Abstract
Objective:
Stroke symptoms in right hemispheric stroke tend to be underestimated in clinical assessment scales, resulting in greater infarct volumes in right as compared to left hemispheric strokes despite similar clinical stroke severity. We hypothesized that patients with right hemispheric nonlacunar stroke are at higher risk for secondary intracerebral hemorrhage after thrombolysis despite similar stroke severity.
Methods:
We analyzed data of 2 stroke cohorts with CT-based and MRI-based imaging before thrombolysis. Initial stroke severity was measured with the NIH Stroke Scale (NIHSS). Lacunar strokes were excluded through either the presence of cortical symptoms (CT cohort) or restriction to patients with prestroke diffusion-weighted imaging (DWI) lesion size >3.75 mL (MRI cohort). Probabilities of having a parenchymal hematoma were determined using multivariate logistic regression.
Results:
A total of 392 patients in the CT cohort and 400 patients in the MRI cohort were evaluated. Although NIHSS scores were similar in strokes of both hemispheres (median NIHSS: CT: 15 vs 13, MRI: 14 vs 16), the frequencies of parenchymal hematoma were higher in right hemispheric compared to left hemispheric strokes (CT: 12.4% vs 5.7%, MRI: 10.4% vs 6.8%). After adjustment for potential confounders (but not pretreatment lesion volume), the probability of parenchymal hematoma was higher in right hemispheric nonlacunar strokes (CT: odds ratio [OR] 2.3; 95% confidence interval [CI] 1.08–4.89; p = 0.032) and showed a borderline significant effect in the MRI cohort (OR 2.1; 95% CI 0.98–4.49; p = 0.057). Adjustment for pretreatment DWI lesion size eliminated hemispheric differences in hemorrhage risk.
Conclusions:
Higher hemorrhage rates in right hemispheric nonlacunar strokes despite similar stroke severity may be caused by clinical underestimation of the proportion of tissue at bleeding risk.
Intracerebral hemorrhage (ICH) is the most serious complication after systemic thrombolysis for ischemic stroke.1 Risk of secondary hemorrhagic transformation increases with the volume of ischemic brain tissue.2–4 Although there is a rough association between the clinical stroke severity and the volume of the affected brain tissue, the recognition of neurologic deficits is different for left and right hemispheric strokes.5 Language disorders, being a typical sign of left hemispheric dysfunction, are more likely to be recognized than predominantly right hemispheric symptoms, such as neglect or anosognosia.6 The most widely used assessment scale for stroke severity is the NIH Stroke Scale (NIHSS). Although its reliability, clinical usefulness, and prognostic validity was shown in several studies,7–10 it overemphasizes left hemispheric symptoms.11 Thus, lesion volume may be substantially higher in right hemispheric as compared to left hemispheric stroke despite similar NIHSS scores.11–13 This may lead to different risk profiles of secondary ICH after thrombolysis.
We used 2 independent cohorts with CT- and MRI-based prethrombolysis brain imaging to investigate whether the risk of parenchymal ICH in nonlacunar strokes differs between patients with left and right hemispheric stroke when adjusted for clinical stroke severity according to the NIHSS.
METHODS
Hypothesis and definitions.
The primary hypothesis was that patients with right hemispheric nonlacunar stroke have a higher hemorrhage risk as compared to patients with left hemispheric nonlacunar stroke despite similar stroke severity assessed by the NIHSS. The secondary hypothesis was that hemorrhage risk is dependent on the prethrombolysis lesion volume irrespective of the side of ischemia.
The analysis was restricted to nonlacunar strokes because the functional difference between the 2 brain hemispheres does not affect the severity of lacunar strokes (not involving task-specific cortical areas). For the nature of the available data, we used 2 different definitions for nonlacunar stroke in the 2 cohorts: In the CT-based cohort, nonlacunar stroke was defined clinically by the positive evidence of cortical symptoms (i.e., dysphasia, neglect, hemianopia). In the MRI-based cohort, a radiologic definition for nonlacunar stroke was applied: all patients with infarct volumes larger than the upper size limit of a lacune (1.5 cm × 1.5 cm × 1.5 cm = 3.38 mL) were included in the present study irrespective of clinical symptoms.
