Abstract
Microarray gene expression experiments often consider specific developmental stages, tissue sources or reproductive technologies. This focus hinders the understanding of the cattle embryo transcriptome. To address this, four microarray experiments encompassing three developmental stages (7 d, 25 d, 280 d), two tissue sources (embryonic or extra-embryonic) and two reproductive technologies (artificial insemination or AI and somatic cell nuclear transfer or NT) were combined using two sets of meta-analyses. The first set of meta-analyses uncovered 434 genes differentially expressed between AI and NT (regardless of stage or source) that were not detected by the individual-experiment analyses. The molecular function of transferase activity was enriched among these genes that included ECE2, SLC22A1 and a gene similar to CAMK2D. Gene POLG2 was over-expressed in AI versus NT 7 d embryos and was under-expressed in AI versus NT 25 d embryos. Gene HAND2 was over-expressed in AI versus NT extra-embryonic samples at 280 d yet under-expressed in AI versus NT embryonic samples at 7 d. The second set of meta-analyses uncovered enrichment of system, organ and anatomical structure development among the genes differentially expressed between 7 d and 25 d embryos from either reproductive technology. Genes PRDX1and SLC16A1 were over-expressed in 7 d versus 25 d AI embryos and under-expressed in 7 d versus 25 d NT embryos. Changes in stage were associated with high number of differentially expressed genes, followed by technology and source. Genes with transferase activity may hold a clue to the differences in efficiency between reproductive technologies.
Keywords: embryo development, gene expression, extra-embryonic tissue, cattle, reproductive technologies, meta-analysis
INTRODUCTION
Hundreds of genes influence embryo development in human and reproduction models (Beyhan et al. 2007; Humpherys et al. 2002; Kues et al. 2008). The cattle embryo is a well-established reproductive model to study gene expression changes in embryonic and maternal tissues. Microarray gene expression experiments have profiled the expression of thousands of genes across conditions such as tissue sources (embryonic or extra-embryonic), developmental stages and, reproductive technologies used to create the cattle embryo (Huang et al., 2010; Pfister-Genskow et al. 2005; Smith et al. 2009; Somers et al. 2006). The detection of genes that are differentially expressed between the embryo and maternal components or at different stages is useful in understanding embryo survival and healthy development. Likewise, the detection of differential gene expression between reproductive technologies within and across developmental stages and tissue sources sheds insights into the lower efficiency of somatic cell nuclear transfer (NT) to produce healthy calves relative to artificial insemination (AI), the standard reproductive technique in cattle (Dinnyes et al. 2008; Everts et al. 2008; Khatib et al. 2009; Marjani et al. 2009).
The identification and understanding of genes and gene pathways that are critical for embryo development has been hindered by the limited size and focus of most experiments on a particular tissue source (Everts et al. 2008), developmental stage (Smith et al. 2005), or reproductive technology (Thelie et al. 2009). Two examples of this situation are the relative expression level of 1) genes that have transferase activity function between reproductive technologies and 2) genes that are part of system development processes between developmental stages. With respect to the former case, multiple studies have reported difference in the expression of genes that have transferase activity function between normal and abnormal pregnancies at particular stages of mammalian development or tissues sources (Prokopenko et al. 2002; El-Bassiouni et al. 2005; Toledo et al. 2006; Rausell et al., 2007; Obolenskaya et al. 2010). However, no study has looked at the differences across stages or tissue sources. With respect to the latter case, several studies have reported differences in genes that are part of the system development process (Ruberte et al., 1993; Cardoso and Lu 2006; Laranjeira and Pachnis 2009). However, no study has compared the patterns across reproductive technologies. The goal of this study was to obtain a more comprehensive understanding of genes, molecular functions and biological processes associated with cattle embryo development and viability. To accomplish this, the information from four cattle microarray experiments was integrated using two sets of meta-analyses that provided complementary understanding (Adams et al. 2008; Rodriguez-Zas et al. 2008). The first set of meta-analyses offered broad insights into the differential gene expression between AI and NT technologies regardless of developmental stage or tissue source. This analysis allowed the profiling of genes with transferase activity between reproductive technologies across all developmental stages and tissue sources considered. The second set of meta-analyses provided detailed knowledge into the differential gene expression between developmental stages or tissue sources within technology under particular conditions. This analysis allowed the profiling of genes belonging to the system development process between developmental stages in both reproductive technologies considered. The genes identified by the meta-analyses and subsequent Gene Ontology analysis allowed the characterization of general and condition-specific expression profiles, functions and processes.
MATERIALS AND METHODS
Data Sets
Four cattle microarray experiments that compared gene expression between reproductive technologies using different developmental stages and tissue sources were integrated. The common comparison between AI and NT studied in all these experiments, the shared platform, and the availability of the cattle genome facilitated the combination of information from these experiments using meta-analysis and subsequent detection of genes associated with embryo development, and interpretation of results. The experiments encompassed AI and NT samples from one of two tissue sources (embryonic or E, and extra-embryonic or X) and one of three developmental stages (day 7 of gestation or d7, day 25 of gestation or d25, and at term or d280). Based on the stage and source studied, the four experiments are denoted d280X (Everts et al. 2008), d7E (Marjani et al. 2009; Smith et al. 2007), d25X (Everts et al. 2007a) and d25E (Everts et al. 2007b). The number of AI (and NT) samples were 9 (20), 5 (6), 6 (6), and 9 (20) for d7E, d25E, d25X, and d280X, respectively. In the d7E study, NT embryos from two cell lines were analyzed as one NT group based on preliminary comparisons that indicated consistent profiles. The microarray platform included 13,257 spotted oligonucleotide (Gene Expression Omnibus platform identifier GPL2853) created from cattle placenta and spleen cDNA libraries. Of the probes, 9,655 have a match on human RefSeq, 1,460 have identification on cattle UniGene, 54 have no hits, and the remainder matched other mouse, human or cattle databases (Loor et al., 2007). All studies used a reference design with dye swap and each sample appeared in two microarrays.
Data processing and normalization was performed as described by Rodriguez-Zas et al. (2006) and Adams et al. (2008) and implemented in Beehive (http://stagbeetle.animal.uiuc.edu/Beehive). Briefly, spots flagged by the scanning software (GenePix Pro 5.0) or that did not surpass the median intensity of the negative control spots were removed and the background-subtracted foreground intensities were log2 transformed. A global LOWESS normalization was used, duplicate spots were averaged and global dye and microarray effects were removed. All data processing and analyses were conducted using the SAS mixed procedure (SAS Institute Inc., NC, USA). The total number of observations analyzed per study was 29, 11, 12 and 29 for d7E, d25E, d25X, and d280X, respectively. Following normalization, two sets of analyses were used to gain a thorough understanding of the cattle embryo transcriptome.
