Abstract
Background:
Retinal vessels provide a unique opportunity to study both systemic and cerebrovascular disease. Smaller retinal arteriolar calibers are strongly related to hypertension, whereas larger retinal venular calibers are more related to inflammation, cerebral hypoperfusion, and cerebrovascular disease. Whether retinal vessel calibers are related to dementia remains unclear.
Methods:
We investigated whether retinal arteriolar and venular calibers are associated with risk of dementia, and its subtypes Alzheimer disease (AD) and vascular dementia, in the prospective population-based Rotterdam Study. Digitized retinal images were available in 5,553 participants aged 55 years or over and dementia-free at baseline (1990–1993). Participants were re-examined in 1993–1994, 1997–1999, and 2002–2004 and were continuously monitored for development of dementia.
Results:
During a mean follow-up of 11.6 years, 655 participants developed dementia. AD was diagnosed in 519 and vascular dementia in 73 participants. Larger venular calibers were associated with an increased risk of dementia, in particular vascular dementia (age- and sex-adjusted hazard ratio per SD increase: 1.31; 95% confidence interval 1.06–1.64), but not AD. The association remained significant after adjustment for stroke and cardiovascular risk factors. Smaller arteriolar calibers were also associated with an increased risk of vascular dementia, yet only when adjusted for venular calibers.
Conclusions:
Retinal venular widening is associated with an increased risk of vascular dementia. Our findings are in line with previous observations in stroke and cerebral small-vessel disease and suggest that the association between larger retinal venular calibers and dementia may reflect cerebral hypoperfusion and subsequent ischemia.
Dementia is a leading cause of morbidity in the elderly, yet the exact causes remain unclear and treatment options are limited. Cerebrovascular disease is thought to play a role in the pathogenesis of dementia and its major subtypes Alzheimer disease (AD) and vascular dementia.1 The cerebral microcirculation is, however, difficult to assess and most noninvasive indicators of vascular pathology relate to vessel beds outside the brain. Retinal vessels provide a unique insight into the brain's microvasculature, because embryologic, anatomic, and physiologic characteristics are similar to the cerebral circulation and the retina is easy to visualize noninvasively.2,3 Moreover, pathologic changes in the retinal microcirculation have been shown in patients with cerebrovascular disease, suggesting that retinal vessels may reflect concomitant cerebral microangiopathy.4,5
During the late 1990s, a semiautomated system became available to reliably quantify retinal arteriolar and venular calibers.6 Several studies have shown that smaller arteriolar calibers were strongly related to higher blood pressure,7–9 whereas larger venular calibers were consistently associated with higher levels of inflammation markers, cholesterol, and both subclinical and clinical atherosclerosis.7,8,10–12 Furthermore, larger venular calibers were associated with an increased risk of stroke and progression of cerebral small-vessel disease.13–17 We studied the associations between retinal arteriolar and venular calibers, and risk of dementia and its major subtypes AD and vascular dementia, using data from a population-based cohort study.
METHODS
Study population.
The study was conducted as part of the Rotterdam Study, a large population-based prospective cohort study among all inhabitants aged 55 years and over of Ommoord, a district of Rotterdam, the Netherlands.18 Of 10,274 eligible subjects, 7,983 (78%) participated in the baseline examinations between 1990 and 1993. Since eye examinations became operational a few months after the baseline examinations had started, a smaller number (n = 6,780) participated in the ophthalmic part of the study. Due to technical reasons (mostly absence of technicians), fundus transparencies were not available for 344 participants. Fundus transparencies were available in 6,436 participants, and of these, 6,432 participants were screened for dementia, of whom 213 were diagnosed with dementia at baseline. Fundus transparencies were ungradable in 666 of the 6,219 participants who were free from dementia and underwent the eye examination at baseline. The cohort at risk of dementia with gradable retinal vessel measurements at baseline thus comprised 5,553 participants. Follow-up examinations were conducted in 1993–1994, 1997–1999, and 2002–2004. In addition, through linkage with records of general practitioners, the total cohort was continuously monitored for morbidity and mortality. Follow-up for dementia was virtually complete until January 1, 2007.