The primary outcome parameter was the percentage of parenchymal hematoma. In both cohorts, hemorrhages were classified according to published criteria14 as hemorrhagic infarctions with small petechial hematoma (HI1), hemorrhagic infarctions with more confluent petechiae (HI2), parenchymal hematoma with <30% of the infarcted area with some mild space-occupying effect (PH1), and parenchymal hematoma ≥30% of the infarcted area with significant space-occupying effect or clot remote from the infarcted area (PH2). PH1 and PH2 hematoma were defined as parenchymal hematoma. Identical criteria of hemorrhage volume and location were used in MRI (T2*) and CT follow-up scans and all hemorrhagic lesions were interpreted as acute except of known residual hemorrhagic defects. In case of more than one follow-up scan within 36 hours, hemorrhage classification was always determined with the scan showing the highest degree of intracranial hematoma.
We used the radiologic definitions as principal outcome parameter to avoid a recognition bias, because hemorrhages (like ischemic lesions) may cause clinical signs to a different extent in both hemispheres. Although there is controversial information about the association of parenchymal hematoma with infarct size,3,4,15 both PH1 and PH2 have been shown to be associated with poor prognosis.4,16 Symptomatic hemorrhage was defined according to the National Institute of Neurological Disorders and Stroke criteria: a hemorrhage was considered to be symptomatic if it was not seen on a previous brain scan and there had subsequently been any decline of neurologic status,1 irrespective of a drop in the NIHSS. Symptomatic ICH were defined to be treatment-related when they were observed within the first 36 hours.
Prethrombolysis CT-based cohort.
The thrombolysis databank of the Telemedical Project for Integrative Stroke Care (TEMPiS) was used to test the primary hypothesis.17 TEMPiS is a TeleStroke network of 2 academic and 12 community hospitals in Germany.18 In the TEMPiS network, standardized protocols for administering tissue plasminogen activator (tPA) within 3 hours were applied according to international guidelines. For selection of tPA candidates and guidance in complication management, all patients treated with tPA in community hospitals are presented to the academic hospitals for standardized telemedical consultation. The consultation includes a clinical examination with NIHSS assessment, check of inclusion and exclusion criteria, as well as transmission of baseline and follow-up imaging. All consecutive thrombolysis treatments were recorded in a quality assessment databank. Previous evaluations of this ongoing registry yielded comparable process quality and outcome of stroke thrombolysis in regional hospitals and stroke centers.17,19
Patients with (suspected) basilar artery occlusion or brainstem infarcts, nonvascular final diagnosis, bilateral hemispheric infarcts, and incomplete data were excluded from further analysis. Information about demographics, comorbidities, and clinical and laboratory values at baseline were collected. All NIHSS raters underwent standardized training and certification in the use of the NIHSS. Mandatory brain imaging was performed on admission and between 24 and 36 hours after tPA administration according to existing guidelines.20 Two different radiologists blinded to clinical data reviewed all brain images. Vital status at day 7 was recorded; patients discharged before day 7 were followed up using the medical documentation after discharge (e.g., letters from rehabilitation hospitals) or by telephone.
MRI-based cohort.
Data of the retrospective magnetic resonance (MR) stroke study cohort were used in order to confirm our hypothesis of increased hemorrhage risk in right hemispheric strokes when adjusted for the NIHSS.3 Furthermore, this dataset was used to test the second hypothesis that the hemorrhage risk is similar in left and right hemispheric stroke when adjusted for the size of the initial ischemic lesion. The MR stroke study cohort comprises the pooled data from 678 patients of 10 academic stroke centers in Europe, the United States, and Asia who underwent MRI before thrombolytic treatment. Follow-up imaging for hemorrhage detection was done in all patients either with CT or with T2* imaging in MRI. This study had previously shown a continuous increase of symptomatic hemorrhage with increased volume of the ischemic lesion (odds ratio [OR] 1.080; 95% confidence interval [CI] 1.012–1.153) in diffusion-weighted imaging (DWI) but did not evaluate hemispheric differences concerning bleeding risk.