First set of meta-analyses to identify differentially expressed genes between AI and NT across developmental stages and tissue sources
Three approaches to combine information across all experiments and identify differentially expressed genes between AI and NT were considered. A detailed description of the complementary properties of these approaches is provided in Rodriguez-Zas et al. (2008) and Adams et al. (2008). In the first approach, the data from each experiment was analyzed separately (individual-experiment analyses). Lists of differentially expressed genes from each experiment were obtained and the genes that overlap two or more lists were identified. In the individual-experiment analyses, each gene was described with a linear mixed-effects model that included the fixed effects of dye, reproductive technique, and the random effects of sample and microarray. Numerous genes were differentially expressed between term and preterm calving samples in experiment d280X (Everts et al. 2007a) and thus this factor was included as fixed effect in the model for this experiment.
Second, a study-level meta-analysis (Study) that combines estimates of differential expression between AI and NT resulting from the individual-experiment analyses was undertaken (Adams et al. 2008). The estimates were standardized by their standard errors to account for differences in the precision between experiments. The linear mixed-effects model used to analyze the standardized estimates of differential expression included an overall mean and the random effect of experiment assuming heterogeneity of variances across experiments and zero co-variance between experiments. Third, a sample-level meta-analysis (Sample) was used to combine the normalized gene expression intensities across all four experiments. A linear mixed-effects model that included the fixed effects of dye, reproductive technology and experiment, and the random effects of sample and microarray was used to describe the expression of each gene. The effect of amplification on the d7E samples was not significant and was removed from the model (Smith et al. 2005).
Second set of meta-analyses to identify differentially expressed genes between developmental stages and tissue sources within reproductive technology
An alternative specification of the sample-level meta-analysis was used to gain complementary information to that resulting from the first set of meta-analysis. The second set of meta-analyses allowed the detection of differentially expressed genes between developmental stages or tissue sources within reproductive technology. The meta-analysis model included the combined effect of tissue source, developmental stage and reproductive technology into a single factor. Contrasts between developmental stages or tissue sources that encompassed multiple experiments were identified. Results from four comparisons between developmental stages within tissue sources and reproductive technologies and two comparisons between tissue sources within developmental stages and reproductive technologies are presented.
Post-analysis mining of results
Genes that had technology, source or stage P-values < 1 × 10−3 (equivalent to False Discovery Rate adjusted P-value < 0.05, Benjamini and Hochberg 1995), and fold change > 1.23 were considered differentially expressed. Four scenarios were investigated using funnel plots; a) genes that were not differentially expressed in any individual experiment, and were detected by the study-level and sample-level meta-analyses; b) genes that had differential expression between AI and NT samples in two experiments, and were not detected by meta-analysis; c) genes that were found differentially expressed in the sample-level meta-analysis and were not in the study-level meta-analysis, and; d) genes that were differentially expressed in the study-level meta-analysis and were not in the sample-level meta-analysis. Gene Set Enrichment Analysis (Al-Shahrour et al. 2006) was undertaken to uncover over-represented Gene Ontology (GO, The Gene Ontology Consortium) biological processes, molecular functions and KEGG pathways (Kanehisa and Goto 2000) using the standardized estimates of differential expression between the conditions (e.g. AI vs NT, 7 d vs 25 d). Among all the genes analyzed, 31.6% were homologous to human genes that have GO annotations.
RESULTS
Genes differentially expressed between AI and NT across developmental stages and tissue sources identified from the first set of meta-analyses
The number of differentially expressed genes between AI and NT identified from the individual-experiment analyses ranged from 36 to 846 (Table 1). Supplementary Materials Table 1 presents the gene identifier, estimate of difference in expression between conditions and P-value from all the individual experiment analyses. No particular sign or pattern of differential expression dominated across the individual-experiment analyses, with the exception of experiment d280X that had 189 and 657 positively and negatively differentially expressed genes, respectively. The percentage of genes differentially expressed and overlapping among individual-experiment analyses ranged from 0% to 13.1% (average of 7.65%) relative to the number of genes identified in one of the experiments.
Table 1.
Number of differentially expressed gene between AI and NT samples by analysis and sign of the pattern.
| Individual analyses | Meta-analyses | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d280X1 | d7E | d25X | d25E | Study | Sample | ||||||||
| + | − | + | − | + | − | + | − | + | − | + | − | ||
| d280X | + | 1892 | − | 03 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 3 | 0 |
| − | − | 657 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 14 | 1 | 19 | |
| d7E | + | 0.0%4 | 1.9% | 52 | − | 0 | 0 | 0 | 4 | 0 | 0 | 26 | 0 |
| − | 1.4% | 2.7% | − | 74 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 26 | |
| d25X | + | 4.5% | 0.0% | 0.0% | 0.0% | 22 | − | 0 | 1 | 0 | 0 | 0 | 0 |
| − | 0.0% | 7.1% | 0.0% | 0.0% | − | 14 | 0 | 0 | 0 | 0 | 0 | 1 | |
| d25E | + | 10.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 10 | − | 0 | 0 | 0 | 0 |
| − | 2.6% | 0.0% | 10.5% | 2.6% | 2.6% | 0.0% | − | 38 | 0 | 1 | 4 | 1 | |
| Study | + | 3.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 66 | − | 9 | 0 |
| − | 0.0% | 13.7% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 1.0% | − | 102 | 0 | 10 | |
| Sample | + | 1.5% | 0.5% | 12.7% | 0.5% | 0.0% | 0.0% | 0.0% | 2.0% | 4.4% | 0.0% | 204 | − |
| − | 0.0% | 10.8% | 0.0% | 14.8% | 0.0% | 0.6% | 0.0% | 0.6% | 0.0% | 5.7% | − | 176 | |
d280X = day 280 extra-embryonic samples, d7E = day 7 embryos, d25X = day 25 extra-embryonic sample, d25E = day 25 embryos.
Number of differentially expressed genes between AI and NT (P-value < 1×10−3, fold change > 1.23) within individual-experiment analyses, study-level standardized (Study), and sample-level (Sample) meta-analysis (diagonals). “+” denotes over-expression in AI relative to NT and “−” denotes under-expression in AI relative to NT.
Upper off-diagonals are the number of genes identified differentially expressed in all pairs of analyses relative to the maximum number of significant genes that can overlap in both analyses.
Lower off-diagonals are the percentage of genes identified differentially expressed in all pairs of analyses relative to the maximum number of significant genes that can overlap in both analyses.
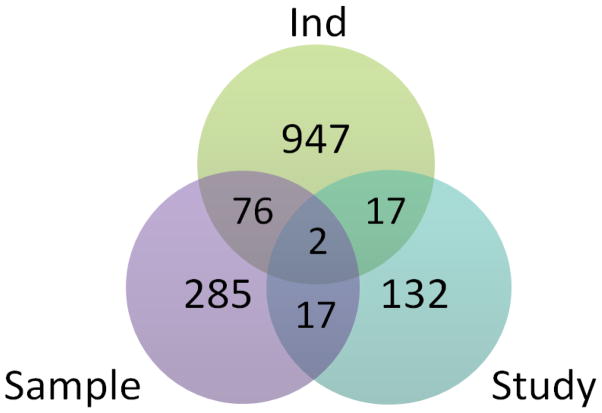
The study-level and sample-level meta-analyses uncovered 168 and 380 differentially expressed genes between AI and NT across experiments, respectively (Table 1). Supplementary Materials Table 2 presents the gene identifier, estimate of difference in expression between conditions and P-value resulting from the study-level and sample-level meta-analyses. The Venn diagram in Figure 1 depicts the overlap of differentially expressed genes from the individual-experiment analyses, study-level and sample-level meta-analyses. A total of 434 differentially expressed genes detected by the sample-level and study-level meta-analysis were not detected by the individual experiment analyses. Two genes were differentially expressed between AI and NT in all three types of analyses. The number of genes differentially expressed in one, two and more individual experiment analyses simultaneously was 1028, 14, and 0 respectively. Eight of the fourteen genes differentially expressed in two experiments had opposite signs (Table 2). Of the 380 and 168 genes differentially expressed between AI and NT detected by the sample and study-level meta-analyses respectively, 376 and 168 genes were not detected in the overlap of two individual-experiment analyses. All (17) or most (81 out of 87) genes that were differentially expressed between AI and NT in the study- and sample-level meta-analysis respectively, had consistent sign with the individual-experiment analyses. From the GO analysis of the results from the meta-analyses, the molecular function transferase activity (GO:0016772, P-value < 0.0008) was over-represented among the genes detected by the sample-level meta-analysis.
