Abstract
The role of the maternal immune system during pregnancy has focused mainly on the aspect of immune tolerance to the invading trophoblast and, therefore, fetus. While this is a critical aspect of reproductive immunology, it is also important to consider the function of the maternal immune system in the promotion of implantation and maintenance of pregnancy. Apoptosis or cell death is not the final stage in tissue development. The quick and effective removal of apoptotic cells by tissue macrophages represents a vital process preventing "leak" of self-antigens and promoting the production of proliferative/survival factors. One of the key requirements of apoptotic cell clearance is the resolution of inflammatory conditions, which, as in the case of pregnancy, may have lethal consequences. This review will focus on decidual macrophages and their role on apoptosis and cell clearance during pregnancy.
Introduction
The role of the maternal immune system during pregnancy has focused mainly on the aspect of immune tolerance to the invading trophoblast and, therefore, fetus. While this is a critical aspect of reproductive immunology, it is also important to consider the function of the maternal immune system in the promotion of implantation and maintenance of pregnancy. This review will focus on decidual macrophages and their role on apoptosis and cell clearance during pregnancy.
Leucocytes at the implantation site
During normal pregnancy the decidua is populated by a variety of leucocytes [1], however, cells of the innate immune system seem to dominate this tissue since the levels of lymphocytes are relatively low (1–3%) [2]. At the time of implantation, many of the leucocytes are NK cells, expressing a phenotype distinct from those found in the periphery [3]. As gestation proceeds, NK cell numbers decline and at term these leucocytes are absent [4]. Macrophages constitute 20–30% of the decidual cells at the site of implantation [5,2,7] and unlike NK cells, remain high throughout pregnancy [2,8]. This evidence suggests that the innate immune system is not indifferent to the fetus and may have a role not only in host protection to infections, but also as important players in the feto-maternal immune adjustment. An important aspect in this process is the establishment of an adequate microenvironment that will promote cell grow and will inhibit hazardous inflammatory immune reactions.
Microenvironment of the implantation site
The local environment of the maternal-fetal interface is characterized, not only by the cell types present, but also by the soluble factors produced therein. The production and effects of cytokines at the implantation site is important for the regulation of trophoblast cell grow, differentiation and invasion [9-11]. In normal pregnancies, particularly at the maternal fetal interface, anti-inflammatory, Th-2, cytokines predominate [12,13] and, therefore, an appropriate balance between pro-inflammatory and anti-inflammatory cytokines is thought to be crucial for determining the success or failure of a pregnancy [11]. It is currently believed that for the continuous normal development of pregnancy, production of inflammatory cytokines such as, inerleukin-2 (IL-2), tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) is suppressed, whereas production of anti-inflammatory cytokines such as, IL-4, IL-6 or IL-10 is enhanced [12]. Placental and decidual tissues from normal pregnancies have been shown to express both pro- and anti-inflammatory cytokines [14-17]. However, pro-inflammatory, Th-1, cytokines appear to be potentially harmful to pregnancy since excess production of TNF-α or IFN-γ has been associated with pre-term delivery [17-20]. Similarly, low levels of decidual IL-4 and IL-10 have been observed in women suffering from unexplained recurrent abortions and where spontaneous abortion has occurred during the first trimester of pregnancy [19].
Immune cells, especially the macrophages, are a main source of cytokines and growth factors and contribute to the maintenance of the adequate balance between Th1 and TH2 cytokines at the placental bed.