Details of the MRI-based cohort were described previously.3 For the present analysis, only patients with complete data for age, NIHSS, time to treatment, kind of thrombolysis (IV or intra-arterial), thrombolytic agent (urokinase or tPA), NIHSS, and DWI lesion volume were included.
Standard protocol approvals, registrations, and patient consents.
The scientific evaluation of the TEMPiS project (CT-based cohort) including safety monitoring of systemic thrombolysis in patients who were not able to give informed consent was approved by the local ethics committees of the Bavarian Chamber of Physicians. The post hoc analysis of anonymized data was approved by the ethics committee of the University of Regensburg.
The standardized MRI study protocols of the MRI-based cohort had been approved by all local ethics committees of the hospitals participating in the MRI study group.
Statistical analysis.
The χ2 test was used to test differences in proportions and the Mann-Whitney U-test for those in continuous variables. To estimate OR and the resulting 95% CI of predictors for parenchymal hematoma, multivariate logistic regression was performed. In the CT imaging cohort, the influence of lesion site (a priori hypothesis), age (age groups), sex, institution (academic or community hospital), comorbidities (diabetes, hypertension, atrial fibrillation), antiplatelets and anticoagulants before stroke, systolic blood pressure and blood glucose levels before treatment (both continuous), time from stroke onset to treatment in minutes (continuous), and NIHSS on risk of ICH were investigated in univariate and multivariate analyses. We used the NIHSS as a continuous variable because there is no rational cutoff value when comparing patients with left and right hemispheric stroke.
In the MRI cohort, the risk of parenchymal hematoma was assessed with adjustment for age (age groups), time to treatment (≤3 hours and >3 hours), thrombolytic agent (tPA or urokinase), intra-arterial vs IV application, and NIHSS (continuous).
In a post hoc, nonprespecified analysis, we pooled the 2 cohorts and included all patients with lacunar and nonlacunar strokes. The same variables as in the MRI cohorts (except of DWI volume) were entered in the multivariable logistic regression analysis in order to determine the effect of lesion side on risk of parenchymal hematoma. Variables in multivariate analyses were eliminated using a backward-elimination procedure. Analyses were restricted to data without missing values.
The statistical significance of the resulting coefficient was tested by the likelihood ratio test. An α level of 5% was considered to be significant. Data were analyzed using SPSS 14.
RESULTS
CT cohort.
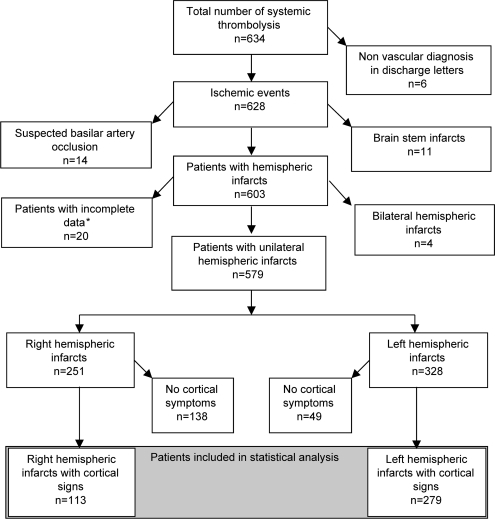
From February 2003 to December 2005, tPA was administered to 634 consecutively admitted patients with suspected ischemic stroke within the TEMPiS network. After exclusion of patients according to predefined criteria (i.e., missing clinical/laboratory values, vertebrobasilar infarction, bilateral infarctions), 392 patients with nonlacunar strokes remained for further analysis (figure 1).
Figure 1. Selection of patients in the CT cohort.
