Mutations in the microtubule-associated protein tau gene (MAPT) cause frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17).1 In addition, the major common MAPT-containing H1 haplotype is associated with increased risk for 2 parkinsonian disorders: progressive supranuclear palsy (PSP) characterized by 4-repeat tau pathology and Parkinson disease (PD) with α-synuclein pathology.2,3 However, the role of MAPT variation in other disorders with similar pathology or disease phenotype is unclear. We investigated the frequency of the MAPT H1 haplotype in both essential tremor (ET) and multiple system atrophy (MSA).
ET is the most common movement disorder, and prior evidence has indicated a common link between ET and PD from clinical, epidemiologic, and pathologic studies as well as some reports of brainstem Lewy bodies at autopsy in patients with ET.4 MSA is a neurodegenerative disorder with α-synuclein pathology with a mixed clinical presentation combining autonomic dysfunction, parkinsonism, and cerebellar or pyramidal symptoms. The initial clinical signs of MSA with prominent parkinsonism can make it difficult to differentially diagnose it from early PD. In addition, up to 30% of patients with MSA with prominent parkinsonism may have a transient response to levodopa therapy.5
Methods.
Genotyping of the MAPT H1 discriminating SNP (rs1052553) and H1c subhaplotype SNP (rs242557) was performed on a Sequenom MassArray iPLEX platform (San Diego, CA) (primer sequences are available on request) and analyzed with Typer 4.0 software. The rate of genotype calls was ≥95% in each population. The series contained 356 patients with clinical ET, 61 patients with pathologically confirmed MSA, and 409 US control subjects; all samples are North American Caucasians. Numerical variables were summarized with the sample mean, SD, and range (table e-1 on the Neurology® Web site at www.neurology.org). Associations between ET and MSA with MAPT rs1052553 and rs242557 were measured by χ2 statistics with Pearson probability estimates and corresponding odds ratios (ORs) with confidence intervals (CIs).
Standard protocol approvals, registrations, and patient consents.
The ethical review boards at each institution approved the study, and all participants provided informed consent.
Results.
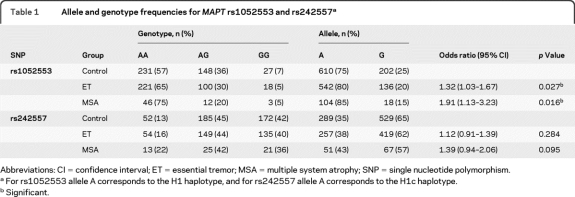
We observed an uncorrected association of MAPT H1 genotype with both ET (p = 0.027) and MSA (p = 0.016) when compared to the frequency in controls (table 1). No significant association was observed with SNP rs242557. The observed OR for ET [1.32 (1.03–1.67)] and MSA [1.91 (1.13–3.23)] are similar to those observed for PD in the same population [1.47 (1.15–1.89)].6
Table 1.
Allele and genotype frequencies for MAPT rs1052553 and rs242557a
Abbreviations: CI = confidence interval; ET = essential tremor; MSA = multiple system atrophy; SNP = single nucleotide polymorphism.
For rs1052553 allele A corresponds to the H1 haplotype, and for rs242557 allele A corresponds to the H1c haplotype.
Significant.
Discussion.
Given the relatively small sample size for both the ET and MSA series, and the possible clinical overlap with PD in the ET series, these results must be treated with caution and require independent replication. However, it is intriguing that while the extended haplotype containing MAPT has now shown association with a number of parkinsonian disorders, there is no evidence of association with AD, which displays abundant tau pathology. Given the presence of α-synuclein pathology in PD, MSA, and ET, these findings indicate a possible interplay between these proteins. It has been previously suggested that α-synuclein induces fibrillization of tau and that coincubation of tau and α-synuclein synergistically promotes fibrillization of both proteins.7
While we observed an association between the MAPT H1 haplotype and both ET and MSA, the functional variant remains to be identified. The expanded H1 haplotype contains MAPT, which is the best candidate gene to explain the observed association; however, other genes are present in this haplotype that cannot be excluded. It is postulated that the alternate MAPT haplotypes affect a differential expression of 3-repeat and 4-repeat tau protein in FTDP-17.2 The SNP displaying the strongest association with risk of the tauopathy PSP (rs242557) does not show the same association with PD, MSA, or ET, suggesting different pathomechanisms for disease risk.2,6
It is now crucial to determine the functional risk variants that are located on the backbone of the extended H1 haplotype containing MAPT. With the established genomic capture and next-generation sequencing approaches, this goal is within reach. Sequencing of the entire extended haplotype will identify the variants influencing risk of PSP, PD, MSA, or ET, and help elucidate the disease pathways involved.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all those who have contributed to their research, particularly the patients and families who donated DNA samples for this work.
Footnotes
Supplemental data at www.neurology.org.