Standard protocol approvals, registrations, and patients consents.
The medical ethics committee at Erasmus University of Rotterdam approved the study and written informed consent was obtained from all participants.
Dementia diagnoses.
Participants were screened for dementia with a 3-step procedure, which was similar at baseline and follow-up examinations.19 First, participants were cognitively screened with the Mini-Mental State Examination (MMSE)20 and the Geriatric Mental State schedule (GMS)21 organic level. Second, if participants scored below 26 on the MMSE or above 0 on the GMS organic level, the Cambridge Examination of Mental Disorders in the Elderly22 was administered, and an informant was interviewed. Finally, participants suspected of having dementia were further examined by a neurologist, a neuropsychologist, and, if possible, had MRI of the brain. In addition, continuous monitoring of the cohort for incident dementia cases took place through direct linkage between the study database and computerized medical records from general practitioners and through surveillance of Regional Institute for Outpatient Mental Health Care reports. The diagnosis of dementia and subtype of dementia was made in accordance with internationally accepted criteria for dementia (DSM-III-R)23, AD (National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association),24 and vascular dementia (National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche en l'Enseignement en Neurosciences criteria).25 As proposed in the latter criteria, we recognized a subgroup of AD with cerebrovascular disease. Diagnoses were made on all available information by an expert panel including the neurologist, neuropsychologist, and research physician.
Grading of retinal vascular calibers.
At the baseline ophthalmic examination, fundus color transparencies were taken centered on the optic disk (20° field, Topcon Optical Company, Tokyo, Japan) after pharmacologic mydriasis and were digitized with a high-resolution scanner (Nikon LS-4000, Nikon Corporation, Japan). For each participant, the digitized image with the best quality of either eye was analyzed with the Retinal Vessel Measurement System (Retinal Analysis, Optimate, WI; Department of Ophthalmology & Visual Science, University of Wisconsin-Madison).6
The rationale and procedures to measure and summarize retinal vascular calibers have been described.6,7 Summary measures for arteriolar and venular calibers were based on improved Parr-Hubbard formulas and were corrected for magnification changes due to refractive errors of the eye. Four trained graders performed the assessments, masked to the clinical characteristics of the participants. A random subsample of 40 transparencies was used to monitor quality of the data at regular intervals. Pearson correlation coefficients for intergrader agreement were 0.67–0.80 (arteriolar calibers) and 0.91–0.94 (venular calibers). For intragrader agreement these figures were 0.69–0.88 (arteriolar calibers) and 0.90–0.95 (venular calibers).
Other variables.
Smoking habits (categorized as current, former, and never smoking) and use of antihypertensive medication were assessed during the baseline interview. Blood pressure was measured twice with a random zero sphygmomanometer at the brachial artery with the subject in sitting position, and the measurements were averaged. Nonfasting serum total cholesterol concentrations were determined by an automated enzymatic procedure. Serum levels of high-sensitive C-reactive protein (CRP) were determined by the Rate Near Infrared Particle Immunoassay method (Immage® high-sensitive CRP, Beckman Coulter). Diabetes mellitus was considered present if participants reported use of antidiabetic medication or when the random or postload serum glucose level was greater than 11.1 mmol/L. History of stroke at baseline was assessed during the baseline interview and verified by reviewing medical records. After enrollment, participants were continuously monitored for incident stroke through automated linkage of the study database with files from general practitioners and the municipality. Additional information was obtained from hospital records. Coronary heart disease was defined as a previous myocardial infarction, percutaneous transluminal coronary angioplasty, or coronary bypass. APOE genotype was assessed on coded DNA samples using PCR without knowledge of the dementia diagnosis.26 APOE ϵ4 carriership was defined as the presence of at least one APOE ϵ4 allele.
Statistical analysis.
