Abstract
Purpose
To determine the maximum-tolerated dose, dose-limiting toxicities and pharmacokinetics of the kinesin spindle protein inhibitor ispinesib in pediatric patients with recurrent or refractory solid tumors.
Subjects and Methods
Ispinesib was administered as 1-hour intravenous infusion weekly × 3, every 28 days. Cohorts of 3-6 patients were enrolled at 5, 7, 9 and 12 mg/m2/dose. Serial plasma samples for pharmacokinetic analyses were obtained after the first dose.
Results
Twenty-four (13 females) patients with a median (range) age of 10 years (1-19) were enrolled on the study. At the 12 mg/m2 dose level dose-limiting neutropenia occurred in 2/6 patients and hyperbilirubinemia in 1/6 patients, while at the 9 mg/m2 dose level 1/6 patients had dose-limiting neutropenia. There were no objective responses, but 3 patients (diagnoses of anaplastic astrocytoma, alveolar soft part sarcoma and ependymoblastoma) had stable disease for 4 to 7 courses. There was substantial inter-patient variation in drug disposition. The median (range) terminal elimination half-life was 16 hours (8-44) and the plasma drug clearance was 5 L/hr/m2 (1-14).
Conclusions
The maximum tolerated and recommended phase II dose for ispinesib administered weekly × 3 every 28 days for children with solid tumors is 9 mg/m2/dose. Plans for a phase II trial in select pediatric solid tumors are in development.
Keywords: Ispinesib, pediatric cancer, solid tumors, Children Oncology Group, phase 1 trials
Introduction
Ispinesib (SB-715992) is a potent inhibitor (binding constant, Ki = 0.6 nM) of the kinesin spindle protein (KSP/HsEg5). KSP functions exclusively during mitosis, driving the spindle pole separation and establishing the mitotic spindle bipolarity.(1, 2) This mitotic motor ATPase is essential for cell cycle progression and its inhibition arrests cells in mitosis.(3-5) KSP's over-expression, on the other hand, can induce a genomic instability leading to tumor formation.(6)
Ispinesib was discovered in the search for small molecule inhibitors of KSP. KSP inhibitors, including ispinesib, prevent the formation of a bipolar mitotic spindle,(7) eventually resulting in apoptotic cell death.(5) The drug has no effects outside mitosis, and thus it does not adversely affect non-dividing cells. As a result, peripheral neuropathy commonly seen with the anti-microtubule-directed vinca alkaloids and taxanes has not been associated with ispinesib.
At nanomolar concentrations, ispinesib was cytotoxic in the majority of tumor cell lines studied in vitro in the Pediatric Preclinical Testing Program, including acute lymphoblastic leukemia, Ewing sarcoma, rhabdomyosarcoma, rhabdoid tumor, neuroblastoma and glioblastoma cell lines.(8, 26) The drug also demonstrated a high level of in vivo anti-tumor activity against Ewing sarcoma, Wilms tumor, glioblastoma, rhabdoid tumor and acute lymphoblastic leukemia xenografts.(8) Percent protein binding in humans ranges from 81.1% to 96.2% (mean, 90.5%).
Four dosing regimens have been explored in adult patients with solid tumors: once every 21 days found the maximum tolerated dose (MTD) to be 18 mg/m2/dose (9), on a weekly × 3 every 28 days schedule the MTD was 7 mg/m2/dose (10), on a day 1, 2 and 3 every 21 days schedule the MTD was 6 mg/m2 (24), and on a day 1 and day 15 every 28 days schedule in patients with breast cancer the MTD was 12 mg/m2 (25). Neutropenia was dose-limiting on the first three schedules; liver transaminase elevations were dose-limiting on the every 14 day schedule. The MTD of ispinesib administered on days 1, 2 and 3 every 21 days in adults with acute leukemia was 10 mg/m2/dose, with dose-limiting neutropenia, hepatotoxicity (hyperbilirubinemia and elevated alanine aminotransferase) and mucositis being observed. Based on the high degree of ispinesib preclinical anti-tumor activity in pediatric tumor models,(7) the current study was performed to determine the MTD and recommended phase II dose of ispinesib, the incidence and severity of toxicities associated with ispinesib administration, and the pharmacokinetics of ispinesib in pediatric patients with recurrent or refractory solid tumors.