Apoptosis and implantation
Apoptosis, or programmed cell death, is a natural mechanism by which the body eliminates unnecessary or potentially dangerous cells in order to maintain normal tissue function. During implantation, apoptosis is important for the appropriate tissue remodeling of the maternal decidua and invasion of the developing embryo [7]. Although, first trimester trophoblasts are resistant to Fas-stimulation, apoptosis has been described in the trophoblast layer of placentas from uncomplicated pregnancies throughout gestation, suggesting that there is a constant cell turnover at the site of implantation necessary for the appropriate growth and function of the placenta [21-23]. In addition, the incidence of trophoblast apoptosis is higher in third trimester villi compared to first trimester placenta [24], suggesting that increasing placental apoptosis may be involved in the process of parturition. In pregnancies complicated by preeclampsia or intrauterine growth restriction (IUGR), there is a greater incidence of placental apoptosis in the first trimester, which is accompanied by insufficient trophoblast invasion [23,25]. Several mechanisms, in addition to apoptosis, have been described to limit extravillous trophoblast invasion into the uteroplacental arteries. These include reduced expression of integrin α1β1 [26] decreased secretion of metalloproteinase (MMP-9) [27] and low cell surface plasminogen activator activity, absent expression of vascular endothelial cadherin [26] and reduced expression of HLA-G [28]. The reduction of these factors, necessary for trophoblast differentiation and invasion is compatible with the hypothesis of increased apoptosis in IUGR because it is known that once the cells, including trophoblast, enter the apoptotic cascade this down regulate their level of transcription [29]. This data suggests that the regulation of placental apoptosis is essential for the normal physiology of pregnancy.
However, cell death by apoptosis is not the end of the story, the clearance of apoptotic bodies represents a critical step in tissue homeostasis, preventing the release of intracellular contents, which may cause tissue damage and the possibility to initiate an inflammatory reaction.
Clearance of apoptotic cells
Different morphological changes accompany the execution of the apoptotic program. Cells first become round and detach from their neighbors. Then, condensation of both the nucleus and cytoplasm occurs without major modification to the other intracellular organelles. Following condensation, nuclear fragmentation and membrane blebbing is observed, resulting in the formation of apoptotic bodies with intact membranes. These morphological changes are a translation of the biochemical modifications, mediated by the activation of the caspase cascade, that are occurring inside of the cell.
Another important cellular change that occurs during apoptosis is the redistribution of membranal proteins, which will allow macrophages to recognize apoptotic cells and direct the phagocytic process. Several receptors have been implicated in the recognition and engulfment of apoptotic cells [30,31], suggesting that the process of phagocytosis is well regulated and functionally relevant.
As discussed above, implantation and trophoblast invasion is characterized by a progressive, continuous induction of apoptosis in the maternal tissue surrounding the fetus [32]. During this period, numerous macrophages are present at the implantation site and this was originally thought to represent an immune response against the invading trophoblast. However, we propose that this may not be the case. We suggest that macrophage engulfment of apoptotic cells prevents the release of potentially pro-inflammatory and pro-immunogenic intracellular contents that occurs during secondary necrosis (See Figure 1). Due to the allogenic nature of the placenta, this process may be essential for the well being of the fetus. Trophoblast cells are carriers of proteins, which are antigenically foreign to the maternal immune system and if released, as result of cell death, may initiate or accelerate immunological responses with lethal consequences for the fetus. Therefore, the appropriate removal of dying trophoblast cells prior to the release of these intracellular components is critical for the prevention of fetal rejection. Macrophages are a key cellular constituent of this process.
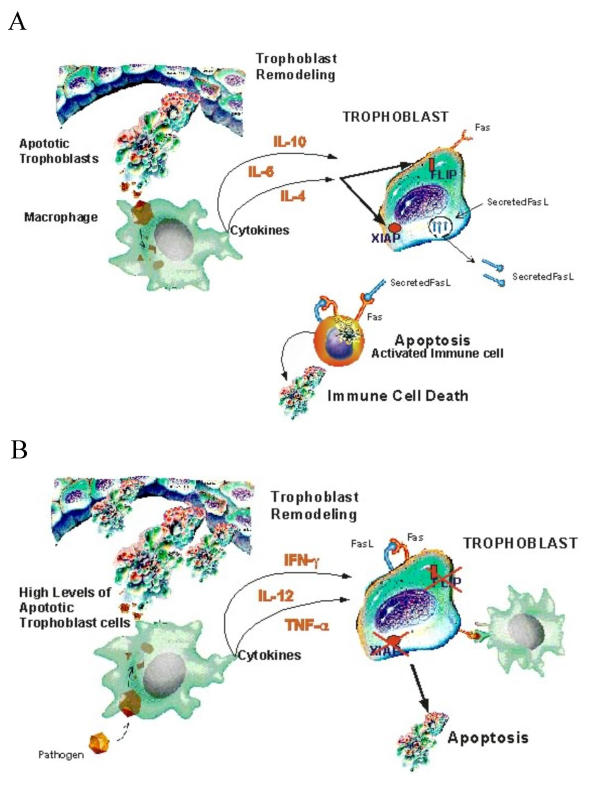
Figure 1.