Figure 1.
Venn diagram of the number of differentially expressed genes detected by one or more individual-experiment analyses (Ind), the study-level meta-analysis (Study), and the sample-level (Sample) meta-analysis.
Table 2.
Gene information for eight cattle genes with significant differential expression in two individual-experiment analyses and opposite signs.
| Transcript | Gene Bank Bovine ID | Gene Name | Over-expressed (+) AI vs NT | Under-expressed (−) AI vs NT |
|---|---|---|---|---|
| OLIGO_07366 | CK769368 | PFDN2 - prefoldin subunit 2 | d7E1 | d25E |
| OLIGO_08124 | CN440810 | POLG2 - polymerase (DNA directed), gamma 2, accessory subunit | d7E | d25E |
| OLIGO_00784 | CR454884 | POLE2 - polymerase (DNA directed), epsilon 2 (p59 subunit) | d7E | d280X |
| OLIGO_03653 | DV784696 | HAND2 - heart and neural crest derivatives expressed 2 | d280X | d7E |
| OLIGO_05820 | BC111227 | POLR3H - polymerase (RNA) III (DNA directed) polypeptide H | d25X | d25E |
| OLIGO_06019 | DT722240 | BRRN1 - non-SMC condensin I complex, subunit H | d7E | d25E |
| OLIGO_06762 | CX948597 | MTFR1 - mitochondrial fission regulator 1 | d280X | d7E |
| OLIGO_08413 | M74083 | FNTA - farnesyltransferase, CAAS box, alpha | d7E | d25E |
d280X = 280 d extra-embryonic samples, d7E = 7 d embryos, d25X = 25 d extra-embryonic samples, d25E = 25 d embryos.
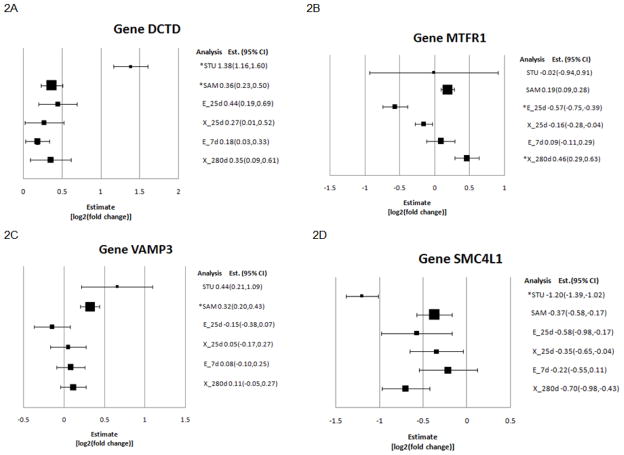
Funnel plots were used to compared the results from the individual-experiment, study-level and sample-level meta-analyses. Figure 2 presents the estimates (or log2-fold change between AI and NT and 95% confidence intervals) of four gene expression scenarios of relevance. The estimate from the study-level meta-analysis appeared distant from the rest because this value was standardized.
Figure 2.
Funnel plots of differential expression estimates (square) and 95% confidence interval limits (horizontal line) for genes deoxycytidylate deaminase (DCTD), mitochondrial fission regulator 1 (MTFR1), vesicle-associated membrane protein 3 (VAMP3), and structural maintenance of chromosomes 4-like 1 (SMC4L1), by individual-experiment, study-level (Study), and sample-level (Sample) meta-analyses. STU denotes study-level meta-analysis of standardized estimates, SAM denotes sample-level meta-analysis, and the individual-experiment analyses are denoted: d25X (25 d extra-embryonic samples), d25E (25 d embryonic samples), d280X (280 d extra-embryonic sample and d7E (7 d embryo samples). The size of the square denoting the estimate corresponds to the number of observations in the experiment (d25X n=12; d25E n=11; d280X n=29; d7E n=29; STU n = 4; SAM n = 89). Analyses detecting significant (P-value <1×10−3, fold change > 1.23) differential expression between AI and NT samples are denoted by an asterisk.
Genes differentially expressed between developmental stages and tissue sources within reproductive technology identified by the second set of meta-analyses
Six contrasts of gene expression between developmental stages and tissue sources within reproductive technology (either AI or NT) were investigated among the second-set of meta-analyses (Table 3). The first four contrasts compared the expression of genes between developmental stages within reproductive technology and tissue source. The last two contrasts compared the expression of genes between tissue sources within reproductive technology and developmental stage. The contrasts (and number of differentially expressed genes) were: 1) 7 d versus 25 d in AI embryos (483 genes), 2) 7 d versus 25 d in NT embryos (1149 genes), 3) 25 d versus 280 d in AI extra-embryonic tissue (340 genes), 4) 25 d versus 280 d in NT extra-embryonic samples (334 genes), 5) embryonic versus extra-embryonic AI samples at 25 d (82 genes), and 6) embryonic versus extra-embryonic NT samples at 25 d (11 genes). Supplementary Materials Tables 3 and 4 present the gene identifier, estimate of difference in expression between conditions and P-value for the first four contrasts and last two contrasts, respectively.
Table 3.
Number of differentially expressed genes between the levels of the conditions: developmental stages (7 d, 25 d and 280 d), tissue sources (extra-embryonic or X and embryonic or E) and reproductive technology (AI and NT).
| Factors | Within technology and source | Within technology and stage | |||||
|---|---|---|---|---|---|---|---|
| Technology | AI | =1 | = | = | |||
| NT | = | = | = | ||||
| Source | E | = | = | ≠ | ≠ | ||
| X | = | = | ≠ | ≠ | |||
| Stage | 7 d | ≠ | ≠ | ||||
| 25 d | ≠ | ≠ | ≠ | ≠ | = | = | |
| 280 d | ≠ | ≠ | |||||
| Contrast label | 7vs25d_AI_E | 7vs25_d_NT_E | 25vs280d_AI_X | 25vs280d_NT_X | EvsX_AI_25d | EvsX_NT_25d | |
| Number of genes2 | 483 | 1149 | 340 | 334 | 82 | 11 | |
Equal (=) symbol denotes the condition levels (stages, sources or technologies) shared by the samples being compared. Different symbol (≠) denotes the condition levels that differ between the samples being compared.