*One patient died within 24 hours prior to follow-up imaging.
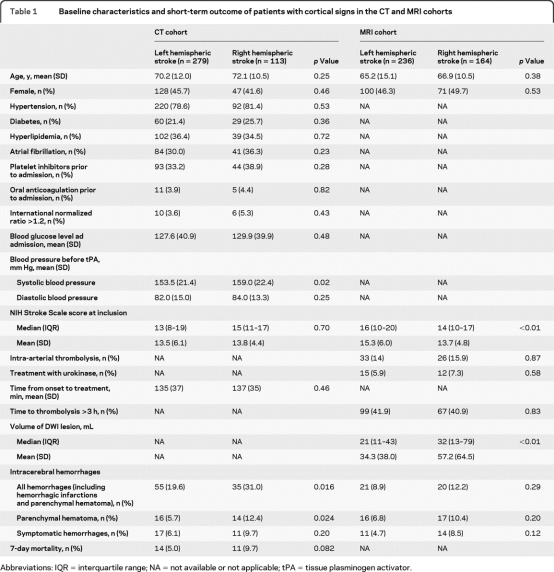
Characteristics of patients with nonlacunar stroke are shown in table 1. There were no significant differences in other baseline characteristics between patients with right and left hemispheric strokes, except for initial systolic blood pressure being higher in right hemispheric nonlacunar strokes (129.9 vs 127.6 mm Hg, p=0.02). Despite similar NIHSS scores in right and left hemispheric nonlacunar strokes (median NIHSS: 15 vs 13, p=0.7), patients with right hemispheric nonlacunar stroke had a twofold higher rate of parenchymal hematoma (12.4% vs 5.7%, p=0.024), higher rates of symptomatic ICH (not significant), and a trend toward a higher 7-day mortality (table 1).
Table 1.
Baseline characteristics and short-term outcome of patients with cortical signs in the CT and MRI cohorts
Abbreviations: IQR = interquartile range; NA = not available or not applicable; tPA = tissue plasminogen activator.
In comparison, patients without cortical signs (left hemispheric n=49, right hemispheric n=138) had lower NIHSS scores (right: median 9, interquartile range [IQR] 7–12; left: median 7, IQR 5–10), low parenchymal hematoma rates (right 4 [2.9%]; left 1 [2.1%]), and low 7-day case fatality rates (right 0 [0%]; left 1 [0.7%]).
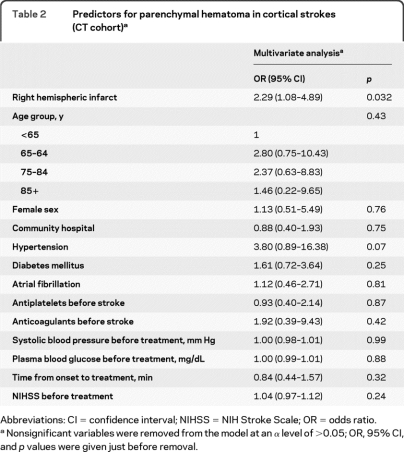
When controlling for multiple clinical factors (but not pretreatment lesion size), the side of the ischemia remained an independent predictor for parenchymal hematoma (OR for right hemispheric nonlacunar stroke 2.29; 95% CI 1.08–4.89) (table 2).
Table 2.
Predictors for parenchymal hematoma in cortical strokes (CT cohort)a
Abbreviations: CI = confidence interval; NIHSS = NIH Stroke Scale; OR = odds ratio.
Nonsignificant variables were removed from the model at an α level of >0.05; OR, 95% CI, and p values were given just before removal.
MRI cohort.