Effect of clearance of apoptotic cells by macrophages. A. Clearance of apoptotic cells induce the expression, by macrophages, of anti-inflammatory cytokines with protective effects on trophoblast survival and immunological tolerance. B. Changes in the cytokine milieu, owing to elevated levels of apoptotic bodies and inefficient clearance, will result in a pro-inflammatory microenvironment that in turn may result in changes in trophoblast resistance to Fas-mediated apoptosis and the maternal immune system
Macrophages and clearance of apoptotic cells
The removal of cellular debris, generated as a result of apoptosis, is a challenging task that must be performed to maintain cellular homeostasis. This clearance process, far from being the end, represents an active and coordinate event which sends specific signals to the remaining cells, either for survival or death [33]. The removal of apoptotic bodies is neither a neutral nor a passive process, but rather an active physiological event that may influence not only immune responses, but also the proliferation and differentiation of surrounding cells [31]. Consequently, a signal from a macrophage to the wrong cell type may have profound consequences for the normal physiology of the tissue [34].
Recent reports have shown that binding to, and ingestion of, apoptotic cells by phagocytes can result in active immunosuppressive and anti-inflammatory responses. Voll et al [35] found that monocyte secretion of TNFα was inhibited, while production of TGFβ and IL-10 was increased following co-culture of monocytes with apoptotic lymphocytes. Furthermore, in vivo studies have clearly demonstrated that the TGFβ released by macrophages ingesting apoptotic cells, has anti-inflammatory effects in inflamed peritoneum and lungs [36]. Furthermore, the capacity for macrophages to influence cell death may be regulated by the extent of uptake of apoptotic cells [31]. Duffield et al demonstrated that the capacity of macrophages stimulated with IFNγ and LPS or TNFα to induce glomerular cells apoptosis could be suppressed by the uptake of apoptotic cells [37].
Macrophages at the maternal-fetal interface
Histological analysis of normal placental beds shows the presence of large number of macrophages, which are localized, in the majority of the cases, in the vicinity of apoptotic cells. Indeed, macrophages are one of the major cell types in both the maternal and fetal compartments of the uteroplacental unit [38]. In humans, during the first weeks of implantation, macrophages are found in high numbers in the maternal decidua and in tissues close in proximity to the placenta [39]. Similarly in rodents, macrophages accumulate at or near the implantation site [40]. The dense macrophage-infiltration at the maternal fetal interface suggests that these cells are also involved in specific pregnancy-associated functions, and not only to perform their usual immunological tasks [41]. Hunt and co-workers have implied that maternal macrophages assist in the tissue remodeling necessary to accommodate expansion of extraembryonic tissue [42]. However, macrophages are not merely scavengers of dying cells, but also actively orchestrate apoptosis of unwanted cells during tissue remodeling [31]. Macrophages synthesize and secrete cytokines and growth factors, which govern the local cellular and tissue interactions [39,42-44]. They also respond to hormonal factors affecting their function and survival [45,46].