Number of differentially expressed genes (P-value < 1×10−3 and fold change >1.23).
The overlap of genes differentially expressed between 7 d and 25 d samples, separate for AI (483 genes) and NT (1149 genes) resulted in 373 genes (372 with GO annotations). Numerous biological processes relevant to embryo development were over-represented among the genes with over-expression at 7 d relative to 25 d, including anatomical structure development (P-value < 7 × 10−5), multicellular organismal development (P-value < 3 × 10−4), system development (P-value < 7 × 10−5), and organ development (P-value < 2 × 10−4).
Several summaries can be drawn from the number of differentially expressed genes detected by the second set of meta-analyses. First, developmental stage (7 d versus 25 d) was associated with high number of differentially expressed genes in NT embryos (1149 genes), distantly followed by AI embryos (483 genes). Second, tissue source (embryonic versus extra-embryonic) was associated with a low number of differentially expressed genes in AI at 25 d (82 genes) and even lower numbers in NT at 25 d (11 genes). Third, the number of genes differentially expressed between 25 and 280 d in extra-embryonic sources was similar in AI and NT samples (340 and 334 genes, respectively).
DISCUSSION
Genes differentially expressed between AI and NT across developmental stages and tissue sources identified by the first set of meta-analyses
The trends on the number of genes differentially expressed between AI and NT detected by the individual-experiment analyses are consistent with previous reports from each experiment (Everts et al. 2008; Everts et al. 2007a; Everts et al. 2007b; Marjani et al. 2009; Smith et al. 2007). The low overlap among the lists of genes from individual-experiment analyses may be due to the limited precision of analyses and demonstrates the potential limitation of simple comparison of lists of genes from individual-experiment analysis to combine experiments (Table 1). The pooling of consistent differential expression between AI and NT attained by the study and sample-level meta-analyses allowed the detection of 434 differentially expressed genes that were not detected by the individual-experiment analyses (Figure 1). The number of genes differentially expressed between AI and NT detected in the first set of meta-analyses is low relative to the total number of genes analyzed. This result suggests that the lower reproductive efficiency and higher incidence of large offspring syndrome in NT may be associated with significant differences in the expression of a few genes and less significant differences in the expression of the reminder genes.
The detection of functional categories and individual genes that exhibit consistent differential expression between AI and NT across developmental stages and tissue sources offers insights into the systematic impact of the technologies on the transcriptome and ultimately on reproductive efficiency. The GO molecular function transferase activity was enriched among the differentially expressed genes detected by the sample-level meta-analysis. The importance of this function on embryo development stems from the role of transferase enzymes catalyzing the transfer of a chemical group or radical from one molecule to another.
Genes that exhibit transfrase activity and were detected in this study are discussed. Gene v-akt murine thymoma viral oncogene homolog 1 (AKT1, AY781100, OLIGO_07021) was over-expressed in AI relative to NT samples. This gene is important in placental development and fetal growth and deficiencies in expression can lead to restricted development and growth (Yang et al. 2003). Likewise, activin A receptor, type IIA (ACVR2A, BF039418, OLIGO_04559) was over-expressed in AI versus NT samples, and is suggested to have multiple roles in murine organogenesis (Feijen et al. 1994). Endothelin converting enzyme 2 (ECE2, AF489575, OLIGO_01033) was over-expressed in NT relative to AI samples. This gene is known to act in human brain and heart development, along with other processes crucial to human embryonic development (Yanagisawa et al. 2000). Lastly, gene polymerase (DNA directed), gamma 2, accessory subunit (POLG2, CN440810, OLIGO_08124) is important during the blastocyst stage for mitochondrial DNA transcription and replication (Lloyd et al. 2009). These results are consistent with multiple studies that reported under-expression of genes with transferase activity in placental and embryo samples from abnormal relative to normal pregnancies in human and mouse (Prokopenko et al. 2002; El-Bassiouni et al. 2005; Toledo et al. 2006; Rausell et al., 2007; Obolenskaya et al. 2010).
Two genes differentially expressed between AI and NT in both meta-analyses and individual experiment analyses were of particular interest. The consistent pattern of these genes may be related to the lower embryo survival and efficiency of NT relative to AI. One of these genes, a gene similar to calcium/calmodulin-dependent protein kinase II delta (CAMK2D, Hs.610896, OLIGO_04736) was over-expressed in NT versus AI samples. Salilew-Wondim et al. (2010) reported CAMK2D as part of the fingerprint of the bovine pre-transfer endometrium and embryo transcriptome fingerprint. The CAMK2D gene has transferase activity and thus this finding is consistent with the over-representation of the transferase activity function among the genes differentially expressed between reproductive technologies reported in this study. The other gene was solute carrier family 22 (organic cation transporter) member 1 (SLC22A1, CK772645, OLIGO_12625), was over-expressed in NT versus AI samples. This gene encodes for membrane-spanning transporter proteins that show tissue-specific expression patterns in embryos (Verhaagh et al. 1999) and is involved in the functional capacity of the placenta (Rivera et al. 2007).
The identification of genes that exhibit opposite patterns between AI and NT across developmental stages and tissue sources offer insights into temporal- or spatial-dependent impact of the reproductive technologies on the transcriptome that could translate into reproductive efficiency differences. Eight genes were differentially expressed genes in two individual-experiment analyses but had opposite sign in each experiment (Table 2). Among them, heart and neural crest derivatives expressed 2 (HAND2, DV784696, OLIGO_03653) was over-expressed in AI versus NT samples in d280X and under-expressed in AI versus NT samples in d7E. Gene HAND2 is crucial to the survival of mouse embryos past 10.5 days post-conception (Srivastava et al. 1997) and is involved in the development of cardiovascular structures (Morikawa and Cserjesi 2008). Our finding suggests potentially different roles of HAND2 across developmental stages and tissue sources and is consistent with the over-representation of transferase activity function among the genes differentially expressed between reproductive technologies found in this study. Gene HAND2 pertains to a helix-loop-helix transcription factor family known to interact with transferase factors and this interaction is essential for modulating transcriptional activity as well as controlling differentiation (Dai and Cserjesi 2002). In addition, four of the eight genes had opposite expression sign between d7E and d25E. The polymerase, gamma 2, accessory subunit (POLG2, CN440810, OLIGO_08124) was over-expressed in AI versus NT samples in d7E, and under-expressed in AI versus NT samples in d25E. This result is consistent with a report that the expression of POLG2 increases dramatically during the blastocyst stage. This gene has also been involved in mitochondrial DNA replication that, in turn, is important for embryo survival (Facucho-Oliveira et al. 2007). The detection of POLG2 is consistent with the over-representation of transferase activity among the genes differentially expressed between reproductive technologies reported in this study because POLG2 has nucleotidyl transferase activity.