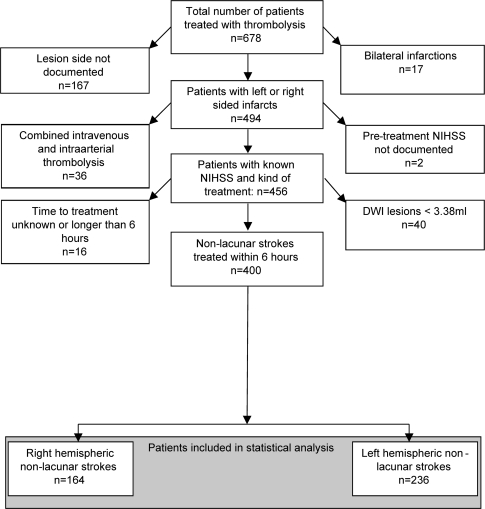
The MR stroke study group databank comprised 678 patients. The selection of patients according to inclusion and exclusion criteria is shown in figure 2.
Figure 2. Selection of patients in the MRI cohort.
DWI=diffusion-weighted imaging; NIHSS=NIH Stroke Scale.
Baseline characteristics and hemorrhage rates are shown in table 1. Patients with right hemispheric nonlacunar stroke had lower baseline NIHSS scores (median NIHSS 14 vs 16, p < 0.01) despite larger initial DWI lesion volumes (32 mL vs 21 mL, p < 0.01). Parenchymal and symptomatic ICH were more frequent in right hemispheric strokes but these differences were not significant (table 1).
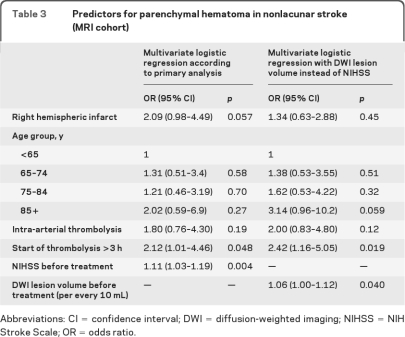
In multivariate analysis, the increased probability of parenchymal hematoma in right hemispheric nonlacunar strokes (OR 2.09, 95% CI 0.98–4.49) was of borderline significance (p=0.057) (table 3). After adjustment for lesion volume instead of NIHSS, lesion side had no association with risk for parenchymal hematoma (OR 1.34, 95% CI 0.63–2.88; p = 0.45) but lesion volume was correlated with hemorrhage risk (table 3).
Table 3.
Predictors for parenchymal hematoma in nonlacunar stroke (MRI cohort)
Abbreviations: CI = confidence interval; DWI = diffusion-weighted imaging; NIHSS = NIH Stroke Scale; OR = odds ratio.
Post hoc analysis of the pooled cohorts including lacunar and nonlacunar strokes.
The pooled cohort comprised 1,019 patients. Beside NIHSS at admission (OR 1.08, 95% CI 0.03–1.13) and intra-arterial thrombolysis (OR 2.17, 95% CI 1.02–4.62), right hemispheric stroke remained a predictor of parenchymal hematoma (OR 1.72, 95% CI 1.03–2.87; p = 0.037).
DISCUSSION
A higher risk of parenchymal hematoma was observed in patients with right hemispheric nonlacunar stroke despite similar clinical stroke severity according to the NIHSS. The rate of parenchymal hematoma in right hemispheric stroke was more than twofold compared to left hemispheric nonlacunar stroke and this relationship was seen in 2 independent patient cohorts. The higher risk for hemorrhages is apparently caused by greater lesion volumes in right hemispheric nonlacunar stroke with similar stroke severity. This is mainly attributable to the fact that right cortical symptoms in right hemispheric strokes are not equally represented in the NIHSS compared to predominantly left hemispheric speech disorders. Of the 42 points available, 7 are directly related to language disturbances. Only 2 points are related to neglect. In addition, dysphasic symptoms are often caused by smaller infarcts restricted to cortical speech areas while neglect is usually associated with extended infarcts.
