Abstract
Objective:
Attention-deficit/hyperactivity disorder (ADHD) is a childhood-onset behavioral diagnosis in which children often fail to meet age norms in development of motor control, particularly timed repetitive and sequential movements, motor overflow, and balance. The neural substrate of this motor delay may include mechanisms of synaptic inhibition in or adjacent to the motor cortex. The primary objective of this study was to determine whether transcranial magnetic stimulation (TMS)–evoked measures, particularly short interval cortical inhibition (SICI), in motor cortex correlate with the presence and severity of ADHD in childhood as well as with commonly observed delays in motor control.
Methods:
In this case-control study, behavioral ratings, motor skills, and motor cortex physiology were evaluated in 49 children with ADHD (mean age 10.6 years, 30 boys) and 49 typically developing children (mean age 10.5 years, 30 boys), all right-handed, aged 8–12 years. Motor skills were evaluated with the Physical and Neurological Examination for Subtle Signs (PANESS) and the Motor Assessment Battery for Children version 2. SICI and other physiologic measures were obtained using TMS in the left motor cortex.
Results:
In children with ADHD, mean SICI was reduced by 40% (p < 0.0001) and less SICI correlated with higher ADHD severity (r = −0.52; p = 0.002). Mean PANESS motor development scores were 59% worse in children with ADHD (p < 0.0001). Worse PANESS scores correlated modestly with less SICI (r = −.30; p = 0.01).
Conclusion:
Reduced TMS-evoked SICI correlates with ADHD diagnosis and symptom severity and also reflects motor skill development in children.
Development of behavioral control and fine motor coordination are 2 fundamental maturational processes in childhood. Children with attention-deficit/hyperactivity disorder (ADHD) have age-inappropriate impulse control and fail to meet age norms on timed repetitive and sequential movements, develop accurate rhythm more slowly, and persist in immature patterns of motor overflow.1 These developmental skills, rated using the Physical and Neurological Examination for Subtle Signs (PANESS),2 consistently distinguish children with ADHD from typically developing (TD) children.3 The neurobiologic underpinnings of these motor delays as well as of the cardinal ADHD behavioral symptoms are incompletely understood. A more quantitative biologically based marker is needed as a foundation for predicting risk for poor ADHD adult outcomes4 and for designing more rational, personalized interventions that prevent these outcomes. Such a marker might have applications more broadly in other neurologic conditions in which executive function is poor.
The aim of the present study was to determine, in a large and well-characterized sample of children with ADHD and TD children, whether transcranial magnetic stimulation (TMS) in the motor cortex could generate quantitative measures that correlate with both ADHD behaviors and ADHD-associated motor development. Several prior studies have shown that ADHD diagnosis or symptoms are associated with reduced TMS-evoked short interval cortical inhibition (SICI) in motor cortex.5–8 These small studies did not comprehensively assess motor skills or cognition. We hypothesized that reduced SICI would be a quantitative, predictive, physiologic biomarker of ADHD, correlating with severity of ADHD symptoms as well as with associated impairments in fine motor skills.
METHODS
This was a 1:1, age- and sex-matched, case-control study of motor development, motor cortex physiology, and behavioral symptoms in 8- to 12-year-old right-handed children with ADHD vs TD control children. The rationales for studying this age group include well-documented, ADHD-related delays in motor skill development,3 but incomplete understanding of the biologic basis in the motor system as well as its relationship to cognitive and behavioral data.
Subjects.
All participants were recruited using institutional review board–approved advertisements placed in clinics, schools, and newspapers, from 2006 to 2009. Parents who responded were initially screened by phone. To be included, children had to be otherwise healthy, with no history of psychiatric or developmental disorders other than ADHD. Psychostimulants, but no other medications, were allowed. For the study, stimulants were discontinued on the day before and the day of cognitive and TMS testing.
Standard protocol approvals, registrations, and patient consents.
Permission for the study was obtained from the Cincinnati Children's Hospital Medical Center and Johns Hopkins Medicine institutional review boards. Written informed consent was obtained from legal guardians and assent was obtained from children.
