophy in patients with AD. Global atrophy of 25%, observed in our patients with AD, falls within the range of alterations detected previously from in vivo MRI6,18 and from postmortem neuropathologic studies,19,20 where 14%–60% lower amygdalar volume was found. This proportion of atrophy is somewhat lower than that of other regions involved in AD, such as the hippocampus and entorhinal cortex, which showed a range of atrophy from 30% to 40%.10,21
Pathologic studies of Alzheimer brains have showed remarkable deposition of amyloid plaques and neurofibrillary tangles in the amygdala3 with a regional selective distribution in the medial part, both dorsally (Me, Ce, cortical and anterior amygdaloid area) and ventrally (BLVM).4 Previous studies have also showed Lewy bodies in the amygdala of AD22,23 without a clear pattern of deposition in specific nuclei. Our results are in agreement with neuropathologic studies, but we also found significant atrophy in the right lateral nucleus. Only one other in vivo study has investigated the regional shape abnormalities in the amygdala of patients with AD24 showing local surface inward deformation in the basolateral complex area, consistent with our findings.
In our sample, the Me and to a lesser extent the anterior cortical (Aco) and posterior cortical (Pco) nuclei were significantly atrophic. Neuropathologic studies in patients with AD show a different pattern: the medial nucleus is much less affected than the cortical nuclei.25 These nuclei project and receive efferents from the olfactory bulb (Aco), olfactory cortex (Me), and accessory olfactory bulb (Pco).26,27 Therefore, seeing as hyposmia is a sign of AD,28 we believe that the structural changes found in Me, Aco, and Pco may relate to the involvement of this olfactory network.
The central nucleus, affected by significant tau deposition,29 is an important output region27,30 with key connections to different modulatory circuits, including the lateral division of the capsular cholinergic pathway.31 This finding reflects the known deficit in acetylcholine pathways that characterizes AD.
Ventrally, the La and BLVM nuclei were primarily involved. The La nucleus is relatively spared from tau deposition in AD, while the BLVM nucleus is more affected.4 The La nucleus emits widespread intra-amygdalar connections, including projections to the basal and central nuclei.27,32,33 Moreover, the La and the BLVM are the main amygdalar nuclei that project and receive information respectively from entorhinal cortex34 and hippocampal regions (CA1–CA3 fields, subiculum, and parasubiculum),35 known to be atrophic in patients with AD.36,37 Each of the outputs from the lateral or basolateral-ventral-medial nuclei provides a slightly different representation of the sensory stimulus to the hippocampal formation.4 Therefore, the atrophy in the La and the BLVM nuclei can explain memory deficits in the encoding of stimuli (pleasant and unpleasant) during learning of explicit memories in patients with AD.38
The amygdalar atrophy has been previously found in other neurodegenerative diseases such as frontotemporal dementia, dementia with Lewy bodies, and vascular dementia.8,39 Future studies comparing the amygdalar atrophy in different kinds of dementias are needed. This will help to understand if the amygdalar pattern of damage may distinguish not only AD from controls but also AD from other dementias.
Some caveats should be noted. First, the sample is small and a replication of the findings in a larger group is needed. A larger sample size will help to better understand whether this measure may consistently distinguish controls from patients with AD. Second, diagnosis of our patients with AD was made clinically and lacked neuropathologic confirmation. Because of low specificity of the NINCDS-ADRDA criteria for AD, we could not exclude co-occurrence of neurodegenerative conditions other than AD.
Third, our mapping of the local tissue differences to neuroanatomic regions is indirect, based on 3-dimensional reconstruction of the amygdala, suggesting that our inferences about the atrophy of specific nuclei may be insufficiently accurate and need at a minimum to be confirmed in independent studies. So far, no in vivo imaging technique has been able to directly segment the amygdalar nuclei, due to their complex organization and relatively small size. Finally, the 3-dimensional atlas that we used is derived from a single healthy subject,17 which therefore does not take into account the normal or pathologic intersubject morphologic variability.
Footnotes
- Aco
- anterior cortical
- AD
- Alzheimer disease
- BLVM
- basolateral ventral medial nucleus
- Ce
- central nucleus
- La
- lateral nucleus
- Me
- medial nucleus
- MMSE
- Mini-Mental State Examination score
- NINCDS-ADRDA
- National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association
- Pco
- posterior cortical
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Enrica Cavedo.
DISCLOSURE
E. Cavedo, Dr. Boccardi, R. Ganzola, E. Canu, and Dr. Beltramello report no disclosures. Dr. Caltagirone has served on scientific advisory boards for Wyeth and Novartis and has received research support from the Italian Ministry of Health RF97. Dr. Thompson serves on editorial advisory boards for IEEE Transactions on Medical Imaging, Human Brain Mapping, Medical Image Analysis, Cerebral Cortex, Current Medical Imaging Reviews, Inverse Problems and Imaging, and Translational Neuroscience; and receives research support from the NIH. Dr. Frisoni reports no disclosures.