Analysis of covariance, adjusted for age and sex, was used to compare baseline characteristics of participants with and without gradable fundus transparencies. Associations between baseline retinal vascular calibers and incident dementia, AD (with or without cerebrovascular disease), and vascular dementia were assessed with Cox proportional hazards models. Participants were followed until diagnosis of dementia, death, or end of study, whichever came first. Hazard ratios (HR) were adjusted for age and sex. Retinal arteriolar and venular calibers were first entered in quintiles of their distribution to check whether their relations with dementia were nonlinear. Since associations did not obviously deviate from linearity, all analyses were subsequently performed entering retinal vascular characteristics as a linear term in the model. HRs were expressed per SD difference in retinal vascular calibers to allow comparison of strength of associations across the different vascular characteristics. We tested the proportional hazard assumption by including the interactions of the vessel characteristics with time as covariate in the model. Interaction terms of both arteriolar and venular calibers with follow-up time were all nonsignificant, indicating that the associations between vascular calibers and dementia did not differ according to length of follow-up. To control for the confounding effect of the other vessel, we subsequently entered both calibers simultaneously in the model.27–29 All analyses were additionally adjusted for the abovementioned cardiovascular risk factors. Stroke before the end of follow-up was included in the model as a time-varying covariate. Because the APOE ϵ4 allele is an important risk factor for AD,30 and may modulate the effects of vascular disease on the brain,31 we also performed the analyses within strata of APOE genotype (carriers vs noncarriers of the ϵ4 allele). All analyses were performed using SPSS statistical software version 15 (SPSS Inc., Chicago, IL).
RESULTS
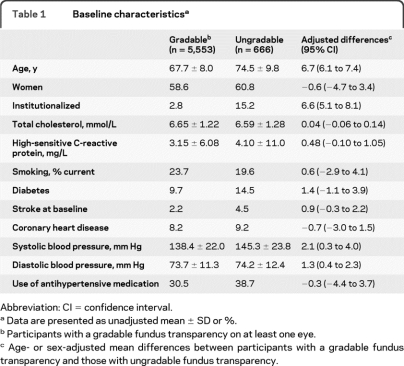
Baseline characteristics of the study population and a comparison between participants with gradable and ungradable fundus transparencies are shown in table 1. Adjusted mean differences show that those with ungradable fundus transparencies were significantly older, more often institutionalized, and had higher blood pressures. There were no significant differences in other risk factors. The mean summated arteriolar caliber was 147.0 μm (SD 14.4 μm; range 92.2–235.7 μm), and the mean summated venular caliber 222.2 μm (SD 20.8 μm; range 135.1–313.6 μm).
Table 1.
Baseline characteristicsa
Abbreviation: CI = confidence interval.
Data are presented as unadjusted mean ± SD or %.
Participants with a gradable fundus transparency on at least one eye.
Age- or sex-adjusted mean differences between participants with a gradable fundus transparency and those with ungradable fundus transparency.
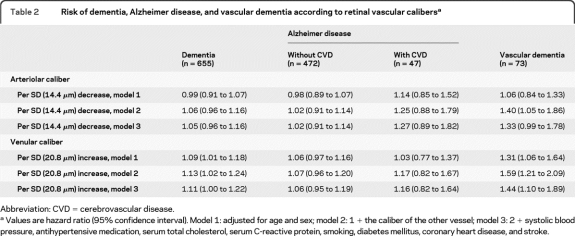
After a follow-up of 64,549 person-years (mean 11.6 years [SD 4.4]), 655 participants had developed dementia, of whom 519 were diagnosed with AD (472 without and 47 with cerebrovascular disease) and 73 with vascular dementia. The remaining 63 cases were ascribed to other subtypes (including dementia in Parkinson disease, multisystem atrophy, and Lewy body dementia). Table 2 shows the association of retinal arteriolar and venular calibers with risk of dementia. When analyses were adjusted only for age and sex, we found no association of arteriolar calibers with risk of dementia, whereas larger venular calibers were associated with a higher risk of dementia. Analyses according to dementia subtype showed that the association of larger venular calibers with an increased risk of dementia was driven by the association with vascular dementia. For every SD increase in venular caliber, risk of vascular dementia increased significantly by 31%. This association became even more pronounced after additional correction for arteriolar caliber (HR per SD increase in venular caliber 1.59, 95% confidence interval [CI] 1.21 to 2.09). Further adjustments for stroke and cardiovascular risk factors only slightly attenuated the association (HR per SD increase in venular caliber 1.44, 95% CI 1.10 to 1.89). Venular calibers were not related to the risk of AD without cerebrovascular disease, regardless of correction for arteriolar caliber (HR per SD increase in venular caliber after correction for arteriolar caliber 1.07, 95% CI 0.96 to 1.20). The risk of AD with cerebrovascular disease increased with 17% per SD increase in venular caliber after correction for arteriolar caliber, although this was nonsignificant.