Smaller proportions of patients with right hemispheric infarcts were seen in both cohorts. On the one hand, this may reflect the difficult clinical recognition of subtle right cortical signs. Conversely, it could also be a consequence of the unawareness (anosognosia) of patients who have mainly right-sided cortical symptoms5 and therefore do not seek medical consultation, in contrast to patients with, i.e., isolated speech disorders. In addition, patients with predominately neglect symptoms are less likely to score 5 or more points in the NIHSS compared to patients with speech disorders. Because strokes with NIHSS <5 are often deemed to be nonsevere strokes, these patients had a (relative) contraindication for IV thrombolysis in the CT imaging cohort.21
Larger infarct volume and the higher parenchymal hematoma rate are also possible reasons for the trend toward increased case fatality in right hemispheric nonlacunar stroke. This is in line with previous observations22 of a twofold increased probability of good outcome (modified Rankin Scale <2) in left hemispheric stroke after systemic thrombolysis, although this analysis was not stratified for cortical and noncortical involvement.
Our study has several limitations. The determination of cortical involvement with neurologic symptoms as used in the CT cohort may not always be reliable because subcortical lesions like thalamic infarcts occasionally cause neuropsychological deficits and infarcts restricted to the motor or sensory cortex may cause “lacunar syndromes.” However, the increased risk of parenchymal hematoma in right hemispheric nonlacunar strokes was confirmed in the MRI cohort using a different definition of nonlacunar stroke. Conversely, restriction to DWI lesions ≥3.75 mL in this MRI cohort will have excluded small cortical infarcts. In our perspective, these limitations are likely to lead to an underestimation of hemisphere impact on hemorrhage risk. DWI lesion volume does not exactly reflect the volume of tissue ischemia but is widely used as an approximation of infracted tissue. Although time from (recognized) symptom onset to imaging was similar in left and right hemispheric strokes in the MRI cohort, delayed recognition of symptoms may have contributed to the differences in DWI lesion volume at baseline.
The differences between the 2 cohorts regarding patient selection, hemorrhage detection, follow-up data, and use of intra-arterial therapy and volume determination in initial imaging make direct comparisons difficult.
The observation that the secondary hemorrhage risk is higher in right hemispheric nonlacunar strokes does not necessarily mean that the risk-benefit ratio is different in ischemic strokes of the 2 hemispheres. Saved brain tissue in the right hemisphere may prognostically be as important as in the left hemisphere and not all parenchymal hemorrhages are symptomatic particularly if they occur in the bed of large infarctions. Hence, the impact on prognosis needs to be analyzed separately with existing data of randomized thrombolysis trials.23,24 A recent analysis of placebo-treated patients included in randomized trials confirmed that right hemisphere stroke is an independent predictor for adverse outcome at 90 days when adjusted for the original NIHSS.25 However, no difference in functional outcome was found between patients with right or left hemisphere stroke when adjusted for a “hemisphere unbiased” NIHSS subscore.
Stroke severity based on the results of the NIHSS is currently one of the most commonly used criteria in selecting patients for thrombolytic treatment. Our study raises the question whether the NIHSS needs to be adjusted for hemispheres in order to provide an adequate assessment of hemorrhage risk. “Tissue-based assessment” for hemorrhage risk appears to be more accurate and should be used for the scientific evaluation of new therapeutic approaches.
ACKNOWLEDGMENT
The authors thank all participating stroke neurologists in the TEMPiS stroke centers; all TEMPiS hospitals (Asklepios Stadtklinik Bad Tölz, Kreiskrankenhaus Burglengenfeld, Kreisklinik Cham, Klinikum Dachau, Kreisklinik Ebersberg GmbH, Kreisklinik Eggenfelden, Klinikum Freising, Kreiskrankenhaus Kelheim, Kreisklinik Mühldorf, Kreisklinik München-Pasing, Klinikum Rosenheim, Klinikum St. Elisabeth Straubing); and all contributing stroke neurologists and radiologists who participated in the MR stroke study.