Clinical diagnostic assessments of children with ADHD and TD children.
All evaluations were conducted by research personnel trained to administer psychiatric interviews and parent questionnaires, and all data were reviewed by the physician investigators. Diagnoses of ADHD vs typical development were confirmed by direct interview, by clinical rating scales, and by the Diagnostic Interview for Children and Adolescents,9 which uses DSM-IV criteria. Children with other diagnoses, e.g., conduct or mood disorders, were excluded. ADHD symptom presence and severity were evaluated with the parent versions of the ADHD Rating Scale IV (ADHD-RS-IV)10 and the Conners' Parent Rating Scale–Revised (CPRS).11 The CPRS ADHD severity T score accounts for age and gender.
Study personnel also administered the Wechsler Intelligence Scale for Children, 4th edition,12 and the Word Reading subtest of the Wechsler Individual Achievement Test, 2nd edition.13 Children were excluded for any speech/language disorder or reading disability, based on school assessments or a statistically significant discrepancy between IQ and reading test subscores or for a Word Reading subtest score below 85. To screen for differences in home environment, the Hollingshead Parent History Questionnaire assessment of socioeconomic status14 was used, and right-handedness was confirmed with the Edinburgh Handedness Inventory15 and the PANESS.2
Motor skill assessments of children with ADHD and TD children.
Study personnel at both sites trained together for consistency and reliability of motor assessments. Motor function was assessed comprehensively using 2 validated, age-normed batteries. The primary battery of interest for this study was the PANESS, as described recently.3 This test measures timed movements, lateral preference, motor overflow, dysrhythmia, coordination, gait, balance, and motor persistence. The Motor Assessment Battery in Children version 2 (MABC–2), developed to assess children's performance in areas of manual dexterity, ball skills, and balance, was used as a secondary measure and has also been validated as a developmental coordination battery in several populations.16
Motor cortex physiology.
TMS was used to measure motor evoked potential (MEP) amplitudes in intrinsic hand muscle (figure 1). We have previously shown that SICI is reduced in children with Tourette syndrome plus ADHD.5 The relationship between SICI and motor skill development in children has not been studied previously.
Figure 1. Transcranial magnetic stimulation (TMS) data from one typically developing child.
Two EMG tracings are shown: one on the left from a single TMS pulse and one on the right from a 3-ms paired pulse, for which the first, conditioning pulse is subthreshold intensity and the second pulse has the same suprathreshold intensity as the single pulse. The motor evoked potential (MEP) amplitudes, which represent a summation of motor cortex system output, are circled. In the dot plot, each point represents the TMS-evoked, peak-to-peak MEP amplitude in mV from a single trial, out of multiple trials in the same child, after tracings with motion artifact have been removed. Trial-to-trial variability is always high, so multiple trials are needed for a good estimate. As shown on the right, amplitudes evoked by the condition/test paired pulses are smaller on average. Short interval cortical inhibition (SICI) for each individual is calculated from the means of the trials under each condition. Thus, SICI equals mean paired/mean single pulse MEP amplitudes. SICI may also be calculated as a percent reduction, e.g., a ratio of 0.58 would be 42% inhibition.
Both sites performed single- and paired-pulse TMS using a Magstim 200 stimulator (Magstim Company, New York, NY) connected through a BiStim module to a double 70-mm coil. The coil was placed tangential to the skull with handle backward, at 45 degrees to the midline and its center near the optimal position and orientation for producing an MEP in the right first dorsal interosseous muscle. An electromyogram was recorded with surface electrodes, amplified, and filtered (100/1,000 Hz) (Coulbourn Instruments, Allentown, PA) before being digitized at 2 kHz and stored for analysis using Signal software and a Micro1401 interface (Cambridge Electronic Design, Cambridge, UK). For single-pulse vs paired-pulse studies, all individual tracings were analyzed blinded and off-line. Additional discussion of TMS methodology and specific methods used to reduce motion artifact are presented in appendix e-1 on the Neurology® Web site at www.neurology.org.