Numerous findings indicate that the capacity for macrophages to influence cell death is regulated by the extent of uptake of apoptotic cells [31]. For example, the cytolysis of tumor cells by activated macrophages is inhibited by the ingestion of apoptotic but not necrotic cells [47]. Similarly, during embryo implantation, uterine epithelial cells surrounding the blastocyst undergo apoptosis and may form an anti-inflammatory environment by increasing Th-2 type cytokines. This may explain the surprising cohabitation of macrophages and trophoblast cells at the implantation site. As stated earlier, the type of cytokines produced by a macrophage depends on its activation state [48]. We propose that during normal pregnancy, the uptake of apoptotic cells suppresses activated macrophages from secreting pro-inflammatory cytokines such as TNF-α and IFN-γ and promotes the release of Th-2 type, anti-inflammatory and immunosuppressive cytokines (Figure 1A). In pregnancies complicated with preeclampsia or IUGR, activated macrophages secrete pro-inflammatory cytokines such as TNF-α and IFN-γ and induce apoptosis in extravillous trophoblast (Figure 1B). This hypothesis is supported by a recent report of Pijnenborg et al [49] who found a higher incidence of cell clusters secreting TNF-α, probably macrophages, in the placental bed of patients with severe forms of preeclampsia.
Macrophages are also located near the spiral arteries during trophoblast invasion and transformation. Previous studies, and ours, of placental bed specimens, demonstrate changes in the distribution of macrophages during pathologic conditions such as preeclampsia [50,51]. While in normal pregnancies macrophages are located in the stroma surrounding the transformed spiral arteries and extravillous trophoblast; in preeclamsia macrophages are located within and around the spiral arteries separating them from the trophoblast cells. Their distribution resembles a barrier between the invading trophoblast and the spiral arteries (See Figure 2). In addition, Resiter et al [51] have reported that macrophages residing in excess in the placental bed of preeclamptic women are able to limit extravillous trophoblast invasion of spiral arteries segments through apoptosis mediated by the secretion of TNFα. We propose a differential role for uterine macrophages during trophoblast invasion/differentiation, according to their stage of activation. In normal pregnancies, macrophages function as support cells by facilitating trophoblast invasion through the placental bed. In pathologic conditions, macrophages function as a barrier for trophoblast invasion and differentiation by inducing trophoblast apoptosis and therefore preventing spiral arteries transformation (Figure 2).
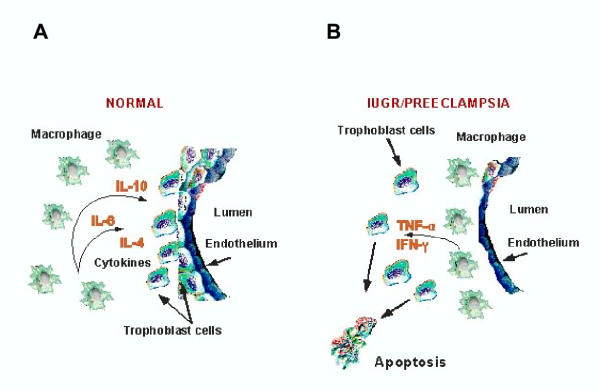
Figure 2.