Footnotes
- CI
- confidence interval
- DWI
- diffusion-weighted imaging
- ICH
- intracerebral hemorrhage
- IQR
- interquartile range
- MR
- magnetic resonance
- NIHSS
- NIH Stroke Scale
- OR
- odds ratio
- TEMPiS
- Telemedical Project for Integrative Stroke Care
- tPA
- tissue plasminogen activator
AUTHOR CONTRIBUTIONS
Biostatistical analysis was conducted by Dr. Heinrich J. Audebert and Dr. Peter U. Heuschmann.
Study Funding
TEMPiS has been supported by the Bavarian health insurance companies, the Bavarian State Ministry for Employment and Social Order, Family and Women, the German Stroke Foundation, and the German Federal Ministry of Research (BMBF) within the Competence Net Stroke. Boehringer Ingelheim Pharma GmbH supplied stroke lyses boxes at initiation of the network. The MR Stroke Study group acts without dedicated funding. Local data sampling was supported by different funding sources. German centers: Bundesministerium für Bildung und Wissenschaft within the Kompetenznetzwerk Schlaganfall (Stroke Imaging Net). Stanford: National Institute of Neurological Disorders and Stroke RO1 NS39325 and K23 NS051372. Los Angeles: NIH Grant 5P50NS044378-03. Paris: PHRC AOM 03 008 EVAL-USINV. Seoul: Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology of Korea (M103KV010010 06K2201 01010). These organizations had no role in the conduct of the study or in the collection, analysis, or interpretation of the data.
DISCLOSURE
Prof. Audebert has received speaker honoraria from Takeda Pharmaceutical Company Limited, sanofi-aventis, Boehringer Ingelheim, Lundbeck Inc., and the University of Greifswald; serves as Associate Editor for Frontiers of Teleneurology; receives research support from Charité Universitätsmedizin Berlin; and has provided expert legal advice to Lundbeck Inc. Dr. Singer and Dr. Gotzler report no disclosures. Dr. Vatankhah has received funding for travel from Boehringer Ingelheim, Bayer Schering Pharma, Merck Serono, and UCB. Dr. Boy reports no disclosures. Dr. Fiehler has received funding for travel and speaker honoraria from Siemens, Boston Scientific, Cordis Corporation, ev3, and Bracco; serves as Neuroradiology Section Editor for Interventional Neuroradiology; and receives research support from the DFG and the EU. Prof. Lansberg has received research support from the NIH/NINDS and served as a consultant to legal firms. Dr. Albers has served on scientific advisory boards for Genentech, Inc., Lundbeck Inc., Merck Serono, and NMT Medical, Inc.; serves on the editorial board of Stroke; and has received research support from NMT Medical, Inc., PhotoThera, AstraZeneca, Forest Laboratories, Inc., and the NIH (NINDS, NIBIB). Dr. Kastrup serves on a scientific advisory board for and has received funding for travel and speaker honoraria from Boehringer Ingelheim. Dr. Rovira has received speaker honoraria from Bayer Schering Pharma and Teva Pharmaceutical Industries Ltd.; serves on the editorial boards of the American Journal of Neuroradiology and Neuroradiology; and serves on the speakers' bureau for Bayer Schering Pharma. Dr. Gass serves on the editorial board of Cerebrovascular Diseases. Dr. Rosso is a coinventor on a patent re: Neurinfarct software, which is protected by an international patent currently own by Intelligence in Medical Technologies, Paris. Dr. Derex and Dr. Kim report no disclosures. Dr. Heuschmann receives research support from the European Union, the German Stroke Foundation, and the Stanley Thomas Johnson Foundation.