Resting motor threshold (RMT) and active motor threshold (AMT) were evaluated and defined using conventional criteria.17 SICI and intracortical facilitation (ICF) were measured together using standard methods.18 In brief, because intertrial variability is high,19 we performed 60 trials, with single or paired pulses administered at intervals of 6 (±10%) seconds, 20 each under one of 3 conditions in randomized order: 1) single (test) pulses; 2) SICI: paired pulses (condition/test) at an inhibitory 3-ms interval; and 3) ICF: paired pulses (condition/test) at an excitatory 10-ms interval. The test pulse intensity was set above RMT at an intensity that yielded MEP amplitudes of 0.5–1.5 mV, usually at about 20% above RMT. The conditioning pulse intensity was set below AMT, at 60% RMT.
The cortical silent period (CSP) was evaluated with the rectified, averaged EMG tracing resulting from 5 pulses at 50% above AMT,20 administered during a moderate first dorsal interosseous muscle contraction (∼50% maximum force). The onset and offset of the silent period, an epoch of EMG suppression/inhibition, were identified visually.
Statistical analyses.
Univariate analyses.
Children with ADHD vs TD children: behavior, motor function, motor physiology, clinical/demographic variables were compared.
Motor and behavioral scales scores were treated as quantitative variables. Demographics and IQ were compared across groups, and clinical and experimental data were compared across the 2 sites using t tests and χ2 tests as appropriate.
Group comparisons.
Behavior (ADHD total scores, inattention, and hyperactivity), motor physiology (thresholds, SICI, ICF, and CSP), and motor function (PANESS total, PANESS subscales, and MABC–2 age percentiles) were characterized (mean and SD) and compared between children with ADHD and typical children, using t tests.
Correlations for age/maturation.
Nonparametric (Spearman) correlations of age (in months), ADHD symptoms, motor physiology, and motor function were explored within the entire sample and stratified by diagnosis. To assess for possibly confounding medication effects (stimulant withdrawal), post hoc t test comparisons were made between the following groups: 1) non-ADHD, 2) ADHD-nontreated, and 3) ADHD treated.
Primary multivariate analyses: SICI and ADHD.
To address collinearity between PANESS and age, age was centered. To further assess for possible bias and confounding effects, cognitive and socioeconomic variables were included in initial regression modeling. Stepwise logistic regression was performed using SAS (version 9.2; SAS Institute Inc, Cary, NC) PROC LOGISTIC with the diagnosis of ADHD as the dependent variable to model associations between age, SICI, other motor physiologic measures, and motor function on the odds of a categorical diagnosis of ADHD. Variables were included on the basis of univariate analysis results. Criteria for model entry were p = 0.1 and to remain p < 0.05.
Stepwise linear regression using ADHD subject data (only) and SAS 9.2 PROC GLM, with the CPRS ADHD T score as the dependent variable, was used to model contributions of SICI, motor function, and age to ADHD severity.
RESULTS
Subjects.
Demographic information about the study sample is shown in table 1. Motor physiology and ratings of motor function did not differ across sites (data not shown). The recruited sample included 104 children (Cincinnati: 43 ADHD and 31 TD; Baltimore: 9 ADHD and 21 TD). Of these, there were 6 children (3 ADHD and 3 TD) in whom no motor physiology results could be obtained because of high RMTs. CSP (at 1.5 × AMT) was obtainable in 77 children (46 TD), and paired-pulse data were evaluable in 82 children (44 TD).
Table 1.