Table 2.
Risk of dementia, Alzheimer disease, and vascular dementia according to retinal vascular calibersa
Abbreviation: CVD = cerebrovascular disease.
Values are hazard ratio (95% confidence interval). Model 1: adjusted for age and sex; model 2: 1 + the caliber of the other vessel; model 3: 2 + systolic blood pressure, antihypertensive medication, serum total cholesterol, serum C-reactive protein, smoking, diabetes mellitus, coronary heart disease, and stroke.
When analyzed separately, arteriolar calibers were neither related to AD nor to vascular dementia. Yet, after correction for venular caliber, we observed a significant association of arteriolar calibers with vascular dementia, but not with AD. The association with vascular dementia became borderline significant after adjustment for stroke and cardiovascular risk factors. The risk of AD with cerebrovascular disease nonsignificantly increased with 25% per SD decrease in arteriolar caliber.
For both arteriolar and venular calibers the association with dementia was similar for participants with or without at least one APOE ϵ4 allele.
DISCUSSION
In our prospective study, we investigated the association of retinal vascular calibers with the risk of developing dementia. One previous study investigated the association of retinal vascular calibers with presence of dementia, but found no association of retinal vascular calibers and cognitive function or dementia.32 This study was, however, cross-sectional. Only a few more studies have investigated the relation between retinal vascular calibers and cognitive function, reporting either no association or an association of larger venular calibers with impaired cognitive function.33–35 In these studies, the most often reported retinal vessel characteristic was the ratio of arteriolar-to-venular caliber, which does not provide information on the individual contribution of the arteriolar and venular calibers.
Our results are in agreement with previous findings showing that larger venular calibers are associated with progression of cerebral small vessel disease and stroke,13–17 both major risk factors for vascular dementia. The observation that the association was less strong and nonsignificant for AD with cerebrovascular disease, and absent for AD without cerebrovascular disease, is also in concordance with these findings.
Larger retinal venular calibers may be related to vascular dementia in several ways. First, they may reflect exposure to clinical stroke or other vascular risk factors, including atherosclerosis, inflammation, diabetes mellitus, and smoking. Since adjusting for these factors did not change results, other mechanisms should be considered. Second, larger retinal venular calibers have been hypothesized to be a general marker of retinal ischemia and by proxy of cerebral ischemia.15 Retinal venular dilatation is observed in the early stages of diabetic and venous stasis retinopathy, both of which are characterized by retinal ischemia.12,36 In turn, retinal ischemia has been related to lower cerebral blood flow.12 In line with these observations, larger retinal venular calibers were found to be associated with several indicators of lower cerebral oxygen supply. We reported lower arteriolar oxygen saturation to be associated with larger retinal venular calibers, in particular in the presence of lower cerebral blood flow.37 In addition, larger venular calibers were found to be associated with severe extracranial carotid artery disease in patients with acute ischemic stroke. This association was confined to retinal venular widening ipsilateral to the carotid artery stenosis.38 Altogether, this suggests that cerebral hypoperfusion may explain the relation between retinal venular widening and increased dementia risk.