REFERENCES
- 1. Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci 2001;5:394–400 [DOI] [PubMed] [Google Scholar]
- 2. Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 2004;14:198–202 [DOI] [PubMed] [Google Scholar]
- 3. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259 [DOI] [PubMed] [Google Scholar]
- 4. Whalen PJ, Phelps EA. The Human Amygdala. New York: The Guilford Press; 2009 [Google Scholar]
- 5. Hirano A, Zimmerman HM. Alzheimer's neurofibrillary changes: a topographic study. Arch Neurol 1962;7:227–242 [DOI] [PubMed] [Google Scholar]
- 6. Jack CR, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology 1997;49:786–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basso M, Yang J, Warren L, et al. Volumetry of amygdala and hippocampus and memory performance in Alzheimer's disease. Psychiatry Res 2006;146:251–261 [DOI] [PubMed] [Google Scholar]
- 8. Horínek D, Varjassyová A, Hort J. Magnetic resonance analysis of amygdalar volume in Alzheimer's disease. Curr Opin Psychiatry 2007;20:273–277 [DOI] [PubMed] [Google Scholar]
- 9. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 10. Frisoni GB, Ganzola R, Canu E, et al. Mapping local hippocampal changes in Alzheimer's disease and normal ageing with MRI at 3 Tesla. Brain 2008;131:3266–3276 [DOI] [PubMed] [Google Scholar]
- 11. Lorenzi M, Beltramello A, Zoccatelli G, et al. Effect of memantine on the activity of the default mode network: a resting fMRI study. Alzheimer's & Dementia 2009;5 (suppl 1): Abst P1–P108 [Google Scholar]
- 12. Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 1980;7:486–488 [DOI] [PubMed] [Google Scholar]
- 13. Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment, 4th ed. New York, NY: Oxford University Press; 2004 [Google Scholar]
- 14. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 15. Pruessner JC, Li LM, Serles W, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex 2000;10:433–442 [DOI] [PubMed] [Google Scholar]
- 16. Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage 2004;22:1754–1766 [DOI] [PubMed] [Google Scholar]
- 17. Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. Academic Press: San Diego; 1997 [Google Scholar]
- 18. Cuénod CA, Denys A, Michot JL, et al. Amygdala atrophy in Alzheimer's disease: an in vivo magnetic resonance imaging study. Arch Neurol 1993;50:941–945 [DOI] [PubMed] [Google Scholar]
- 19. Herzog AG, Kemper TL. Amygdaloid changes in aging and dementia. Arch Neurol 1980;37:625–629 [DOI] [PubMed] [Google Scholar]
- 20. Scott SA, Dekosky ST, Scheff SW. Volumetric atrophy of the amygdala in Alzheimer's disease: quantitative serial reconstruction. Neurology 1991;41:351–356 [DOI] [PubMed] [Google Scholar]
- 21. Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry 2001;71:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lippa CF, Fujiwara H, Mann DM, et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer's disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 1998;153:1365–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 2000;10:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiu A, Fennema-Notestine C, Dale AM, Miller MI. Alzheimer's Disease Neuroimaging Initiative: regional shape abnormalities in mild cognitive impairment and Alzheimer's disease. Neuroimage 2009;45:656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kromer Vogt LJ, Hyman BT, Van Hoesen GW, Damasio AR. Pathological alterations in the amygdala in Alzheimer's disease. Neuroscience 1990;37:377–385 [DOI] [PubMed] [Google Scholar]
- 26. Price JL. Comparative aspects of amygdala connectivity. Ann NY Acad Sci 2003;985:50–58 [DOI] [PubMed] [Google Scholar]
- 27. LeDoux J. The amygdala. Curr Biol 2007;17:R868–R874 [DOI] [PubMed] [Google Scholar]
- 28. Serby M. Olfactory deficits in Alzheimer's disease. J Neural Transm Suppl 1987;24:69–77 [PubMed] [Google Scholar]
- 29. Sahin HA, Emre M, Ziabreva I, Perry E, Celasun B, Perry R. The distribution pattern of pathology and cholinergic deficits in amygdaloid complex in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol 2006;111:115–125 [DOI] [PubMed] [Google Scholar]
- 30. Aggleton JP. The Amygdala: A Functional Analysis, 2nd ed. New York: Oxford University Press; 2000: 425–441 [Google Scholar]
- 31. Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 1998;121:2249–2257 [DOI] [PubMed] [Google Scholar]
- 32. Pitkänen A, Amaral DG. Demonstration of projections from the lateral nucleus to the basal nucleus of the amygdala: a PHA-L study in the monkey. Exp Brain Res 1991;83:465–470 [DOI] [PubMed] [Google Scholar]
- 33. Stefanacci L, Farb CR, Pitkänen A, Go G, LeDoux JE, Amaral DG. Projections from the lateral nucleus to the basal nucleus of the amygdala: a light and electron microscopic PHA-L study in the rat. J Comp Neurol 1992;323:586–601 [DOI] [PubMed] [Google Scholar]
- 34. Pitkänen A, Kelly JL, Amaral DG. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus 2002;12:186–205 [DOI] [PubMed] [Google Scholar]
- 35. Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol 1999;403:229–260 [PubMed] [Google Scholar]
- 36. Frisoni GB, Sabattoli F, Lee AD, Dutton RA, Toga AW, Thompson PM. In vivo neuropathology of the hippocampal formation in AD: a radial mapping MR-based study. Neuroimage 2006;32:104–110 [DOI] [PubMed] [Google Scholar]
- 37. DeToledo-Morrell L, Stoub TR, Wang C. Hippocampal atrophy and disconnection in incipient and mild Alzheimer's disease. Prog Brain Res 2007;163:741–753 [DOI] [PubMed] [Google Scholar]
- 38. Schultz RR, de Castro CC, Bertolucci PH. Memory with emotional content, brain amygdala and Alzheimer's disease. Acta Neurol Scand 2009;120:101–110 [DOI] [PubMed] [Google Scholar]
- 39. Barber R, Ballard C, McKeith IG, Gholkar A, O'Brien JT. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology 2000;54:1304–1309 [DOI] [PubMed] [Google Scholar]