Subjects and Methods
Subject Eligibility
Subjects > 12 months and ≤ 21 years of age with a histologically confirmed recurrent or refractory solid tumor, including CNS tumors and lymphoma, were eligible. Subjects with intrinsic brainstem gliomas were excluded from the requirement for histological verification. Other eligibility criteria included: the presence of measurable or evaluable disease; a Karnofsky or Lansky performance score of ≥ 60; recovery from the acute toxicities of prior therapies; no chemotherapy for > 3 weeks (6 weeks for nitrosourea); no growth factors or biologic agents for > 7 days; no local radiation for ≥ 2 weeks; no bone marrow radiation for ≥ 6 weeks; no total body, craniospinal or pelvic (> 50% of the pelvis) radiation for ≥ 6 months; no stem cell transplant for ≥ 3 months; no active graft vs. host disease; adequate bone marrow function [absolute neutrophil count (ANC) ≥ 1,000/μL, platelet count ≥ 100 × 103/μL, and hemoglobin concentration ≥ 8.0 gm/dL]; adequate renal function (creatinine clearance ≥ 70 mL/min/1.73 m2 or normal serum creatinine for age and gender); and adequate hepatic function (bilirubin ≤ 1.5 times upper limit of normal; alanine aminotransferase (ALT) ≤ 110 units/L (upper limit of normal, 45 units/L); and serum albumin ≥ 2.0 g/dL]. Study exclusion criteria included pregnancy, breast-feeding, and uncontrolled infection. In addition, use of enzyme-inducing anticonvulsants (e.g., phenytoin, phenobarbital, felbamate, primdone, oxcarbazepine or carbamazepine) or agents known to inhibit CYP3A4 (e.g., itraconazole, ketoconazole and voriconazole) were prohibited since ispinesib is metabolized by CYP3A4 (GlaxoSmithKline internal report). This trial was approved by local Institutional Review Boards, and all patients or their legal guardians signed a document of informed consent; when appropriate, assent was obtained according to individual institutional guidelines.
Drug Administration
Ispinesib was administered as an intravenous infusion over 1 hour on days 1, 8 and 15 of each 28 day course. Ispinesib was supplied by the Pharmaceutical Branch, National Cancer Institute (Bethesda, MD) in vials containing 4 mg, 5 mg or 10 mg in an isotonic 1 mg/mL solution. The calculated dose was added to 5% dextrose in water to attain a final concentration of ≤ 150 μg/mL. Concentrations > 48 but ≤ 150 μg/mL were administered in plastic or glass containers and those < 48 μg/mL in glass, PVC or non-DEHP containers with low-sorbing tubing.
Trial Design
The starting dose was 5 mg/m2/dose, approximately 80% of the adult MTD, with planned escalations to 7, 9 and 12 mg/m2/dose. Courses were repeated every 28 days if the patient had at least stable disease and met the inclusion criteria.
A patient was considered fully evaluable for toxicity if they experienced a DLT or received at least 85% of the total dose during course 1. A minimum of three patients were entered at each dose level, and the dose level was expanded to six patients if one patient experienced DLT during the first course of therapy. When DLT was observed in two patients of a cohort of three to six patients receiving the same dose of drug, the MTD was exceeded. If necessary, an additional three patients were added at the dose level immediately below the dose level at which the unacceptable level of toxicity was observed. The MTD of ispinesib was defined as the dose level immediately below the level at which at least two patients in a cohort of three to six experienced dose-limiting toxicity.
Toxicities were graded according to the NCI Common Toxicity Criteria (version 3.0). Dose-limiting non-hematologic toxicity was defined as any grade 3 or 4 adverse event attributable to the study drug with the specific exclusions of: 1) nausea or vomiting of < 3 days; 2) elevated transaminases that returned to levels that met the eligibility criteria within 7 days of study drug interruption and did not recur upon re-administration of the drug; and 3) fever or infection of < 5 days. Dose-limiting non-hematological toxicity included any grade 2 adverse event that persisted for ≥ 7 days and required treatment interruption, or any adverse event that required drug interruption for > 7 days or recurred upon drug re-administration. Hematologic dose-limiting toxicity was defined as grade 4 thrombocytopenia (platelet count < 25 × 103/μL) on 2 separate days, thrombocytopenia requiring platelet transfusion on 2 separate days in a 7-day period, and grade 4 neutropenia for > 7 days or occurring prior to day 15 dose.