Differential Distribution of macrophages in normal pregnancy and pregnancy complicated with preeclamsia and IUGR. While in normal pregnancies macrophages are located in the stroma surrounding the transformed spiral arteries and extravillous trophoblast (A) ; in pre-eclampsia macrophages are located within and around the spiral arteries separating them from the trophoblast cells (B). In the normal condition, macrophages promote trophoblast survival; while in the pathologic state induce apoptosis
Role of apoptotic cell phagocytosis in pregnancy-associated diseases
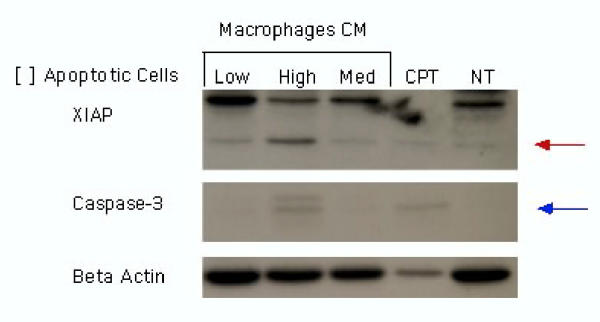
The anti-inflammatory action of phagocytic clearance of apoptotic cells may be perturbed in disease processes. Anti-phospholipid antibodies in apoptotic cells may bind to surface expressed Fc receptors on macrophages resulting in secretion of pro-inflammatory cytokines such as, TNF-α, [52]. Therefore, during pregnancy, cytokine production by macrophages and other cells at the maternal-fetal interface may be drastically altered [53]. The results of our studies are in concert with this concept and indicate that enhanced levels of pro-inflammatory macrophage products increase Fas expression and enhance trophoblast sensitivity to Fas-mediated apoptosis [54,55].We have also demonstrated that the factors produced as a result of phagocytosis of apoptotic cells have a significant impact on viable trophoblast cells and that this is dependent on the levels of apoptotic cells. We have developed an in vitro system consisting of monocyte-derived macrophages and apoptotic first trimester trophoblast cells. We find that macrophages successfully engulf apoptotic trophoblast cells in vitro (Figure 3). In addition, trophoblasts cells treated with conditioned media collected from monocyte-derived macrophages, co-cultured with low numbers of apoptotic trophoblast expressed the active form of XIAP, an inhibitor of apoptosis, and showed no caspase-3 activation. This supports the hypothesis that clearance of apoptotic trophoblast cells by macrophages may protect the expanding trophoblast population from cell death during pregnancy. However, trophoblasts cells treated with conditioned media collected from macrophages co-cultured with high levels of apoptotic trophoblast cells expressed the inactive from of XIAP and the active forms of caspase-3 (Figure 4). This suggests that an excess of apoptotic bodies may stimulate macrophages to produce pro-apoptotic factors. Alternatively, apoptotic cells undergoing secondary necrosis as a result of a failure of efficient clearance may directly influence trophoblast cell viability. Therefore, an increase in the levels of trophoblast apoptosis, possibly a result of infection, may initiate an inflammatory event that will further promote trophoblast cell death preventing normal trophoblast invasion, spiral arteries transformations and fetal survival. Such may be the case in pathologies such as preeclampsia and IUGR.
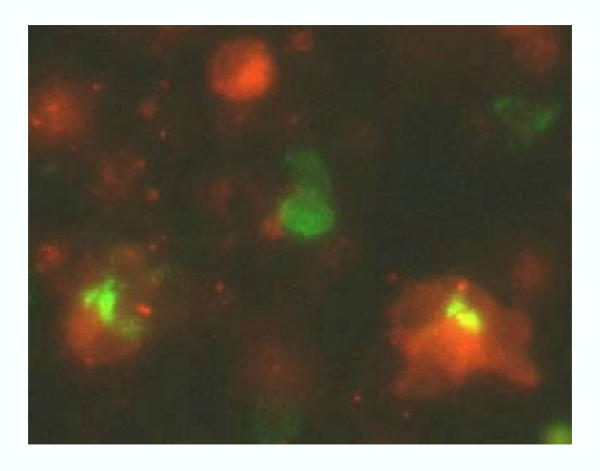
Figure 3.
Macrophages phagocytose apoptotic first trimester trophoblasts. Apoptotic trophoblasts cells (green) are engulfed by THP-1-differentiated macrophages (red).
Figure 4.
Phagocytosis of apoptotic cells by macrophages influences trophoblast apoptosis. Trophoblast cells were treated with macrophage condition media. Caspase 3 and XIAP expression was determined after 24 h incubation. NT: Control, CPT: Trophoblast cells treated with camptothecin. Low, High Med: Condition media from macrophages treated with either low, high and medium concentration of apoptotic cells.