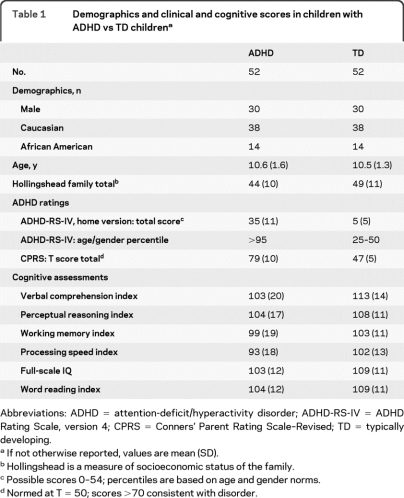
Demographics and clinical and cognitive scores in children with ADHD vs TD childrena
Abbreviations: ADHD = attention-deficit/hyperactivity disorder; ADHD-RS-IV = ADHD Rating Scale, version 4; CPRS = Conners' Parent Rating Scale–Revised; TD = typically developing.
If not otherwise reported, values are mean (SD).
Hollingshead is a measure of socioeconomic status of the family.
Possible scores 0–54; percentiles are based on age and gender norms.
Normed at T = 50; scores >70 consistent with disorder.
Motor function and physiology in children with ADHD vs TD children.
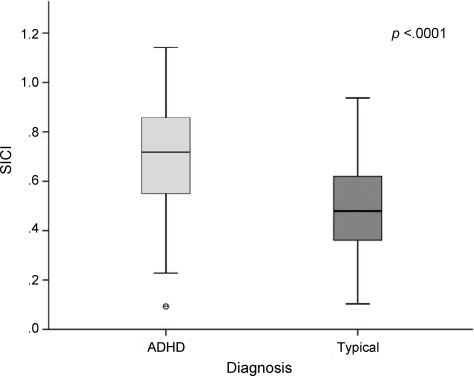
Motor physiology and function are shown in table 2. SICI was significantly less in children with ADHD than in TD children (figure 2), and total PANESS scores were higher (table 2), demonstrating greater motor dysfunction in children with ADHD than in TD children aged 8–12 years. Children with ADHD who were currently being treated (chronically taking stimulants but discontinued temporarily for the study) and untreated (no recent stimulant use) were statistically no different (p = 0.65). Treated (p < 0.0001) and untreated (p = 0.0001) ADHD groups both had less SICI than the TD group. Motor thresholds diminish significantly between ages 8 and 12 years. Age effects on motor function and physiology are shown in table e-1.
Table 2.
Motor physiology and motor function in children with ADHD vs TD children
Abbreviations: ADHD = attention-deficit/hyperactivity disorder; AMT = active motor threshold; CSP = cortical silent period; ICF = intracortical facilitation; MABC–2 = Motor Assessment Battery for Children version 2; PANESS = Physical and Neurological Examination for Subtle Signs; RMT = resting motor threshold; SICI = short interval cortical inhibition; TD = typically developing.
SICI ratio numerator is group mean motor evoked potential amplitude after 3-ms paired pulses (20 trials per subject) and denominator is group motor evoked potential amplitude after single pulses (20 trials per subject), so ratio of 1.0 indicates no inhibition.
Significant differences.
SICI % is (1 − ratio) × 100.
Raw scores; higher scores indicate more significant delays.
Percentiles are for age strata.
Figure 2. Short interval cortical inhibition (SICI) is significantly reduced in children with attention-deficit/hyperactivity disorder (ADHD) vs typical children.
Larger ratios indicate less paired-pulse vs single-pulse inhibition (see figure 1).
Univariate correlations between SICI, ADHD symptom severity, and motor function.
Within the ADHD group, less SICI (higher ratios) correlates with more severe ADHD symptoms (figure 3). Additional correlations between ADHD subscale scores and motor physiologic measures are shown in table e-2.
Figure 3. Short interval cortical inhibition (SICI) correlates with behavioral ratings in children with attention-deficit/hyperactivity disorder (ADHD).
Higher Conners' Parent Rating Scale–Revised T scores indicate more severe ADHD symptoms, adjusted for age and gender. Higher SICI ratios indicate less inhibition.
Multivariate analysis: Predictors of ADHD categorical diagnosis and severity.
Categorical ADHD.
In the final logistic regression model, higher PANESS total score (p < 0.0001), less SICI (p = 0.0026), and shorter CSP (p = 0.013) were associated with odds of ADHD (model Wald χ2 = 19.3, p = 0.0002). Gender, age, socioeconomic factors, other MABC–2 measures, and other physiologic measures were not significant.