Subjects who had hematologic DLT received the next lowest dose level for the subsequent courses. Subjects who had grade 4 neutropenia on day 8 or 15 did not receive the drug on these days and were considered to have DLT.
Criteria for Assessment of Response
The NCI Response Evaluation Criteria in Solid Tumors (RECIST) were used for assessment of radiographic response.(11)
Pharmacokinetic Studies
In consenting patients, serial blood samples were collected during course one prior to the first dose of ispinesib, immediately after completion of infusion, and 15 min, 30 min, 1 hr, 2 hr, 4 hr, 6 hr, 8 hr, 20-24 hr, and 44-48 hr following the infusion. All samples were collected in potassium-EDTA tubes and centrifuged at 2,500g for 10 min at 5°C. Plasma was frozen at -20°C until analysis. Ispinesib (molecular weight, 517.1) quantification in human plasma was performed using a high pressure liquid chromatography-mass spectrometry (LC/MS/MS) assay validated (Cedra Corporation) over a range of 0.1 to 100 ng/mL (∼0.19 to 193 nM). Ispinesib was harvested from 0.2 mL human plasma by liquid-liquid extraction, using 2.1 mL (total volume) of 1:1 acetonitrile:dH2O, 1.0 M sodium carbonate, and methyl tert-butyl ether containing 10 ng of an isotopically labeled internal standard, ispinesib-D4. Following centrifugation, the upper organic layer was removed and evaporated. The sample was then reconstituted in the mobile phase (acetonitrile:dH2O:formic acid:ammonium hydroxide; 900:100:1.00:0.25) and an aliquot was analyzed using Sciex API 4000 HPLC-MS-MS equipped with a TurboIonSpray™ interface along with quality control samples (0.30, 20.0, and 80.0 ng/mL). The peak area of the mass-to-charge ratio (m/z) 517→247 of ispinesib product ion was measured against peak area of the m/z 521→251 of ispinesib-D4 internal standard product ion. Quantitation was performed using a weighted (1/x2) linear least squares regression analysis generated from calibration standards prepared immediately prior to each run. Pharmacokinetic analysis was performed using WinNonlin® (Enterprise Edition version 4.0) non-compartmental methods.
Concentration-time data that were below the limit of quantification were treated as zero (0.00 ng/mL) in the data summarization and descriptive statistics. Nominal protocol sample times were used for all pharmacokinetic and statistical analyses.
Results
From September 2006 to January 2008, 24 patients, 19 fully evaluable for toxicity, were enrolled on the study (Table I). The five toxicity inevaluable patients did not complete the first course of therapy secondary to early disease progression but did not develop dose limiting toxicity.
Table I. Patient characteristics (n = 24).
| Characteristic | |
|---|---|
| Male/female | 11/13 |
| Age (years) | |
| Median | 10 |
| Range | 1-19 |
| Race | |
| White | 19 (79%) |
| African-American | 4 (17%) |
| Unknown | 1 (4%) |
| Ethnicity | |
| Non-Hispanic | 17 (71%) |
| Hispanic | 4 (17%) |
| Unknown | 3 (12%) |
| Diagnoses | |
| Non-CNS tumors | |
| Soft tissue sarcoma | 2 |
| Rhabdomyosarcoma | 2 |
| Osteosarcoma | 1 |
| Wilms tumor | 2 |
| Hepatoblastoma | 3 |
| Lymphoepithelial carcinoma | 1 |
| Adenocarcinoma | 1 |
| Neuroendocrine hepatic carcinoma | 1 |
| Pancreatoblastoma | 1 |
| CNS tumors | |
| Malignant glioma | 5 |
| Astrocytoma | 1 |
| Ependymoma | 3 |
| Atypical teratoid/rhabdoid tumor | 1 |
| Prior therapies | |
| Chemotherapy regimens | |
| Median | 1 |
| Range | 0-6 |
| Radiation | 15 |
Table II summarizes the DLTs observed in the first course of therapy. At the 7 mg/m2 dose level, one patient with hepatoblastoma experienced grade 3 elevated ALT that was initially attributed to the underlying tumor. Dose escalation proceeded, but upon further review the toxicity attribution was modified to be considered possibly related to ispinesib. At the 12 mg/m2 dose level, 3 of 6 evaluable patients had DLT, two with grade 4 neutropenia and one with grade 3 hyperbilirubinemia. The grade 4 neutropenia occurred prior to day 15 in one patient and on day 15 in the other, precluding administration of the day 15 dose. The hyperbilirubinemia was observed in a patient with Wilms tumor and liver metastasis; a possible relationship to study drug could not be completely excluded. At the 9 mg/m2 dose level, 1 of 6 evaluable patients experienced dose-limiting neutropenia (grade 4 on day 15), defining this as the MTD.