Comparison of the results from the individual-experiment analyses (Table 1) indicate that higher number of differentially expressed genes between AI and NT can be detected in embryo compared to extra-embryonic tissue and at earlier compared to later stages of development. These results provide insights into the adjustments of the embryonic and extra-embryonic system associated with reproductive technologies. Specific examples of these genes are given. Cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1, CB170385, OLIGO_08251) was under-expressed in NT versus AI samples in d280X, however was not differentially expressed in d25X. Our finding is consistent with reports of the importance of this gene in placental progesterone synthesis (Hu et al. 2004). Additional sex combs like 1 (ASXL1, DT817577, OLIGO_04004) was under-expressed in NT versus AI samples in d7E, but was not differentially expressed in d25E. Mutations in this gene were associated with myelodysplastic syndromes (Gelsi-Boyer et al. 2009) that encompass ineffective blood production in the embryo and with reduced vascularization in placental tissues. These phenotypes may be associated with the frequency of large offspring syndrome in fetuses produced by NT or with failed pregnancies. E1A binding protein p300 (EP300, Hs.517517, OLIGO_05064) was under-expressed in AI compared to NT d25E. This protein is involved in nervous system development, homeostasis, and apoptosis (Bundy et al. 2006; Byun et al. 2009). Lastly, neuropeptide gene oxytocin (OXT, M25648, OLIGO_12229) was under-expressed in AI compared to NT d280X samples (P-value < 0.005). Oxytocin is involved in smooth muscle contractions during parturition and lactation, and in maternal behavior and cardiovascular function (Gimpl and Fahrenholz 2001). Large amounts of OXT are produced by the corpus luteum in the presence of high levels prostaglandin (PGF2a), decreasing embryonic survival (Seals et al. 1998). Prostaglandin increases during times of stress usually encountered through embryos created using assisted reproductive techniques (Schallenberger et al. 1989). The previous genes are potential reliable markers for embryo survival across technologies based on the findings from our study and literature review of the role of these genes on embryo development.
Among the genes consistently differentially expressed between AI and NT in two experiments, protein phosphatase 1, regulatory subunit 12C (PPP1R12C, BM364003, OLIGO_05509) was over-expressed in NT versus AI in d7E and d25E, and was not differentially expressed in either d25X or d280X. Choong et al. (2007) reported that a miRNA targeting PPP1R12C was differentially expressed during hematopoietic differentiation obtained from erythroid cultures from umbilical cord blood. Conversely, two genes were only differentially expressed between AI and NT in extra-embryonic sources (d25X, d280X). The first gene, lymphocyte antigen 6 complex locus G6C (LY6G6C, CV798714, OLIGO_08173) is part of the Major Histocompatibility Complex III and was over-expressed in AI versus NT in both d25X and d280X. This association was confirmed by a report that LY6G6C was up-regulated in the cattle endometrium of pregnant versus control cows at 18 d of gestation (Klein et al., 2006). The second gene, thyroid hormone receptor associated protein 6 (THRAP6 or Trap25 or MED30 mediator complex subunit 30, BC110250, OLIGO_06132) was under-expressed in AI versus NT in both d25X and d280X. This finding is consistent with reports of defects in Trap function affecting nuclear receptor signaling and resulting in severe defects during embryonic development (Rienzo et al. 2010).
The study-level and sample-level meta-analyses identified genes differentially expressed between AI and NT that were detected on previously reports of the individual experiments. These genes included: polo-like kinase 4 (PLK4, CN437097, OLIGO_02307), structural maintenance of chromosomes 4-like-1 (SMC4L1, CV981642, OLIGO_02116), EBNA1 binding protein 2 (EBNA1BP2, BC102356, OLIGO_07973), topoisomerase II binding protein 1 (TOPBP1, CK977601, OLIGO_03810), SMC4L1(CV981642, OLIGO_02116), and vesicle-associated membrane protein 3 (VAMP3, BC105399, OLIGO_10402) and were reported by Everts et al. (2008).
The results from all analyses for four genes that have differential expression between AI and NT are summarized in funnel plots (Figure 2). These plots confirm the complementary properties of the individual-experiment, study-level and sample-level meta-analyses reported by Rodriguez-Zas et al. (2008) and Adams et al. (2008). Deoxycytidylate deaminase (DCTD or DCMP, CR550800, OLIGO_00155) was detected to be over-expressed in AI relative to NT by the study-level and sample-level meta-analyses yet it was not detected in any of the four individual-experiment analyses because of insufficient precision (Figure 2A). This result is in agreement with accounts that inhibition of DCTD results in the inhibition of growth in chicken embryos (Roth et al., 1963). Mitochondrial fission regulator 1 (MTFR1, CX948597, OLIGO_06762) exhibited differential expression between AI and NT in two individual-experiment analyses (d280X, d25E) but with different sign (Figure 2B). Neither the study-level nor the sample-level meta-analyses was able to detect differential expression for this gene because of the opposite expression pattern across the individual-experiment analyses. The differential expression found in the d25E experiment is consistent with previous reports of association between this protein, mitochondrial fission and embryo development (Chan 2006; Monticone et al. 2007). Vesicle-associated membrane protein 3 (VAMP3, BC105399, OLIGO_10402) was over-expressed in AI relative to NT in the sample-level meta-analysis (Figure 2C). The limited information from the combination of four estimates prevented the detection of differential expression by the study-level meta-analysis. The detection of VAMP3 by the sample-level meta-analysis is supported by studies indicating that this protein is involved in cell migration, an essential process for embryonic development (Luftman et al. 2009). Chromosomes 4-like 1 gene (SMC4L1, CV981642, OLIGO_02116) was detected to be under-expressed in AI relative to NT in the study-level meta-analysis (Figure 2D). The consideration of heterogeneity of variance among experiments in the study-level meta-analysis allowed this approach to uncover differential expression for this gene. This finding is supported by previous work that detected over-expression of SMC4L1 in human fetal wounds relative to postnatal wounds (Colwell et al. 2008).
Genes differentially expressed between developmental stages and tissue sources within reproductive technology identified by the second set of meta-analyses
The high number of differentially expressed genes detected among the first four contrasts between developmental stages within reproductive technology and the low number of differentially expressed genes detected in the last two contrasts between tissue sources within reproductive technology from the second set of meta-analysis indicates that developmental stage is associated with more changes in the transcriptome than tissue source. This result suggests that embryonic and extra-embryonic tissues may exhibit similar differences in gene expression between reproductive technologies or developmental stages.
The detection of differentially expressed genes between developmental stages or between tissue sources within reproductive technology offer insights into the plasticity of the transcriptome and impact of reproductive technology at particular developmental stages (Table 3). Among the genes identified as differentially expressed by the second-set of meta-analysis, peroxiredoxin 1 (PRDX1, BT021073, OLIGO_07962) and SLC16A1 (BC104598, OLIGO_12806) were over-expressed in 7 d relative to 25 d AI embryos and under-expressed in 7 d relative to 25 d NT embryos. The patterns for PRDX1 and SLC16A1 may be associated with more frequent developmental problems in NT relative to AI embryos. Supporting this hypothesis, Mourot et al. (2006) reported that PRDX1 is involved in antioxidant defenses and developmental competence in bovine embryos. Similarly, Somers et al. (2006) found SLC16A1 to be under-expressed in NT relative to non-NT embryos, and suggested that a reduced expression of the gene, specifically during the early blastocyst stage, may affect embryonic viability. These results are consistent with multiple studies that reported changes in genes pertaining to the system development biological process between developmental stages in various mammalian tissues (Ruberte et al., 1993; Cardoso and Lu 2006; Laranjeira and Pachnis 2009).