Venous stasis may cause cerebral hypoperfusion and ischemia, in particular in the periventricular region of the brain through diminished clearance of cellular metabolites, and as such contributes to the development of white matter lesions and ultimately dementia.39 Because brain imaging was not performed routinely in all participants, we were not able to investigate whether white matter lesions account for the association we found between larger venular calibers and dementia risk.
Retinal arteriolar narrowing was also associated with vascular dementia, albeit to a lesser extent. Smaller arteriolar calibers are strongly related to hypertension,7,8 which is one of the strongest risk factors for both stroke and vascular dementia. An association of smaller arteriolar calibers with vascular dementia was therefore expected. Yet, smaller arteriolar calibers were related to an increased risk of vascular dementia only after adjustment for venular calibers. The absence of an association in the overall analysis may well be the result of opposing effects of arteriolar narrowing caused by hypertension on the one hand and arteriolar widening caused by endothelial dysfunction and ischemia on the other hand. Due to increased arterial stiffness as a result of vasoconstriction, intimal thickening, medial hyperplasia, hyalinization, and sclerosis at higher age, widening of retinal arterioles may be less pronounced than widening of retinal venules in conditions reflecting ischemia.15 In addition, arteriolar and venular calibers are correlated and persons with larger venular calibers in general also have larger arteriolar calibers. The effect of smaller arteriolar calibers on dementia risk is therefore masked by a confounding effect of larger venular calibers in model 1.27
Important strengths of our study are the population-based design and the long follow-up, which was virtually complete with regard to the dementia diagnosis. Other advantages of our study are the detailed assessment of retinal vascular calibers on 20° stereoscopic transparencies obtained after pharmacologic mydriasis and the adjustment for refractive errors of the eye. This enabled us to estimate the intraluminal arteriolar and venular calibers in more detail, where others reported uncorrected calibers in pictures with smaller magnification.6
Some further methodologic issues should be discussed. Participants who did not visit the study center to undergo the ophthalmic examination and participants with an ungradable fundus transparency were on average older and more often institutionalized. Given the long duration and the completeness of follow-up in our study, distortion of the reported associations by selection bias is unlikely. Limitations related to the semiautomated system assessing the retinal vascular calibers have been described.6 Because assessment of retinal calibers was unrelated to clinical characteristics of the participants, these limitations most likely led to an underestimation of our effects due to random misclassification.
Future studies are needed to confirm our findings of an association between larger retinal venular caliber and vascular dementia risk. Imaging techniques such as CT perfusion and magnetic resonance angiography studies should be added in order to determine whether cerebral hypoperfusion indeed provides the mechanism underlying the association of venular widening with an increased risk of vascular dementia.
Footnotes
- AD
- Alzheimer disease
- CI
- confidence interval
- CRP
- C-reactive protein
- DSM-III-R
- Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- GMS
- Geriatric Mental State
- HR
- hazard ratio
- MMSE
- Mini-Mental State Examination
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Elisabeth M.C. Schrijvers.
STUDY FUNDING
The Rotterdam Study is supported by the Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research (NWO), the Netherlands Organization for Health Research and Development (ZonMw), The Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. This study was further financially supported by the Netherlands Organization for Scientific Research (NWO) grant 904–61-155; Alzheimer Nederland grant V-2001–015; Swart van Essen, Rotterdam; Blindenpenning, Amsterdam; Blindenhulp, The Hague; Algemene Nederlandse Vereniging ter Voorkoming van Blindheid (ANVVB), Doorn; Stichting Oogfonds Nederland, Utrecht; Stichting Lijf en Leven, Krimpen aan de Lek; Rotterdamse Vereniging Blindenbelangen, Rotterdam; MD Fonds, Utrecht; Oogfonds Nederland, Utrecht; Laméris Ootech BV, Nieuwegein; Medical Workshop, de Meern; and Topcon Europe BV, Capelle aan de IJssel, all in the Netherlands. The sponsors or funding organizations had no role in the design, conduct, analysis, or publication of this research.