Table II. DLTs in the first course at each dose level.
| Dose Level (mg/m2/dose) | No. of patients entered | No. of evaluable patients | No. of patients with DLT | DLT (n) |
|---|---|---|---|---|
| 5 | 4 | 3 | 0 | None |
| 7 | 5 | 4 | 1 | Elevated ALT, AST (1)* |
| 9 | 9 | 6 | 1 | Neutropenia (1) |
| 12 | 6 | 6 | 3 | Neutropenia (2) Hyperbilirubinemia (1) |
The elevated ALT and AST were retrospectively attributed to ispinesib by the treating physician.
Ispinesib was generally well tolerated although the median number of administered courses was only 1 (range 1-7). Grade 3 or 4 hematologic toxicities over all courses are shown in Table III. Non-hematological toxicities attributed to ispinesib that occurred in >10% of evaluable patients, with the exception of the DLTs noted above are shown in Table IV.
Table III. No. of courses with grade 3 or 4 hematological toxicities (independent of frequency or attribution) observed in the patients evaluable for hematological toxicities.
| Toxicity Type | Course 1 (19 courses) |
Courses 2-7 (15 courses) |
||
|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Anemia | 1 | 0 | 0 | 0 |
| Lymphopenia | 2 | 1 | 2 | 0 |
| Neutropenia | 5 | 3* | 1 | 1* |
| Thrombocytopenia | 1 | 0 | 0 | 0 |
Dose-limiting
Table IV. No. of courses with non-hematologic, non-dose-limiting toxicities related to protocol therapy that occurred in > 10% of evaluable patients.
| Toxicity | Course 1 (19 courses) |
Courses 2-7 (15 courses) |
|
|---|---|---|---|
| Grades 1 and 2 | Grade 3 | Grades 1 and 2 | |
| Fatigue | 3 | 0 | 1 |
| Constipation | 2 | 0 | 0 |
| Diarrhea | 2 | 0 | 0 |
| Nausea | 4 | 0 | 1 |
| Vomiting | 3 | 0 | 1 |
| Hypoalbuminemia | 3 | 0 | 0 |
| ALT | 3 | 1 | 0 |
| AST | 2 | 1 | 0 |
| Hyperbilirubinemia | 1 | 1 | 0 |
| Alkaline phosphatase | 2 | 0 | 0 |
| Electrolyte abnormality | 4 | 0 | 0 |
There were no objective tumor responses. Three of the 24 patients evaluable for response had stable disease and received 4 (12 mg/m2 dose level, anaplastic astrocytoma), 5 (9 mg/m2 dose level, alveolar soft part sarcoma) and 7 (7 mg/m2 dose level, ependymoblastoma) complete courses of therapy. The patient with an anaplastic astrocytoma was removed from protocol therapy with stable disease for toxicity after the recurrence of dose-limiting neutropenia following an initial dose reduction.
Ispinesib pharmacokinetic parameters for the eleven patients who consented to participation in the pharmacokinetic studies are presented in Table V. There was a high degree of inter-patient variability observed across dose levels (Figure 1). Protocol deviations included 4 patients in whom pharmacokinetic samples were obtained from the same venous access site through which the drug was administered, creating the potential for an overestimate of plasma drug concentrations. For all patients studied, the median (range) terminal elimination half-life (t1/2) was 16 (8-44) hours, plasma drug clearance was 4.8 (1.2-13.5) L/hr/m2, and the volume of distribution at steady state (Vss) was 84 (23-193) L/m2.