Among the genes differentially expressed between 25 d and 280 d (Table 3), major histocompatibility complex class II DQ beta 1 (HLA-DQB1, AJ580584, OLIGO_10954) was under-expressed in 25 d relative to 280 d in both AI and NT extra-embryonic samples. The result for HLA-DQB1 is consistent with reports that this gene is expressed in the trophoblast (outer layer cells of the blastocyst that will form the placenta) of cloned pregnancies after the fifth month of pregnancy (Hill et al. 2002). Expression of HLA-DQB1 within cattle trophoblast tissue has not been found during the first trimester pregnancies and this is supported by the lower expression of HLA-DQB1 in early extra-embryonic tissue at 25 d compared to 280 d in both technologies.
In summary, the information from four microarray experiments that profiled cattle gene expression from two reproductive technologies at different developmental stages and tissue source was integrated. The meta-analyses allowed the detection of differentially expressed genes and to gain insights into the embryo-extra embryonic transcriptomic system that would have been missed from the individual-experiment analyses. Genes affiliated to system development processes were over-represented among the genes differentially expressed between developmental stages. Genes with transferase activity function were over-represented among the genes differentially expressed between reproductive technologies. This finding is consistent with long-standing and abundant reports of differential expression on genes with transferase activity between abnormal and normal pregnancies (Gibbs et al. 1984; Mirlesse et al. 1996). The ontology categories and genes identified in this study provide candidates for further defining the differences in embryo viability between reproductive technologies and developmental stages.
Supplementary Material
Acknowledgments
Sincere appreciation is expressed to Dr. Rudi Appels for advice and editorial suggestions.
Funding: The support of the David H. and Norraine A. Baker Graduate Fellowship in Animal Sciences (HA Adams), NCI (Grant Number: 1R03CA143975), and NIH/NIDA (Grant Number: R21DA027548 and P30DA018310) (SL Rodriguez-Zas, BR Southey) are greatly appreciated.
References
- Adams HA, Southey BR, Robinson GE, Rodriguez-Zas SL. Meta-analysis of genome-wide expression patterns associated with behavioral maturation in honey bees. BMC Genomic. 2008;9:503–517. doi: 10.1186/1471-2164-9-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shahrour F, Minguez P, Tarraga J, Montaner D, Alloza E, Vaquerizas JMM, Conde L, Blaschke C, Vera J, Dopazo J. BABELOMICS: a systems biology perspective in the functional annotation of genome-scale experiments. Nucleic Acids Research (Web Server issue) 2006;34:W472–W476. doi: 10.1093/nar/gkl172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Beyhan Z, Ross PJ, Iager AE, Kocabas AM, Cunniff K, Rosa GJ, Cibelli JB. Transcriptional reprogramming of somatic cell nuclei during preimplantation development of cloned bovine embryos. Dev Biol. 2007;305:637–649. doi: 10.1016/j.ydbio.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Bundy JG, Iyer G, Gentile MS, Hu DE, Kettunen M, Maia AT, Thorne NP, Brenton JD, Caldas C, Brindle KM. Metabolic consequences of p300 gene deletion in human colon cancer cells. Cancer Res. 2006;66:7606–7614. doi: 10.1158/0008-5472.CAN-05-2999. [DOI] [PubMed] [Google Scholar]
- Byun JS, Wong MM, Cui W, Idelman G, Li Q, De Siervi A, Bilke S, Haggerty CM, Player A, Wang YH, Thirman MJ, Kaberlein JJ, Petrovas C, Koup RA, Longo D, Ozato K, Gardner K. Dynamic bookmarking of primary response genes by p300 and RNA polymerase II complexes. PNAS. 2009;106:19286–19291. doi: 10.1073/pnas.0905469106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Colwell AS, Longaker MT, Peter Lorenz H. Identification of differentially regulated genes in fetal wounds during regenerative repair. Wound Repair Regen. 2008;16:450–459. doi: 10.1111/j.1524-475X.2008.00383.x. [DOI] [PubMed] [Google Scholar]
- Cui X, Kerr MK, Churchill GA. Transformations of cDNA microarray data. Stat Appl Genet Mol Biol. 2003;2 doi: 10.2202/1544-6115.1009. Article 4. [DOI] [PubMed] [Google Scholar]
- Dai Y-S, Cserjesi P. The basic helix-loop-helix factor, hand2, functions as a transcriptional activator by binding to e-boxes as a heterodimer. J Bio Chem. 2002;277:12604–12612. doi: 10.1074/jbc.M200283200. [DOI] [PubMed] [Google Scholar]
- Dinnyes A, Tian XC, Yang X. Epigenetic regulation of foetal development in nuclear transfer animal models. Reprod Domest Anim. 2008;43:302–309. doi: 10.1111/j.1439-0531.2008.01178.x. [DOI] [PubMed] [Google Scholar]
- El-Bassiouni EA, Helmy MH, Abou Rawash N, El-Zoghby SM, Kamel MA, Abou Rayah AN. Embryopathy in experimental diabetic gestation: assessment of oxidative stress and antioxidant defence. Br J Biomed Sci. 2005;62:71–76. doi: 10.1080/09674845.2005.11732688. [DOI] [PubMed] [Google Scholar]
- Everts RE, Band MR, Liu ZL, Kumar CG, Liu L, Loor JJ, Oliveira R, Lewin HA. A 7872 cDNA microarray and its use in bovine functional genomics. Vet Immunol Immunopathol. 2005;105:235–245. doi: 10.1016/j.vetimm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Everts RE, Chavatte-Palmer P, Razzak A, Hue I, Green CA, Oliveira R, Vignon X, Rodriguez-Zas SL, Tian XC, Yang X, Renard JP, Lewin HA. Aberrant gene expression patterns in placentomes are associated with phenotypically normal and abnormal cattle cloned by somatic cell nuclear transfer. Physiol Genomics. 2008;33:65–77. doi: 10.1152/physiolgenomics.00223.2007. [DOI] [PubMed] [Google Scholar]
- Everts RE, Sommers A, Green CA, Oliveira R, Rodriguez-Zas SL, Sung LY, Du F, Evans ACO, Boland M, Fair T, Lonergan P, Renard JP, Yang X, Tian X, Lewin HA. Major differences in gene expression profiles revealed in day-25 placental tissues collected from cows carrying cloned fetuses. Plant & Animal Genomes XV Conference Abstract; San Diego, CA. 2007a. Available: http://www.intl-pag.org/pag/15/abstracts/PAG15_P05k_520.html. [Google Scholar]
- Everts RE, Sommers A, Green CA, Oliveira R, Rodriguez-Zas SL, Sung LY, Du F, Evans ACO, Boland M, Fair T, Lonergan P, Renard JP, Yang X, Tian X, Lewin HA. Major differences in gene expression profiles revealed in day-25 trophoblast but not embryonic discs collected from cattle clones. 2nd International Meeting on Mammalian Embryogenomics Conference Abstract; Paris, France. 2007b. [Google Scholar]
- Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC. Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci. 2007;120:4025–4034. doi: 10.1242/jcs.016972. [DOI] [PubMed] [Google Scholar]
- Farin CE, Farin PW, Piedrahita JA. Development of fetuses from in vitro-produced and cloned bovine embryos. J Anim Sci. 2004;82(E Suppl):E53–E62. doi: 10.2527/2004.8213_supplE53x. [DOI] [PubMed] [Google Scholar]
- Farin PW, Piedrahita JA, Farin CE. Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology. 2006;65:178–191. doi: 10.1016/j.theriogenology.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Feijen A, Goumans MJ, van den Eijnden-van Raaij AJM. Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development. 1994;120:3621–3637. doi: 10.1242/dev.120.12.3621. [DOI] [PubMed] [Google Scholar]
- Gelsi-Boyer V, Trouplin V, Adelaide J, Bonansea J, Cervera N, Carbuccia N, Lagarde A, Prebet T, Nezri M, Sainty D, Olschwang S, Xerri L, Chaffanet M, Mozziconacci MJ, Vey N, Birnbaum D. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145:788–800. doi: 10.1111/j.1365-2141.2009.07697.x. [DOI] [PubMed] [Google Scholar]
- The Gene Ontology Consortium. Gene Ontology: tool for the unification of biology. Nature Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DA, McFadyen IR, Crawfurd MD, De Muinck Keizer EE, Headhouse-Benson CM, Wilson TM, Farrant PH. First-trimester diagnosis of Lesch-Nyhan syndrome. Lancet. 1984;2:1180–1183. doi: 10.1016/s0140-6736(84)92743-0. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Hashizume K, Ishiwata H, Kizaki K, Yamada O, Takahashi T, Imai K, Patel OV, Akagi S, Shimizu M, Takahashi S, Katsuma S, Shiojima S, Hirasawa A, Tsujimoto G, Todoroki J, Izaike Y. Implantation and placental development in somatic cell clone recipient cows. Cloning Stem Cells. 2002;4:197–209. doi: 10.1089/15362300260339485. [DOI] [PubMed] [Google Scholar]
- Hill JR, Schlafer DH, Fisher PJ, Davies CJ. Abnormal expression of trophoblast major histocompatibility complex class I antigens in cloned bovine pregnancies is associated with a pronounced endometrial lymphocytic response. Biol Reprod. 2002;67:55–63. doi: 10.1095/biolreprod67.1.55. [DOI] [PubMed] [Google Scholar]
- Hu MC, Jsu HJ, Guo IC, Chung B. Function of Cyp11a1 in animal models. Molecular and Cellular Endocrinology. 2004;215:95–100. doi: 10.1016/j.mce.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Huang W, Yandell BS, Khatib H. Transcriptomic profiling of bovine IVF embryos revealed candidate genes and pathways involved in early embryonic development. BMC Genomics. 2010;11:23. doi: 10.1186/1471-2164-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpherys D, Eggan K, Akutsu H, Friedman A, Hochedlinger K, Yanagimachi R, Lander ES, Golub TR, Jaenisch R. Abnormal gene expression in cloned mice derived from embryonic stem cell and cumulus cell nuclei. PNAS. 2002;99:12889–12894. doi: 10.1073/pnas.192433399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib H, Huang W, Wang X, Tran AH, Bindrim AB, Schutzkus V, Monson RL, Yandell BS. Single gene and gene interaction effects on fertilization and embryonic survival rates in cattle. J Dairy Sci. 2009;92:2238–2247. doi: 10.3168/jds.2008-1767. [DOI] [PubMed] [Google Scholar]
- Kleber M, Sommer L. Wnt signaling and the regulation of stem cell function. Cell Biology. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Klein C, Bauersachs S, Ulbrich SE, Einspanier R, Meyer HHD, Schmidt SEM, Reichenbach H-D, Vermehren M, Sinowatz F, Blum H, Wolf E. Monozygotic twin model reveals novel embryo-induced transcriptome changes of bovine endometrium in the preattachment period. Bio Repro. 2006;74:253–264. doi: 10.1095/biolreprod.105.046748. [DOI] [PubMed] [Google Scholar]
- Kues WA, Sudheer S, Herrmann D, Carnwath JW, Havlicek V, Besenfelder U, Lehrach H, Adjaye J, Niemann H. Genome-wide expression profiling reveals distinct clusters of transcriptional regulation during bovine preimplantation development in vivo. PNAS. 2008;105:19768–19773. doi: 10.1073/pnas.0805616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira C, Pachnis V. Enteric nervous system development: Recent progress and future challenges. Auton Neurosci. 2009;151:61–69. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Lloyd RE, Romar R, Matas C, Gutierrez-Adan A, Holt WV, Coy P. Effects of oviductal fluid on the development, quality, and gene expression of porcine blastocysts produced in vitro. Reprod. 2009;137:679–687. doi: 10.1530/REP-08-0405. [DOI] [PubMed] [Google Scholar]
- Loor JJ, Everts RE, Bionaz M, Dann HM, Morin DE, Oliveira R, Rodriguez-Zas SL, Drackley JK, Lewin HA. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol Genomics. 2007;32:105–116. doi: 10.1152/physiolgenomics.00188.2007. [DOI] [PubMed] [Google Scholar]
- Luftman K, Hasan N, Day P, Hardee D, Hu C. Silencing of VAMP3 inhibits cell migration and integrin-mediated adhesion. Biochem and Biophys Res Communications. 2009;380:65–70. doi: 10.1016/j.bbrc.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjani SL, Le Bourhis D, Vignon X, Heyman Y, Everts RE, Rodriguez-Zas SL, Lewin HA, Renard JP, Yang X, Tian XC. Embryonic gene expression profiling using microarray analysis. Reprod Fertil Dev. 2009;21:22–30. doi: 10.1071/rd08217. [DOI] [PubMed] [Google Scholar]
- Mirlesse V, Jacquemard F, Daffos F, Forestier F. Fetal gammaglutamyl transferase activity: clinical implication in fetal medicine. Biol Neonate. 1996;70:193–198. doi: 10.1159/000244364. [DOI] [PubMed] [Google Scholar]
- Monticone M, Tonachini L, Tavella S, Degan P, Biticchi R, Palombi F, Puglisi R, Boitani C, Cancedda R, Castagnola P. Impaired expression of genes coding for reactive oxygen species scavenging enzymes in testes of Mtfr1/Chppr-deficient mice. Reprod. 2007;134:483–492. doi: 10.1530/REP-07-0199. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Cserjesi P. Cardiac neural crest expression of Hand2 regulates outflow and second heart field development. Circulation Res. 2008;103:1422–1429. doi: 10.1161/CIRCRESAHA.108.180083. [DOI] [PubMed] [Google Scholar]
- Mourot M, Dufort I, Gravel C, Algriany O, Dieleman S, Sirard MA. The influence of follicle size, fsh-enriched maturation medium, and early cleavage on bovine oocyte maternal mRNA levels. Mol Reprod Dev. 2006;73:1367–1379. doi: 10.1002/mrd.20585. [DOI] [PubMed] [Google Scholar]