ADHD severity.
Multivariate regression within the full cohort, using the ADHD-RS-IV scores (range 0–54) as a dependent variable (to evaluate ADHD symptoms as a dimension in all children), shows that PANESS (p < 0.0001), SICI (p = 0.0080), and CSP (p = 0.033) account for the variance. Using the square of the partial correlations, we estimate that PANESS accounts for 26.3%, SICI for 13.3%, and CSP for 6.7% of the variance of ADHD severity within the full cohort. Within the ADHD cohort, by using the CPRS T score (accounts for age and gender) as the dependent variable, in the final linear regression model only SICI accounted for ADHD severity (r2 = 0.24; F1,30 = 9.45, p = 0.0045).
Likewise, using PANESS total as the dependent variable in the whole cohort, only categorical diagnosis and age remained in the model: PANESStotal = diagnosis(ADHD, typical) + age (r2 = 0.29; F2,85 = 17.47, p < 0.0001).
DISCUSSION
This study identified significantly diminished SICI in dominant motor cortex in 8- to 12-year-old children with ADHD. We identified this finding after comprehensive assessments of cognition, learning, and behavior, including screening for and statistically analyzing IQ, reading ability, and socioeconomic status, in a cohort in which other neurologic, psychiatric, developmental, or medical problems that might act as confounders were excluded. This finding was statistically robust in both univariate and multivariate analyses. Correlation with parent-rated hyperactivity symptoms was more substantial than that for inattention. This is the largest and most completely characterized sample of children to undergo TMS testing. Our results suggest that motor cortex SICI is a quantitative, biologically based marker of both the categorical diagnosis of ADHD and the severity of ADHD symptoms in children at an age during which rapid motor and physiologic development is occurring.
SICI also correlated, although less strongly, with development of motor skills. The more modest degree of SICI/motor function correlation is not surprising. The neural substrate for the PANESS is diffuse, involving not only frontal lobes but also other cerebral cortical, basal ganglia, and cerebellar structures. In contrast, SICI probably reflects more localized inhibitory neurons in or adjacent to the motor cortex. These are more anatomically proximate to prefrontal regions involved in attention, impulse control, and behavioral regulation. In relating a physiologic marker to behavioral or motor scales, it is important to keep in mind that rating scales are only semiquantitative. More importantly, behavioral diagnoses are syndromes that may result from many processes and in which symptoms will overlap with typical peer behavior. Therefore, there will always be overlap between case patients and control subjects and at most modest correlations.21
SICI is believed to result from γ-aminobutyric acid (GABA)A-mediated inhibition at the level of the primary motor cortex.22 The surround inhibition produced by GABAergic interneurons and modulated by dopamine is believed to provide an increased signal-to-noise ratio in the frontal cortex.23 This finding may be important for refining cortical signals involved in the accurate selection and control of motor responses. Because in healthy adults SICI is upregulated by both dopaminergic agonists24 and GABAA agonists22 and because psychostimulants also alter SICI (although in complex ways influenced possibly by age, dose, diagnosis, and genetic factors), we speculate that SICI may provide a window into further investigations to the role of both GABA and GABA/dopamine interactions in frontal cortex behavioral and motor dysfunction in ADHD. Studies of SICI in healthy adults suggest that this may be relevant to motor task inhibition25 and reward expectation.26 Difficulties with response selection and control (including increased inhibitory failure and variability) are hallmarks of ADHD,27 as are alterations in reward motivation.28 Refinement of neural signals critical to developing appropriate response selection and control, particularly in response to reward, may be critical to development of appropriate control of impulsive, hyperactive, and off-task behavior. Decreased SICI may thereby reflect a mechanism critical to the pathophysiology of hyperactive impulsive behavior.