Table V. Ispinesib plasma pharmacokinetic parameters by dose level.
| Dose level mg/m2/dose | Patient | Cmax (ng/mL) |
AUClast (hr*ng/mL) |
AUCINF (hr*ng/mL) |
t1/2 (hr) |
Clearance (L/hr/m2) |
Vss (L/m2) |
|
|---|---|---|---|---|---|---|---|---|
| 5 | 737478** | 86 | 945 | 1662 | 44 | 3.0 | 177 | * |
| 757897 | 145 | 908 | 938 | 9 | 5.3 | 61 | ||
| 7 | 744676 | 181 | 1319 | 1449 | 14 | 4.8 | 84 | * |
| 758892** | 244 | 3716 | 5733 | 32 | 1.2 | 51 | ||
| 767535 | 1900 | 4090 | 4596 | 18 | 1.5 | 24 | * | |
| 769075 | 75 | 851 | 970 | 15 | 7.2 | 145 | ||
| 9 | 763246 | 1880 | 4814 | 5243 | 16 | 1.7 | 23 | * |
| 763770 | 281 | 2506 | 3811 | 33 | 2.4 | 102 | ||
| 769877 | 149 | 1526 | 1865 | 19 | 4.8 | 118 | ||
| 12 | 764399 | 149 | 819 | 890 | 13 | 13.5 | 193 | |
| 771172** | 204 | 732 | 1690 | 8 | 7.1 | 83 | ||
Protocol deviation with samples obtained from same venous access site as drug administration; concentrations obtained may be overestimates;
AUC extrapolated values for these patients were >30% (fewer data points were available for these patients).
Figure 1.
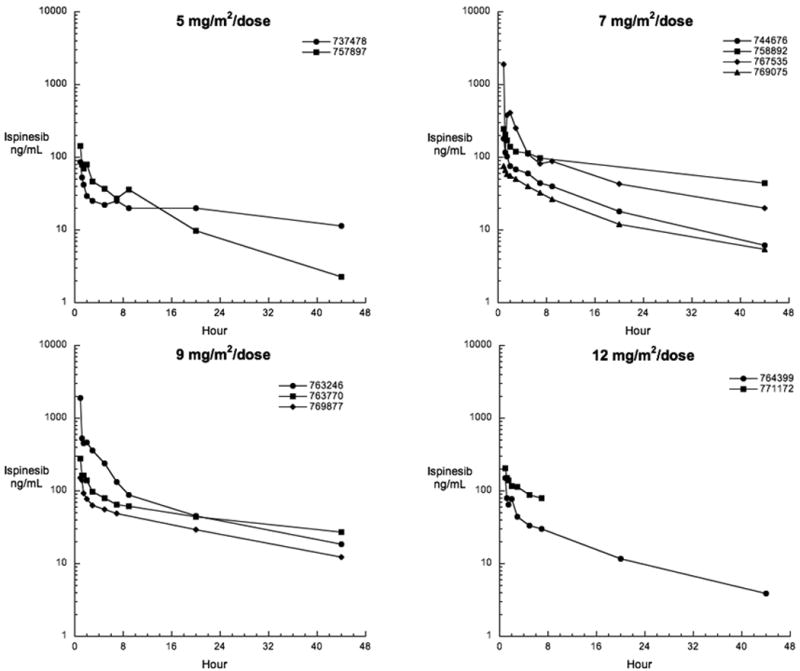
Plasma concentration time curves for individual patients receiving ispinesib at the indicated dose levels.
Discussion
In this trial, 24 pediatric patients received 1-hour intravenous infusion of ispinesib as monotherapy at 5, 7, 9 or 12 mg/m2/dose weekly × 3, every 28 days. The MTD was 9 mg/m2/dose, ∼30% higher than the recommended adult phase II dose of 7 mg/m2/dose. Overall, ispinesib appeared to be well tolerated. The primary dose-limiting adverse events were neutropenia and hepatotoxicity. The neutropenia was rapidly reversible; it was dose-limiting because it precluded administration of all three doses of ispinesib during the first course. A recent adult phase I trial with another KSP inhibitor, SB-743921 (given on days 1 and 15, every 28 days), showed 50% increase in MTD with prophylactic filgrastim (unpublished data). Thus, it may be possible to increase ispinesib dose on the weekly schedule by addition of filgrastim.
The observation of dose-limiting hepatotoxicity in two patients on this trial was confounded by the presence of involvement of the liver by tumor. No objective responses were observed, although 3 patients were noted to have stable disease, receiving 3 to 7 courses of therapy.