DISCLOSURE
Dr. F.J. de Jong, Dr. Schrijvers, and Dr. Ikram report no disclosures. Dr. Koudstaal receives royalties from the publication of Neurologie (Elsevier, 4th edition, 2010) and Anamnese en lichamelijk onderzoek (Elsevier, 4th edition, 2006); has received travel and honoraria from Servier; and receives research support from the Neurovascular Research Fund Rotterdam. Dr. P.T.V.M. de Jong reports no disclosures. Dr. Hofman has received funding for travel from GlaxoSmithKline; serves as Editor-in-Chief for the European Journal of Epidemiology; receives royalties from the publication of Grondslagen der epidemiologie (Elsevier, 2008), Klinische epidemiologie (Elsevier, 2000), and Investigating Neurological Disease (Cambridge University Press, 1996); and receives research support from the Netherlands Genomics Initiative for the Rotterdam Study and from the Ministry of Health for the Generation R study. Dr. Vingerling reports no disclosures. Dr. Breteler serves on editorial advisory boards for Neuroepidemiology and Alzheimer's & Dementia; received research support from Pfizer for biomarker research in Alzheimer disease; is funded by the Netherlands Organization for Scientific Research grant 948-00-010 and grant 918-46-615; and received research support from the Erasmus MC, Alzheimer's Association USA, the NIH, the Dutch Cancer Society, the Dutch Parkinsonfonds, and the Netherlands Brain Foundation.
REFERENCES
- 1. Knopman DS. Cerebrovascular disease and dementia. Br J Radiol 2007;80:S121–S127 [DOI] [PubMed] [Google Scholar]
- 2. Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol 2001;46:59–80 [DOI] [PubMed] [Google Scholar]
- 3. Kwa VI. Our eyes: windows to our souls or crystal balls? Lancet Neurol 2006;5:108–110 [DOI] [PubMed] [Google Scholar]
- 4. Goto I, Katsuki S, Ikui H, Kimoto K, Mimatsu T. Pathological studies on the intracerebral and retinal arteries in cerebrovascular and noncerebrovascular diseases. Stroke 1975;6:263–269 [DOI] [PubMed] [Google Scholar]
- 5. Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol 2004;3:179–183 [DOI] [PubMed] [Google Scholar]
- 6. Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999;106:2269–2280 [DOI] [PubMed] [Google Scholar]
- 7. Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 2004;45:2129–2134 [DOI] [PubMed] [Google Scholar]
- 8. Liew G, Sharrett AR, Wang JJ, et al. Relative importance of systemic determinants of retinal arteriolar and venular caliber: the Atherosclerosis Risk in Communities Study. Arch Ophthalmol 2008;126:1404–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong TY, Mitchell P. The eye in hypertension. Lancet 2007;369:425–435 [DOI] [PubMed] [Google Scholar]
- 10. de Jong FJ, Ikram MK, Witteman JC, Hofman A, de Jong PT, Breteler MM. Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Ann Neurol 2007;61:491–495 [DOI] [PubMed] [Google Scholar]
- 11. Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol 2006;124:87–94 [DOI] [PubMed] [Google Scholar]
- 12. Klijn CJ, Kappelle LJ, van Schooneveld MJ, et al. Venous stasis retinopathy in symptomatic carotid artery occlusion: prevalence, cause, and outcome. Stroke 2002;33:695–701 [DOI] [PubMed] [Google Scholar]
- 13. Doubal FN, MacGillivray TJ, Hokke PE, Dhillon B, Dennis MS, Wardlaw JM. Differences in retinal vessels support a distinct vasculopathy causing lacunar stroke. Neurology 2009;72:1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ikram MK, de Jong FJ, Bos MJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology 2006;66:1339–1343 [DOI] [PubMed] [Google Scholar]
- 15. Ikram MK, De Jong FJ, Van Dijk EJ, et al. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain 2006;129:182–188 [DOI] [PubMed] [Google Scholar]