Disclosure: Dr. Vilariño-Güell and Dr. Soto-Ortolaza report no disclosures. Dr. Rajput has served on scientific advisory boards for Novartis and UCB; has received funding for travel and speaker honoraria from Novartis; serves on the editorial board of the Canadian Journal of Neurological Sciences; and has received research support from Novartis, Allergan Inc., the NIH (NINDS U01 NS050324-01A1 [local PI]), CIHR, International Essential Tremor Foundation, Regina Curling Classic for Parkinson's Research, and Parkinson's disease and movement disorders endowment through RUH Foundation. Dr. Mash serves on scientific advisory boards for NIH PMDA, Boris Sokolov, and SRA; has received funding for travel or speaker honoraria from the Institute for the Prevention of In Custody Deaths; has patents pending re: Noribogaine and Biomarkers of Parkinson disease; is employed as Chief Scientific Officer for DEMERx Inc., and serves as a consultant for Phylogeny, Inc. Dr. Papapetropoulos serves as Associate Editor for Yearbook of Neurology and on the editorial boards of Open Journal of Neurology and Open Journal of Neurosurgery; has a patent pending re: MRPS6 Gene in PD; is employed as Senior Medical Director, Medical Affairs for Allergan, Inc.; and owns stock in Allergan, Inc. and Biogen Idec. Dr. Pahwa has served on scientific advisory boards and as a consultant for Teva Pharmaceutical Industries Ltd., Merck Serono, Schering-Plough Corp., Novartis, Medtronic, Inc., GE Healthcare, Biogen Idec, Boehringer Ingelheim, IMPAX Laboratories, Inc., and Ceregene; serves as Co-Editor in Chief of the International Journal of Neuroscience; receives publishing royalties for Pocket Note Series (Oxford University Press, 2009, 2010); serves on speakers' bureaus for Teva Pharmaceutical Industries Ltd., Medtronic, Inc., GlaxoSmithKline, and Novartis; receives research support from GlaxoSmithKline, Novartis, IMPAX Laboratories, Inc., Merck Serono, Boehringer Ingelheim, Schering-Plough Corp., the NIH/NINDS, and the National Parkinson Foundation; and served as an expert witness in a welding-related legal case. Dr. Lyons serves on a scientific advisory board for St. Jude Medical; serves as Co-Editor in Chief for the International Journal of Neuroscience; and serves as a consultant for Teva Pharmaceutical Industries Ltd. and Novartis. Dr. Uitti serves as an Associate Editor of Neurology®; has received research support from Advanced Neuromodulations Systems and from the NIH; and his institution receives annual royalties from Lundbeck Inc. from the licensing of the technology related to PARK8/LRRK2. Dr. Wszolek serves as Co-Editor-in-Chief of Parkinsonism and Related Disorders, Regional Editor of the European Journal of Neurology, and on the editorial boards of Neurologia i Neurochirurgia Polska, Advances in Rehabilitation, the Medical Journal of the Rzeszow University, and Clinical and Experimental Medical Letters; holds and has contractual rights for receipt of future royalty payments from patents re: A novel polynucleotide involved in heritable Parkinson's disease; receives royalties from publishing Parkinsonism and Related Disorders (Elsevier, 2007, 2008, 2009), and the European Journal of Neurology (Wiley-Blackwell, 2007, 2008, 2009); and receives research support from Allergan, Inc., the NIH, the Pacific Alzheimer Research Foundation (Canada), the CIHR, the Mayo Clinic Florida Research Committee CR program, and the gift from Carl Edward Bolch, Jr., and Susan Bass Bolch. Dr. Dickson serves on the editorial boards of the American Journal of Pathology, Journal of Neuropathology and Experimental Neurology, Brain Pathology, Neurobiology of Aging, Journal of Neurology Neurosurgery and Psychiatry, Annals of Neurology, and Neuropathology; and receives research support from the NIH. Dr. Farrer serves on a scientific advisory board for the Michael J. Fox Foundation; serves/has served on the editorial boards of Neurobiology of Disease and Parkinsonism and Related Disorders; is co-inventor on patents re: LRRK2 gene and mutations; receives institutional research support from Lundbeck Inc.; has received research support from the NIH (NS40256 [Project and Core PI]), the Pacific Alzheimer Research Foundation, and the Michael J Fox Foundation; and his institution receives annual royalties from Lundbeck Inc. from the licensing of the technology related to PARK8/LRRK2. Dr. Ross serves on the editorial board of Open Longevity Science.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Carles Vilariño-Güell.
References
- 1. Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998;393:702–705 [DOI] [PubMed] [Google Scholar]
- 2. Rademakers R, Melquist S, Cruts M, et al. High-density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum Mol Genet 2005;14:3281–3292 [DOI] [PubMed] [Google Scholar]
- 3. Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet 2009;41:1308–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007;130:3297–3307 [DOI] [PubMed] [Google Scholar]
- 5. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wider C, Vilarino-Guell C, Jasinska-Myga B, et al. Association of the MAPT locus with Parkinson's disease. Eur J Neurol 2010;17:483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 2003;300:636–640 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.