Conclusion
Apoptosis or cell death is not the final stage in tissue development. The quick and effective removal of apoptotic cells by tissue macrophages represents a vital process preventing "leak" of self-antigens and promoting the production of proliferative/survival factors. One of the key requirements of apoptotic cell clearance is the resolution of inflammatory conditions, which, as in the case of pregnancy, may have lethal consequences. The clearance of the apoptotic trophoblasts at the implantation site by macrophages and its effect on the well being of the placenta throughout pregnancy is a novel area, which warrants investigation. The field of apoptotic cell clearance is beginning to flourish, and many questions remain unanswered.
Acknowledgments
Acknowledgement
These studies were supported by grants from the National Institutes of Health: RO1HD37137 and RO1-CA092435-01
Contributor Information
Gil Mor, Email: gil.mor@yale.edu.
Vikki M Abrahams, Email: Vikki.Abrahams@yale.edu.
References
- Mincheva-Nilsson L, Baranov V, Yeung M, Hammarstrom S, Hammarstrom ML. Immunomorphologica studies in human decidua-associated lymphoid cells in normal early pregnancy. J Immuno. 1994;152:2020–2032. [PubMed] [Google Scholar]
- Lessin DL, Hunt JS, King CR, Wood GW. Antigen expression by cells near the maternal-fetal interface. Am J Reprod Immunol Microbiol. 1988;16:1–7. doi: 10.1111/j.1600-0897.1988.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Loke YW, King A. Immunology of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:827–837. doi: 10.1053/beog.2000.0122. [DOI] [PubMed] [Google Scholar]
- Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- Kabawat SE, Mostoufi-Zadeh M, Driscoll SG, Bhan AK. Implantation site in normal pregnancy. A study with monoclonal antibodies. Am J Pathol. 1985;118:76–84. [PMC free article] [PubMed] [Google Scholar]
- Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev. 1988;28:1599–1613. doi: 10.1051/rnd:19881006. [DOI] [PubMed] [Google Scholar]
- Uckan D, Steele A, Cherry. Wang BY, Chamizo W, Koutsonikolis A, Gilbert-Barness E, Good RA. Trophoblasts express Fas ligand: a proposed mechanism for immune privilege in placenta and maternal invasion. Mol Hum Reprod. 1997;3:655–662. doi: 10.1093/molehr/3.8.655. [DOI] [PubMed] [Google Scholar]
- Khong TY. Immunohistologic study of the leukocytic infiltrate in maternal uterine tissues in normal and preeclamptic pregnancies at term. Am J Reprod Immunol Microbiol. 1987;15:1–8. doi: 10.1111/j.1600-0897.1987.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Simon C, Martin JC, Meseguer M, Caballero-Campo P, Valbuena D, Pellicer A. Embryonic regulation of endometrial molecules in human implantation. J Reprod Fertil Suppl. 2000;55:43–53. [PubMed] [Google Scholar]
- Simon C, Moreno C, Remohi J, Pellicer A. Cytokines and embryo implantation. J Reprod Immunol. 1998;39:117–131. doi: 10.1016/S0165-0378(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Formby B. Immunologic response in pregnancy. Its role in endocrine disorders of pregnancy and influence on the course of maternal autoimmune diseases. Endocrinol Metab Clin North Am. 1995;24:187–205. [PubMed] [Google Scholar]
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Aschkenazi S, Naftolin F, Mor G. Menopause, Sex Hormones and the Immune System. Menopause Management. 2000;9:6–13. [Google Scholar]
- Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- Tranchot-Diallo J, Gras G, Parnet-Mathieu F, Benveniste O, Marce D, Roques P, Milliez J, Chaouat G, Dormont D. Modulations of cytokine expression in pregnant women. Am J Reprod Immunol. 1997;37:215–226. doi: 10.1111/j.1600-0897.1997.tb00218.x. [DOI] [PubMed] [Google Scholar]