- Obolenskaya MY, Teplyuk NM, Divi RL, Poirier MC, Filimonova NB, Zadrozna M, Pasanen MJ. Human placental glutathione S-transferase activity and polycyclic aromatic hydrocarbon DNA adducts as biomarkers for environmental oxidative stress in placentas from pregnant women living in radioactivity- and chemically-polluted regions. Toxicol Lett. 2010;196:80–86. doi: 10.1016/j.toxlet.2010.03.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister-Genskow M, Myers C, Childs LA, Lacson JC, Patterson T, Betthauser JM, Goueleke PJ, Koppang RW, Lange G, Fisher P, Watt SR, Forsberg EJ, Zheng Y, Leno GH, Schultz RM, Liu B, Chetia C, Yang X, Hoeschele I, Eilertsen KJ. Identification of differentially expressed genes in individual bovine preimplantation embryos produced by nuclear transfer: improper reprogramming of genes required for development. Biol Reprod. 2005;72:546–555. doi: 10.1095/biolreprod.104.031799. [DOI] [PubMed] [Google Scholar]
- Prokopenko VM, Partsalis GK, Pavlova NG, Burmistrov SO, Arutyunyan AV. Glutathione-dependent system of antioxidant defense in the placenta in preterm delivery. Bull Exp Biol Med. 2002;133:442–443. doi: 10.1023/a:1019845217485. [DOI] [PubMed] [Google Scholar]
- Rausell F, Pertusa JF, Gómez-Piquer V, Hermenegildo C, García-Pérez MA, Cano A, Tarín JJ. Beneficial effects of dithiothreitol on relative levels of glutathione S-transferase activity and thiols in oocytes, and cell number, DNA fragmentation and allocation at the blastocyst stage in the mouse. Mol Reprod Dev. 2007;74:860–869. doi: 10.1002/mrd.20569. [DOI] [PubMed] [Google Scholar]
- Rienzo M, Nagel J, Casamassimi A, Giovane A, Dietzel S, Napoli C. Mediator subunits: gene expression pattern, a novel transcript identification and nuclear localization in human endothelial progenitor cells. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbagrm.2010.05.001. In Press. [DOI] [PubMed] [Google Scholar]
- Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17:1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Zas SL, Ko Y, Adams HA, Southey BR. Advancing the understanding of the embryo transcriptome co-regulation using meta-, functional, and gene network analysis tools. Reprod. 2008;135:213–224. doi: 10.1530/REP-07-0391. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Zas SL, Southey BR, Whitfield CW, Robinson GE. Semiparametric approach to characterize unique gene expression trajectories across time. BMC Genomics. 2006;7:233. doi: 10.1186/1471-2164-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JS, Buccino G, Klein NW. Inhibition of growth of chick embryo by inhibition of deoxycytidylate deaminase. Science. 1963;13:1473–1474. doi: 10.1126/science.142.3598.1473. [DOI] [PubMed] [Google Scholar]
- Ruberte E, Friederich V, Chambon P, Morriss-Kay G. Retinoic acid receptors and cellular retinoid binding proteins. III. Their differential transcript distribution during mouse nervous system development. Development. 1993;118:267–282. doi: 10.1242/dev.118.1.267. [DOI] [PubMed] [Google Scholar]
- Salilew-Wondim D, Holker M, Rings F, Ulas-Cinar M, Peippo J, Tholen E, Looft C, Schellander K, Tesfaye D. Bovine pre-transfer endometrium and embryo transcriptome fingerprints as 2 predictors of pregnancy success after embryo transfer. Physiol Genomics. doi: 10.1152/physiolgenomics.00047.2010. In Press. [DOI] [PubMed] [Google Scholar]
- Schallenberger E, Schams D, Meyer HHD. Sequences of pituitary, ovarian and uterine hormone secretion during the first 5 weeks of pregnancy in dairy cattle. J Reprod Fertil. 1989;37(Suppl):277–286. [PubMed] [Google Scholar]
- Seals RC, Lemaster JW, Hopkins FM, Schrick FN. Effects of elevated concentrations of prostaglandin F2 alpha on pregnancy rates in progestogen supplemented cattle. Prostaglandins Other Lipid Mediat. 1998;56:377–389. doi: 10.1016/s0090-6980(98)00063-x. [DOI] [PubMed] [Google Scholar]
- Smith SL, Le Bourhis D, Vignon X, Heyman Y, Lewin H, Tian XC, Renard JP, Yang X. Global gene expression profiling of single, cloned bovine embryos with different developmental competencies: the good, the bad, and the ugly. Biol Reprod. 2008;77 (1 Suppl):217. [Google Scholar]
- Smith SL, Everts RE, Sung LY, Du F, Page RL, Henderson B, Rodriguez-Zas SL, Nedambale TL, Renard JP, Lewin HA, Yang X, Tian XC. Gene expression profiling of single bovine embryos uncovers significant effects of in vitro maturation, fertilization and culture. Mol Reprod Dev. 2009;76:38–47. doi: 10.1002/mrd.20927. [DOI] [PubMed] [Google Scholar]
- Smith SL, Everts RE, Tian XC, Du F, Sung LY, Rodriguez-Zas SL, Jeong BS, Renard JP, Lewin HA, Yang X. Global gene expression profiles reveal significant nuclear reprogramming by the blastocyst stage after cloning. PNAS. 2005;102:17582–17587. doi: 10.1073/pnas.0508952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers J, Smith C, Donnison M, Wells DN, Henderson H, McLeay L, Pfeffer PL. Gene expression profiling of individual bovine nuclear transfer blastocysts. Reprod. 2006;131:1073–1084. doi: 10.1530/rep.1.00967. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- Thelie A, Papillier P, Perreau C, Uzbekova S, Hennequet-Antier C, Dalbies-Tran R. Regulation of bovine oocyte-specific transcripts during in vitro oocyte maturation and after maternal-embryonic transition analyzed using a transcriptomic approach. Mol Reprod Dev. 2009;76:773–782. doi: 10.1002/mrd.21031. [DOI] [PubMed] [Google Scholar]
- Toledo MT, Ventrucci G, Marcondes MC. Cancer during pregnancy alters the activity of rat placenta and enhances the expression of cleaved PARP, cytochrome-c and caspase 3. BMC Cancer. 2006;26:168. doi: 10.1186/1471-2407-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaagh S, Schweifer N, Barlow DP, Zwart R. Cloning of the mouse and human solute carrier 22a3 (Slc22a3/SLC22A3) identifies a conserved cluster of three organic cation transporters on mouse chromosome 17 and human 6q26–q27. Genomics. 1999;55:209–218. doi: 10.1006/geno.1998.5639. [DOI] [PubMed] [Google Scholar]
- Wolfinger RD, Gibson G, Wolfinger ED, Bennett L, Hamadeh H, Bushel P, Afshari C, Paules RS. Assessing gene significance from cDNA microarray expression data via mixed models. J Comput Biol. 2001;8:625–637. doi: 10.1089/106652701753307520. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S, Clouthier DE, Yanagisawa M. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105:1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein Kinase B alpha/Akt1 regulates placental development and fetal growth. J Bio Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.