SICI appears to be heritable.29 ADHD also tends to run in families, and our findings show SICI to be robustly predictive of its core diagnostic features. Still, SICI reflects processes important in other brain disorders as well. Diminished SICI has been described in Tourette syndrome,30 Parkinson disease,31 dystonia,32 schizophrenia,33 and juvenile myoclonic epilepsy in the morning.34 However, to date, the only strong correlation between SICI and symptom severity has been demonstrated with ADHD, as we have previously noted in smaller samples of children with Tourette syndrome.5 A novel and important contribution of the present study is characterization of a relationship between SICI and a highly relevant, developmental motor function, the PANESS score. The diversity of associations between neurologic diagnoses and SICI suggests that SICI may reflect fundamental problems in the frontal lobe–based executive function shared in many neurologic and psychiatric conditions, possibly in some cases as they relate to dopamine or to GABA neurotransmission.
Both ADHD and TD cohorts were samples of convenience and may include some unknown referral biases. We found no evidence that the SICI/ADHD effect is accounted for by use of stimulant medication or medication withdrawal, because, after subdividing the ADHD group by current stimulant treatment status, both groups remained robustly different from the non-ADHD group. Based on our sample size and detailed phenotypic analysis, we believe that our findings are probably generalizable. An additional possible confounder in this or any hyperkinetic disorder is activity level during the TMS study. Movement or premovement preparation increases MEP amplitudes and decreases SICI.25,35 If children with ADHD were more likely to move during the study, this movement would increase the group differences. We do not think increased ADHD-related fidgeting and movement explain our findings, however, based on the measures we took, as described in greater detail in appendix e-1.
Methodologic decisions about TMS stimulus intensities as well as interstimulus intervals probably affected paired-pulse ratios but did not substantially affect group differences or correlations in this study. The conditioning pulse intensity affects the magnitude of SICI and ICF in healthy adults.36 However, because the ADHD vs TD group differences and correlation with symptom severity we found were similar to those in prior studies,5,6 and, in addition, demonstrated correlation with PANESS scores, we think this conditioning pulse intensity yields valid, generalizable results for children.
Facilitatory effects in paired-pulse studies are also of some relevance. In this study, neither group generated much ICF. This observation means that early ICF effects probably did not “contaminate” the SICI result, but higher conditioning intensity would probably have been a better choice for evaluating ICF.36 Likewise, short interval cortical facilitation, which can occur at 3-ms interstimulus intervals, tends to occur more efficiently at far higher conditioning stimulus intensities.37,38 Therefore, our study was not optimal for assessing paired-pulse facilitation but probably yielded SICI data that were relatively uncontaminated by facilitatory processes.
High resting thresholds reduced our final sample size and may have reduced the precision of our results in the youngest children. In addition, although the comprehensive evaluation and strict exclusions in this study may have enhanced our ability to detect ADHD-related differences, it is possible that some components of our findings will not generalize well to a broader ADHD population with more complex emotional or learning problems. In addition, it is not known to what extent, if any, psychostimulants affect SICI after washout. We do not think that this effect is likely to be profound, because our results are similar to those in small studies of treated patients with more complex conditions5 as well as untreated populations.39
This study was cross-sectional, and a longitudinal study might provide more readily interpretable insights into the relationship between age-related motor development, motor physiology, and ADHD. Given that ADHD symptoms are well established by age 8 years, it would be helpful to evaluate children at a much younger age. This work will probably have to wait for advances in coil design and comfort. Large longitudinal studies might clarify the extent to which SICI or other measures indicate compensatory processes40 vs deficiencies.39 In addition, studies in which SICI is evaluated during tasks may be important. For example, a recently published, small study in healthy adults showed that SICI is reduced when an individual is attending to a task and then increases in response to anticipation of reward during this task.26 This finding suggests that mechanisms measured by SICI may engage in attention and reward processing. Such studies in ADHD in children might further enhance our understanding of SICI as a quantitative, biologically based marker of ADHD symptoms.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Martha Denckla for guidance, Drs. Eric Wassermann, Marjorie Garvey, and Robert Chen for discussions about the data, research coordinators for technical assistance, and the children and parents for their time and participation.