- von Rango U, Classen-Linke I, Raven G, Bocken F, Beier HM. Cytokine microenvironments in human first trimester decidua are dependent on trophoblast cells. Fertil Steril. 2003;79:1176–1186. doi: 10.1016/S0015-0282(02)04829-X. [DOI] [PubMed] [Google Scholar]
- Bennett WA, Lagoo-Deenadayalan S, Stopple JA, Barber WH, Hale E, Brackin MN, Cowan BD. Cytokine expression by first-trimester human chorionic villi. Am J Reprod Immunol. 1998;40:309–318. doi: 10.1111/j.1600-0897.1998.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J Clin Invest. 1989;83:430–436. doi: 10.1172/JCI113901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey ML, Cox SM, Word RA, MacDonald PC. Cytokines and infection-induced preterm labour. Reprod Fertil Dev. 1990;2:499–509. doi: 10.1071/rd9900499. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, Nelson DM. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186:1056–1061. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]
- Ratts VS, Tao XJ, Webster CB, Swanson PE, Smith SD, Brownbill P, Krajewski S, Reed JC, Tilly JL, Nelson DM. Expression of BCL-2, BAX and BAK in the trophoblast layer of the term human placenta: a unique model of apoptosis within a syncytium. Placenta. 2000;21:361–366. doi: 10.1053/plac.1999.0486. [DOI] [PubMed] [Google Scholar]
- Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 1997;177:1395–1401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- Smith SC, Baker PN, Symonds EM. Placental apoptosis in normal human pregnancy. Am J Obstet Gynecol. 1997;177:57–65. doi: 10.1016/s0002-9378(97)70438-1. [DOI] [PubMed] [Google Scholar]
- Allaire AD, Ballenger K, Wells SR, MCMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstetrics and Gynecoly. 2000;96:271–276. doi: 10.1016/S0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, McCrae KR. Altered expression of gelatinase and surface-associated plasminogen activator activity by trophoblast cells isolated from placentas of preeclamptic patients. Am J Obstet Gynecol. 1996;175:555–562. doi: 10.1053/ob.1996.v175.a74404. [DOI] [PubMed] [Google Scholar]
- Hara N, Fujii T, Yamashita T, Kozuma S, Okai T, Taketani Y. Altered expression of human leukocyte antigen G (HLA-G) on extravillous trophoblasts in preeclampsia: immunohistological demonstration with anti-HLA-G specific antibody "87G" and anti-cytokeratin antibody "CAM5.2". Am J Reprod Immunol. 1996;36:349–358. doi: 10.1111/j.1600-0897.1996.tb00185.x. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schroder W, Kaufmann P, Rath W. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta. 1999;20:229–233. doi: 10.1053/plac.1998.0373. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Chimini G. The phagocytosis of apoptotic cells. Semin Immunol. 2001;13:365–372. doi: 10.1006/smim.2001.0333. [DOI] [PubMed] [Google Scholar]
- Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- Piacentini M, Autuori F. Immunohistochemical localization of tissue transglutaminase and Bcl-2 in rat uterine tissues during embryo implantation and post-partum involution. Differentiation. 1994;57:51–61. doi: 10.1046/j.1432-0436.1994.5710051.x. [DOI] [PubMed] [Google Scholar]
- Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- Mor G, Straszewski S, Kamsteeg M. The role of the Fas/FasL system in reproductive organs: Survival and apoptosis. Biochemical Pharmacology. 2002;64:1305–1315. doi: 10.1016/S0006-2952(02)01267-4. [DOI] [PubMed] [Google Scholar]