- 16. McGeechan K, Liew G, Macaskill P, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol 2009;170:1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet 2001;358:1134–1140 [DOI] [PubMed] [Google Scholar]
- 18. Hofman A, Breteler MM, van Duijn CM, et al. The Rotterdam Study: 2010 objectives and design update. Eur J Epidemiol 2009;24:553–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ott A, Breteler MM, van Harskamp F, Stijnen T, Hofman A. Incidence and risk of dementia: The Rotterdam Study. Am J Epidemiol 1998;147:574–580 [DOI] [PubMed] [Google Scholar]
- 20. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 21. Copeland JR, Kelleher MJ, Kellett JM, et al. A semi-structured clinical interview for the assessment of diagnosis and mental state in the elderly: the Geriatric Mental State Schedule. I. Development and reliability. Psychol Med 1976;6:439–449 [DOI] [PubMed] [Google Scholar]
- 22. Roth M, Tym E, Mountjoy CQ, et al. CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986;149:698–709 [DOI] [PubMed] [Google Scholar]
- 23. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington, DC: American Psychiatric Association; 1987 [Google Scholar]
- 24. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 25. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260 [DOI] [PubMed] [Google Scholar]
- 26. Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet 1991;337:1158–1159 [DOI] [PubMed] [Google Scholar]
- 27. Liew G, Wong TY, Mitchell P, Wang JJ. Are narrower or wider retinal venules associated with incident hypertension? Hypertension 2006;48:e10. [DOI] [PubMed] [Google Scholar]
- 28. Ikram MK, Witteman JC, Vingerling JR, Breteler MM, Hofman A, de Jong PT. Response to: Are narrower or wider retinal venules associated with incident hypertension? Hypertension 2006;48:e11. [DOI] [PubMed] [Google Scholar]
- 29. Liew G, Sharrett AR, Kronmal R, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci 2007;48:52–57 [DOI] [PubMed] [Google Scholar]
- 30. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–923 [DOI] [PubMed] [Google Scholar]
- 31. Slooter AJ, van Duijn CM, Bots ML, et al. Apolipoprotein E genotype, atherosclerosis, and cognitive decline: the Rotterdam Study. J Neural Transm Suppl 1998;53:17–29 [DOI] [PubMed] [Google Scholar]
- 32. Baker ML, Marino Larsen EK, Kuller LH, et al. Retinal microvascular signs, cognitive function, and dementia in older persons: the Cardiovascular Health Study. Stroke 2007;38:2041–2047 [DOI] [PubMed] [Google Scholar]
- 33. Ding J, Patton N, Deary IJ, et al. Retinal microvascular abnormalities and cognitive dysfunction: a systematic review. Br J Ophthalmol 2008;92:1017–1025 [DOI] [PubMed] [Google Scholar]
- 34. Lesage SR, Mosley TH, Wong TY, et al. Retinal microvascular abnormalities and cognitive decline: the ARIC 14-year follow-up study. Neurology 2009;73:862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liew G, Mitchell P, Wong TY, et al. Retinal microvascular signs and cognitive impairment. J Am Geriatr Soc 2009;57:1892–1896 [DOI] [PubMed] [Google Scholar]
- 36. Zatz R, Brenner BM. Pathogenesis of diabetic microangiopathy. The hemodynamic view Am J Med 1986;80:443–453 [DOI] [PubMed] [Google Scholar]
- 37. de Jong FJ, Vernooij MW, Ikram MK, et al. Arteriolar oxygen saturation, cerebral blood flow, and retinal vessel diameters: The Rotterdam Study. Ophthalmology 2008;115:887–892 [DOI] [PubMed] [Google Scholar]
- 38. De Silva DA, Liew G, Wong MC, et al. Retinal vascular caliber and extracranial carotid disease in patients with acute ischemic stroke: the Multi-Centre Retinal Stroke (MCRS) study. Stroke 2009;40:3695–3699 [DOI] [PubMed] [Google Scholar]
- 39. Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: association with leukoaraiosis. Radiology 1995;194:469–476 [DOI] [PubMed] [Google Scholar]