- ADHD
- attention-deficit/hyperactivity disorder
- ADHD-RS-IV
- ADHD Rating Scale IV
- AMT
- active motor threshold
- CPRS
- Conners' Parent Rating Scale–Revised;
- CSP
- cortical silent period
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- GABA
- γ-aminobutyric acid
- ICF
- intracortical facilitation
- MABC–2
- Motor Assessment Battery in Children version 2
- MEP
- motor evoked potential
- PANESS
- Physical and Neurological Examination for Subtle Signs
- RMT
- resting motor threshold
- SICI
- short interval cortical inhibition
- TD
- typically developing
- TMS
- transcranial magnetic stimulation.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Donald L. Gilbert.
DISCLOSURE
Dr. Gilbert has received honoraria from the Tourette Syndrome Association/Centers for Disease Control and Prevention, the Movement Disorder Society, the American Academy of Neurology, and the American Academy of Pediatrics; serves on the medical advisory board for the Tourette Syndrome Association; writes board review questions for PREP SA (American Academy of Pediatrics); and has received research support from the NIH (NIMH R01 MH078160 , NIMH R01 MH08185, and NINDS NS056276) and from the Cincinnati Children's Hospital Research Foundation, the University of Cincinnati, and the Tourette Syndrome Association. K.M. Isaacs, M. Augusta, and L.K. MacNeil report no disclosures. Dr. Mostofsky has served on a scientific advisory for Bristol-Myers Squibb; serves on the editorial board of Neurocase; and receives research support from the NIH (NIMH R01 MH085328 [PI], NINDS R01 NS048527 [PI], NIMH R01 MH078160 [PI], and NICHD P50 HD052121 [coinvestigator]) and Autism Speaks.
REFERENCES
- 1. Denckla MB, Rudel RG. Anomalies of motor development in hyperactive boys. Ann Neurol 1978;3:231–233 [DOI] [PubMed] [Google Scholar]
- 2. Denckla MB. Revised neurological examination for subtle signs (1985). Psychopharmacol Bull 1985;21:773–800 [PubMed] [Google Scholar]
- 3. Cole WR, Mostofsky SH, Larson JCG, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology 2008;71:1514–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Molina BS, Flory K, Hinshaw SP, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. J Am Acad Child Adolesc Psychiatry 2007;46:1028–1040 [DOI] [PubMed] [Google Scholar]
- 5. Gilbert DL, Bansal AS, Sethuraman G, et al. Association of cortical disinhibition with tic, ADHD, and OCD severity in Tourette syndrome Mov Disord 2004;19:416–425 [DOI] [PubMed] [Google Scholar]
- 6. Gilbert DL, Wang Z, Ridel KR, et al. Dopamine transporter genotype influences the physiological response to medication in ADHD. Brain 2006;129:2038–2046 [DOI] [PubMed] [Google Scholar]
- 7. Buchmann J, Wolters A, Haessler F, Bohne S, Nordbeck R, Kunesch E. Disturbed transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD). Clin Neurophysiol 2003;114:2036–2042 [DOI] [PubMed] [Google Scholar]
- 8. Moll GH, Heinrich H, Trott G, Wirth S, Rothenberger A. Deficient intracortical inhibition in drug-naive children with attention-deficit hyperactivity disorder is enhanced by methylphenidate. Neurosci Lett 2000;284:121–125 [DOI] [PubMed] [Google Scholar]
- 9. Reich W. Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry 2000;39:59–66 [DOI] [PubMed] [Google Scholar]
- 10. DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklist, Norms, and Clinical Interpretations. New York: Guilford Press; 1998 [Google Scholar]
- 11. Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners' Parent Rating Scale (CPRS–R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:257–268 [DOI] [PubMed] [Google Scholar]
- 12. Wechsler D. Wechsler Intelligence Scale for Children, 4th ed. San Antonio, TX: Psychological Corporation; 2003 [Google Scholar]
- 13. Wechsler D. Wechsler Individual Achievement Test Examiner's Manual, 2nd ed. San Antonio, TX: Psychological Corporation; 2002 [Google Scholar]
- 14. Hollingshead AA. Four-factor index of social status. New Haven, CT: Yale University; 1975. Available at: http://www.yale-university.com/sociology/faculty/docs/hollingshead_socStat4factor.pdf Accessed March 1, 2006 [Google Scholar]