Similar to adult trials, (9, 12-14) we observed a high degree of interpatient variability in the drug disposition (Table V, Figure 1). The estimated median (range) terminal t1/2 of 16 hours (8-44 hours) in children appeared to be independent of dose (5-12 mg/m2). This high degree of interpatient variation is similar to that observed in adult trials, with reports of terminal half-lives ranging from 17 to 56 hours with doses of 1-21 mg/m2,(9) 20 to 53 hours with doses of 1-21 mg/m2,(15) and 13 to 96 hours with doses of 1-8 mg/m2.(14) Thus the lower median terminal half-life observed in children should be interpreted with caution, as the small number of subjects studied and limited duration of sampling in both adult and pediatric subjects cannot allow us to conclude that true differences exist in drug disposition. The number of patients studied and the limited number developing DLT did not allow for study of PK-PD relationships.
Phase II trials of ispiniseb, all using a dose of 18 mg/m2 on the once every 21 day schedule, have been performed in adult patients with breast, ovarian, non-small cell lung cancer, squamous cell carcinoma of the head and neck, melanoma, renal cell carcinoma, prostate cancer, colorectal cancer, and hepatocellular carcinoma.(13, 16-23) Four of 45 (9%) previously treated patients with metastatic breast cancer had partial responses, and one of 19 patients (5%) with platinum/taxane refractory ovarian cancer had a radiographic partial response. No significant activity has been observed to date for other adult tumor indications.
In conclusion, ispinesib administered as 1-hour intravenous infusion at 9 mg/m2/dose weekly × 3, every 28 days is well tolerated in pediatric patients. Plans for a phase II trial in select pediatric solid tumors are in development.
Acknowledgments
We thank members of the study committee Rajen Mody, MD, Sharon Bauer, and Michelle Giglio for their contributions to the study protocol. We additionally thank Shanila Faghfoor and Dori Triplett for outstanding administrative and data management support throughout the development and conduct of this trial.
Supported by National Cancer Institute Grant No. U01 CA97452
Footnotes
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30 – June 3, 2008.
Conflict of Interest Disclosure. Dr. Maureen G. Conlan is employed at Cytokinetics. The remaining authors have no conflicts of interest.
References
- 1.Blangy A, Lane HA, d'Herin P, et al. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–69. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 2.Kapitein LC, Peterman EJ, Kwok BH, et al. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114–8. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- 3.Marcus AI, Peters U, Thomas SL, et al. Mitotic kinesin inhibitors induce mitotic arrest and cell death in Taxol-resistant and -sensitive cancer cells. J Biol Chem. 2005;280:11569–77. doi: 10.1074/jbc.M413471200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakowicz R, Finer JT, Beraud C, et al. Antitumor activity of a kinesin inhibitor. Cancer Res. 2004;64:3276–80. doi: 10.1158/0008-5472.can-03-3839. [DOI] [PubMed] [Google Scholar]
- 5.Tao W, South VJ, Zhang Y, et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8:49–59. doi: 10.1016/j.ccr.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Castillo A, Morse HC, 3rd, Godfrey VL, Naeem R, Justice MJ. Overexpression of Eg5 causes genomic instability and tumor formation in mice. Cancer Res. 2007;67:10138–47. doi: 10.1158/0008-5472.CAN-07-0326. [DOI] [PubMed] [Google Scholar]
- 7.Lad L, Luo L, Carson JD, et al. Mechanism of inhibition of human KSP by ispinesib. Biochemistry. 2008;47:3576–85. doi: 10.1021/bi702061g. [DOI] [PubMed] [Google Scholar]
- 8.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–40. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 9.Chu QS, Holen KD, Rowinsky EK, et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV Q 21 days. ASCO Meeting Abstracts. 2004;22:2078. [Google Scholar]