REFERENCES
- 1. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2. Selim M, Fink JN, Kumar S, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke 2002;33:2047–2052 [DOI] [PubMed] [Google Scholar]
- 3. Singer OC, Humpich MC, Fiehler J, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol 2008;63:52–60 [DOI] [PubMed] [Google Scholar]
- 4. Dzialowski I, Pexman JH, Barber PA, et al. Asymptomatic hemorrhage after thrombolysis may not be benign: prognosis by hemorrhage type in the Canadian alteplase for stroke effectiveness study registry. Stroke 2007;38:75–79 [DOI] [PubMed] [Google Scholar]
- 5. Foerch C, Misselwitz B, Sitzer M, et al. Difference in recognition of right and left hemispheric stroke. Lancet 2005;366:392–393 [DOI] [PubMed] [Google Scholar]
- 6. Fink JN. Underdiagnosis of right-brain stroke. Lancet 2005;366:349–351 [DOI] [PubMed] [Google Scholar]
- 7. Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol 1989;46:660–662 [DOI] [PubMed] [Google Scholar]
- 8. Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH Stroke Scale using video training: NINDS TPA Stroke Study Group. Stroke 1994;25:2220–2226 [DOI] [PubMed] [Google Scholar]
- 9. Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870 [DOI] [PubMed] [Google Scholar]
- 10. Adams HP, Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126–131 [DOI] [PubMed] [Google Scholar]
- 11. Lyden P, Claesson L, Havstad S, Ashwood T, Lu M. Factor analysis of the National Institutes of Health Stroke Scale in patients with large strokes. Arch Neurol 2004;61:1677–1680 [DOI] [PubMed] [Google Scholar]
- 12. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke 1999;30:2355–2359 [DOI] [PubMed] [Google Scholar]
- 13. Fink JN, Selim MH, Kumar S, et al. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke 2002;33:954–958 [DOI] [PubMed] [Google Scholar]
- 14. Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999;30:2280–2284 [DOI] [PubMed] [Google Scholar]
- 15. Thomalla G, Sobesky J, Kohrmann M, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke 2007;38:313–318 [DOI] [PubMed] [Google Scholar]
- 16. Molina CA, Alvarez-Sabin J, Montaner J, et al. Thrombolysis-related hemorrhagic infarction: a marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke 2002;33:1551–1556 [DOI] [PubMed] [Google Scholar]
- 17. Audebert HJ, Kukla C, Vatankhah B, et al. Comparison of tissue plasminogen activator administration management between Telestroke Network hospitals and academic stroke centers: the Telemedical Pilot Project for Integrative Stroke Care in Bavaria/Germany. Stroke 2006;37:1822–1827 [DOI] [PubMed] [Google Scholar]
- 18. Audebert HJ, Schenkel J, Heuschmann PU, Bogdahn U, Haberl RL. Effects of the implementation of a telemedical stroke network: the Telemedic Pilot Project for Integrative Stroke Care (TEMPiS) in Bavaria, Germany. Lancet Neurol 2006;5:742–748 [DOI] [PubMed] [Google Scholar]
- 19. Schwab S, Vatankhah B, Kukla C, et al. Long-term outcome after thrombolysis in telemedical stroke care. Neurology 2007;69:898–903 [DOI] [PubMed] [Google Scholar]
- 20. Toni D, Chamorro A, Kaste M, et al. Acute treatment of ischaemic stroke: European Stroke Initiative. Cerebrovasc Dis 2004;17(suppl 2):30–46 [DOI] [PubMed] [Google Scholar]
- 21. Audebert HJ, Kukla C, Clarmann VC, et al. Telemedicine for safe and extended use of thrombolysis in stroke: the Telemedic Pilot Project for Integrative Stroke Care (TEMPiS) in Bavaria. Stroke 2005;36:287–291 [DOI] [PubMed] [Google Scholar]
- 22. Di Legge S, Saposnik G, Nilanont Y, Hachinski V. Neglecting the difference: does right or left matter in stroke outcome after thrombolysis? Stroke 2006;37:2066–2069 [DOI] [PubMed] [Google Scholar]
- 23. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–774 [DOI] [PubMed] [Google Scholar]
- 24. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329 [DOI] [PubMed] [Google Scholar]
- 25. Fink JN, Frampton CM, Lyden P, Lees KR. Does hemispheric lateralization influence functional and cardiovascular outcomes after stroke? Stroke 2008;39:3335–3340 [DOI] [PubMed] [Google Scholar]