- 15. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 16. Henderson SE, Sugden DA. Movement Assessment Battery for Children: Manual. London: Harcourt Assessment; 1992 [Google Scholar]
- 17. Mills KR, Nithi KA. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve 1997;20:570–576 [DOI] [PubMed] [Google Scholar]
- 18. Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;471:501–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol 2001;112:931–937 [DOI] [PubMed] [Google Scholar]
- 20. Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol 2004;115:1076–1082 [DOI] [PubMed] [Google Scholar]
- 21. Gilbert DL. Design and analysis of motor-evoked potential data in pediatric neurobehavioural disorder investigations. In: Wassermann EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH. eds. The Oxford Handbook of Transcranial Magnetic Stimulation. Oxford, UK: Oxford University Press; 2008:389–400 [Google Scholar]
- 22. Di Lazzaro V, Pilato F, Dileone M, et al. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol 2006;575:721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci 2004;27:683–690 [DOI] [PubMed] [Google Scholar]
- 24. Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroencephalogr Clin Neurophysiol 1997;105:430–437 [DOI] [PubMed] [Google Scholar]
- 25. Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 2006;95:3371–3383 [DOI] [PubMed] [Google Scholar]
- 26. Kapogiannis D, Campion P, Grafman J, Wassermann EM. Reward-related activity in the human motor cortex. Eur J Neurosci 2008;27:1836–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wodka EL, Mahone EM, Blankner JG, et al. Evidence that response inhibition is a primary deficit in ADHD. J Clin Exp Neuropsychol 2007;29:345–356 [DOI] [PubMed] [Google Scholar]
- 28. Volkow ND, Wang GJ, Kollins SH, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA 2009;302:1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pellicciari MC, Veniero D, Marzano C, et al. Heritability of intracortical inhibition and facilitation. J Neurosci 2009;29:8897–8900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orth M, Rothwell JC. Motor cortex excitability and comorbidity in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry 2009;80:29–34 [DOI] [PubMed] [Google Scholar]
- 31. Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Ann Neurol 1995;37:181–188 [DOI] [PubMed] [Google Scholar]
- 32. Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry 1995;59:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry 2002;59:347–354 [DOI] [PubMed] [Google Scholar]
- 34. Badawy RA, Macdonell RA, Jackson GD, Berkovic SF. Why do seizures in generalized epilepsy often occur in the morning? Neurology 2009;73:218–222 [DOI] [PubMed] [Google Scholar]
- 35. Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol 1998;44:317–325 [DOI] [PubMed] [Google Scholar]
- 36. Orth M, Snijders AH, Rothwell JC, et al. The variability of intracortical inhibition and facilitation. Clin Neurophysiol 2003;114:2362–2369 [DOI] [PubMed] [Google Scholar]
- 37. Udupa K, Ni Z, Gunraj C, Chen R. Effect of long interval interhemispheric inhibition on intracortical inhibitory and facilitatory circuits. J Physiol 2010;588:2633–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peurala SH, JF MM-D, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF). Clin Neurophysiol 2008;119:2291–2297 [DOI] [PubMed] [Google Scholar]
- 39. Moll GH, Heinrich H, Trott G, Wirth S, Bock N, Rothenberger A. Children with comorbid attention-deficit-hyperactivity disorder and tic disorder: evidence for additive inhibitory deficits within the motor system. Ann Neurol 2001;49:393–396 [PubMed] [Google Scholar]
- 40. Gilbert DL, Zhang J, Lipps TD, et al. Atomoxetine treatment of ADHD in Tourette syndrome: reduction in motor cortex inhibition correlates with clinical improvement. Clin Neurophysiol 2007;118:1835–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.