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI200211638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Ware CF, Ryffel B, Savill J. Suppression by apoptotic cells defines tumor necrosis factor-mediated induction of glomerular mesangial cell apoptosis by activated macrophages. Am J Pathol. 2001;159:1397–1404. doi: 10.1016/S0002-9440(10)62526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De M, Wood GW. Influence of Oestrogen and Progesterone on Macrophage Distribution in the Mouse Uterus. Journal of Endocrinology. 1990;126:417–424. doi: 10.1677/joe.0.1260417. [DOI] [PubMed] [Google Scholar]
- Miller L, Hunt J. Sex steroids hormones and macrophage function. Life Sciences. 1996;59:1–14. doi: 10.1016/0024-3205(96)00122-1. [DOI] [PubMed] [Google Scholar]
- Tachi C, Tachi S. Macrophages and implantation. Ann N Y Acad Sci. 1986;476:158–182. doi: 10.1111/j.1749-6632.1986.tb20929.x. [DOI] [PubMed] [Google Scholar]
- Ben-Hur H, Gurevich P, Berman V, Tchanyshev R, Gurevich E, Zusman I. The secretory immune system as part of the placental barrier in the second trimester of pregnancy in humans. In Vivo. 2001;15:429–435. [PubMed] [Google Scholar]
- Hunt JS. Cytokine networks in the uteroplacental unit: Macrophages as pivotal regulatory cells. Journal of Reproductive Immunology. 1989;16:1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Velasco J, Arici A, Naftolin F, Zreick T, Mor G. Macrophage-derived growth factors regulate FasL expression in endometrial stromal cells. Molecular Human Reproduction. 1998;5:642–650. doi: 10.1093/molehr/5.7.642. [DOI] [PubMed] [Google Scholar]
- Mor G, Wei Y, Santen R, Gutierrez L, Eliza M, Lev B, Harada N, Wang J, Lysiak J, Diano S, Naftolin F. Macrophages, estrogen and the microenvironment of breast cancer. Journal of Steroid Biochemistry and Molecular Biology. 1998;67:403–411. doi: 10.1016/S0960-0760(98)00143-5. [DOI] [PubMed] [Google Scholar]
- Mor G, Naftolin F. Oestrogen, menopause and the immune system. The Journal of the British Menopause Society. 1998;4:4–8. [Google Scholar]
- Mor G, Sapi E, Abrahams VM, Rutherford T, Song J, Hao XY, Muzaffar S, Kohen F. Interaction of the Estrogen Receptors with the Fas Ligand Promoter in Human Monocytes. J Immunol. 2003;170:114–122. doi: 10.4049/jimmunol.170.1.114. [DOI] [PubMed] [Google Scholar]
- Reiter I, Krammer B, Schwamberger G. Differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. J Immunol. 1999;163:1730–1732. [PubMed] [Google Scholar]
- Cavaillon JM. Cytokines and macrophages. Biomed Pharmacother. 1994;48:445–453. doi: 10.1016/0753-3322(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, McLaughlin PJ, Vercruysse L, Hanssens M, Johnson PM, Keith J. C., Jr., Van Assche FA. Immunolocalization of tumour necrosis factor-alpha (TNF-alpha) in the placental bed of normotensive and hypertensive human pregnancies. Placenta. 1998;19:231–239. doi: 10.1016/s0143-4004(98)90054-6. [DOI] [PubMed] [Google Scholar]
- Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–1142. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–1152. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- Manfredi AA, Rovere P, Galati G, Heltai S, Bozzolo E, Soldini L, Davoust J, Balestrieri G, Tincani A, Sabbadini MG. Apoptotic cell clearance in systemic lupus erythematosus. I. Opsonization by antiphospholipid antibodies. Arthritis Rheum. 1998;41:205–214. doi: 10.1002/1529-0131(199802)41:2<205::AID-ART4>3.3.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- Aschkenazi S, Straszewski S, Verwer KM, Foellmer H, Rutherford T, Mor G. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol Reprod. 2002;66:1853–1861. doi: 10.1095/biolreprod66.6.1853. [DOI] [PubMed] [Google Scholar]
- Neale D, Demasio K, Illuzi J, Chaiworapongsa T, Romero R, Mor G. Maternal serum of women with pre-eclampsia reduces trophoblast cell viability: evidence for an increased sensitivity to Fas-mediated apoptosis. J Matern Fetal Neonatal Med. 2003;13:39–44. doi: 10.1080/jmf.13.1.39.44. [DOI] [PubMed] [Google Scholar]