- 10.Burris HA, Lorusso P, Jones S, et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV days 1, 8, 15 q 28 days. ASCO Meeting Abstracts. 2004;22:2004. [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Blagden SP, Molife LR, Seebaran A, et al. A phase I trial of ispinesib, a kinesin spindle protein inhibitor, with docetaxel in patients with advanced solid tumours. Br J Cancer. 2008;98:894–9. doi: 10.1038/sj.bjc.6604264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CW, Belanger K, Rao SC, et al. A phase II study of ispinesib (SB-715992) in patients with metastatic or recurrent malignant melanoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2008;26:249–55. doi: 10.1007/s10637-007-9097-9. [DOI] [PubMed] [Google Scholar]
- 14.LoRusso P, Jones S, Gadgeel S, et al. A phase I study to determine the safety and pharmacokinetics of intravenous administration of SB-715992, a novel kinesin spindle protein (KSP) inhibitor, on a once weekly for three consecutive weeks schedule in patients with refractory solid tumors. European Cancer Conference (ECCO) Annual Meeting; 2003; 2003. [Google Scholar]
- 15.Chu Q, Holen KD, Rowinsky EK, et al. A phase I study to determine the safety and pharmacokinetics of IV administered SB-715992, a novel kinesin spindle protein (KSP) inhibitor, in patients (pts) with solid tumors. ASCO Meeting Abstracts. 2003;22 Abstract 525. [Google Scholar]
- 16.Beekman KW, Dunn R, Colevas D, et al. ASCO Meeting Abstracts. Vol. 25. 2007. University of Chicago Consortium phase II study of ispinesib (SB-715992) in patients (pts) with advanced renal cell carcinoma (RCC) p. 15573. [Google Scholar]
- 17.Beer TM, Goldman B, Synold TW, et al. Southwest Oncology Group phase II study of ispinesib in androgen-independent prostate cancer previously treated with taxanes. Clin Genitourin Cancer. 2008;6:103–9. doi: 10.3816/CGC.2008.n.016. [DOI] [PubMed] [Google Scholar]
- 18.El-Khoueiry AB, Iqbal S, Singh DA, et al. A randomized phase II non-comparative study of Ispinesib given weekly or every three weeks in metastatic colorectal cancer. A California Cancer Consortium Study (CCC-P) ASCO Meeting Abstracts. 2006;24:3595. [Google Scholar]
- 19.Knox JJ, Gill S, Synold TW, et al. A phase II and pharmacokinetic study of SB-715992, in patients with metastatic hepatocellular carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG IND.168) Invest New Drugs. 2008;26:265–72. doi: 10.1007/s10637-007-9103-2. [DOI] [PubMed] [Google Scholar]
- 20.Lee RT, Beekman KE, Hussain M, et al. A University of Chicago consortium phase II trial of SB-715992 in advanced renal cell cancer. Clin Genitourin Cancer. 2008;6:21–4. doi: 10.3816/CGC.2008.n.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philco M, Falcon S, Gomez H, et al. A phase I-II open-label trial of ispinesib on an alternate dosing schedule in chemotherapy-naive patients with locally advanced or metastatic breast cancer (MBC) ASCO Meeting Abstracts. 2008;26:1143. [Google Scholar]
- 22.Shahin MS, Braly P, Rose P, et al. A phase II, open-label study of ispinesib (SB-715992) in patients with platinum/taxane refractory or resistant relapsed ovarian cancer. ASCO Meeting Abstracts. 2007;25:5562. [Google Scholar]
- 23.Tang PA, Siu LL, Chen EX, et al. Phase II study of ispinesib in recurrent or metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2008;26:257–64. doi: 10.1007/s10637-007-9098-8. [DOI] [PubMed] [Google Scholar]
- 24.Heath EI, Alousi A, Eder JP, Valdivieso M, Vasist LS, Appleman L, Bhargava P, Colevas AD, LoRusso PM, Shapiro G. A phase I dose escalation trial of ispinesib (SB-715992) administered days 1-3 of a 21-day cycle in patients with advanced solid tumors. American Society of Clinical Oncology (ASCO) 2006 June; [Google Scholar]
- 25.Gómez H, Philco M, Castaneda C, Pimentel P, Escandón R, Seroogy J, Saikali K, Wolff AA, Conlan MG. American Society of Clinical Oncology (ASCO) Orlando, FL: May, 2009. A Phase I/II Trial of ispinesib, a kinesin spindle protein inhibitor, dosed Q14D in patients with advanced breast cancer previously untreated with chemotherapy for metastatic disease or recurrence. [Google Scholar]
- 26.Carol H, Lock R, Houghton PJ, Morton CL, Kolb A, Gorlick R, Reynolds CP, Maris JM, Keir ST, Billups CA, Smith MA. Initial testing (stage 1) of the kinesin spindle protein inhibitor ispinesib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2009;53:1255–1263. doi: 10.1002/pbc.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]


