Abstract
Objectives
Low-level cadmium exposure, e.g., urinary cadmium < 2.0 μg/g creatinine, is widespread; recent data suggest nephrotoxicity even at these lower levels. Few studies have examined the impact of low-level cadmium exposure in workers who are occupationally exposed to other nephrotoxicants such as lead.
Methods
We evaluated associations of urine cadmium, a measure of cumulative dose, with four glomerular filtration measures and N-acetyl-β-D-glucosaminidase (NAG) in lead workers. Recent and cumulative lead dose was assessed via blood and tibia lead, respectively.
Results
In 712 lead workers, mean (SD) blood and tibia lead, urine cadmium, and estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease equation were 23.1 (14.1) μg/dl, 26.6 (28.9) μg Pb/g bone mineral, 1.15 (0.66) μg/g creatinine, and 97.4 (19.2) ml/min/1.73m2, respectively. After adjustment for age, sex, body mass index, urine creatinine, smoking, alcohol, education, annual income, diastolic blood pressure, current or former lead worker job status, new or returning study participant, and blood and tibia lead, higher ln-urine cadmium was associated with higher calculated creatinine clearance, eGFR (β = 8.7 ml/min/1.73 m2; 95% CI = 5.4, 12.1) and ln-NAG but lower serum creatinine.
Conclusions
Potential explanations for these results include a normal physiologic response in which urine cadmium levels reflect renal filtration; the impact of adjustment for urine dilution with creatinine in models of kidney outcomes; and cadmium-related hyperfiltration.
Keywords: cadmium, kidney function, lead exposure, urine creatinine
INTRODUCTION
Low-level, environmental cadmium exposure, i.e., urinary cadmium < 2.0 μg/g creatinine, is widespread. Similar to lead, cadmium is a kidney proximal tubular toxicant that accumulates in the body resulting in chronic endogenous exposure. A known cause of chronic kidney disease (CKD) in the occupational setting[1], recent data indicate nephrotoxicity at lower levels of exposure.[2], [3, 4] Furthermore, in a recent US general population analysis, participants with higher levels of both blood lead and cadmium had increased risk for estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2 compared to those with lower levels of both metals.[4] Thus, environmental cadmium co-exposure may contribute to nephrotoxicity in lead workers.[5] To address this hypothesis, we performed a cross-sectional analysis examining associations between urine cadmium, a measure of cumulative dose, and kidney function in 712 current and former lead workers in the Republic of Korea.
MATERIALS AND METHODS
Study overview and design
We performed a cross-sectional analysis of data from current and former lead workers who completed the fourth evaluation in a longitudinal study of inorganic lead exposed workers. Evaluations were performed between April 8, 2004 and September 24, 2005. All participants provided written, informed consent. The study protocol was approved by Institutional Review Boards at the SoonChunHyang University School of Medicine and the Johns Hopkins University Bloomberg School of Public Health. Participation in the study was voluntary, and workers were paid approximately $50 for their time and effort.
Study population
As previously described,[6, 7] participants in the initial cohort of this study were recruited between 1997 and 1999 (phase I study participants) from 26 facilities including secondary lead smelters and plants that produced lead batteries, lead oxide, lead crystal, or radiators. The population is 100% Korean. The original participants were followed longitudinally for three annual evaluations. In 2004, recruitment for three additional annual evaluations was begun; 498 of the 803 (62 %) lead workers in the original phase I cohort were re-enrolled. Due to the economic conditions in Asia during the late 1990s, many workers in the initial study cohort were laid off and lost to follow-up. In addition, 279 new participants were recruited (phase II study participants) from 18 of the original facilities and 4 new facilities including automobile battery, instrument and lead crystal manufacturers and a primary lead smelter. Inclusion criteria included occupational lead exposure and, for new participants, age ≥ 40 years in order to enrich the study with participants at greater risk for adverse kidney outcomes. No medical exclusionary criteria were used. At the end of this second enrollment phase (September 24, 2005), 778 current and former lead workers had completed the fourth of six evaluations in the overall longitudinal study. In order to optimize study data for both cross-sectional and longitudinal cadmium analyses while addressing funding constraints, urine cadmium was measured in fourth evaluation samples in the 712 workers who came to both the fourth and fifth evaluations. Cross-sectional analysis of fourth evaluation data in these 712 workers is the focus of the current analysis.
Data collection
Data collection was completed at the study plants for large employers. Workers from smaller facilities and former lead workers were evaluated at the Institute of Industrial Medicine of the SoonChunHyang University in Asan, the University hospital in Chonan or hospitals near their current or former workplaces. A standardized, interviewer-administered questionnaire was used to elicit information on demographics; medical history including physician diagnoses; medications; smoking and alcohol use; education; income; and occupational history. Blood pressure was measured with the IntelliSense™ blood pressure monitor (Model HEM-907; Omron; Vernon Hills, IL) using an appropriately sized cuff via a standardized protocol in which the participant was seated for 5 minutes before three measurements at 30 second intervals were obtained. Data and biologic specimens also included: height and weight measurements to assess body mass index (BMI); a blood specimen (for serum creatinine and blood lead as a recent dose measure); four-hour urine collection (for creatinine to calculate creatinine clearance and cadmium [cumulative exposure measure except in cadmium nephropathy]); a spot urine sample collected just before starting the four-hour urine collection (for NAG and creatinine), and tibia lead as a cumulative dose measure.
Laboratory methods
Urine cadmium was measured in the Trace Elements section of the Laboratory of Inorganic and Nuclear Chemistry at the New York State (NYS) Department of Health’s Wadsworth Center (Albany, NY, USA) which is NYS’s principal reference laboratory for the measurement of trace metals in urine. Urine specimens for cadmium analysis were collected and frozen in containers that were pre-certified for low-level trace element measurements by the analyzing laboratory and stored at −80°C in 5-mL Nalgene Cryogenic polypropylene tubes until analyzed. The analysis was carried out using a Perkin Elmer Sciex ELAN DRC II inductively coupled plasma-mass spectrometer (PerkinElmer Life and Analytical Sciences, Shelton, CT) equipped with dynamic reaction cell (DRC-ICP-MS) technology. The ICP-MS was operated according to a standard operating procedure that is certified and approved for use in New York State.[8]
Briefly, 500 μL of urine was diluted 1+19 with 2% (v/v) HNO3 (Veritas double-distilled; GFS Chemicals, Powell, OH), 0.005% Triton X-100 as a surfactant (Sigma-Aldrich, St. Louis, MO), 1 mg/L gold and 10 μg/L gallium, rhodium, yttrium, and iridium (Spex Certiprep, Inc., Mutuchen, NJ) as internal standards. Multi-element calibration standards were prepared from a National Institute of Science and Technology (NIST)-traceable stock solution (High Purity Standards, Charleston, SC) and a six-point calibration curve was used for each element. Pooled human urine was used to matrix-match the calibration standards. Calibration solutions, reagents and urine samples were prepared under conditions (Clean Room and Class IIB Biosafety Cabinet) certified as Class 100 or better. Urine 114Cd was measured in the standard mode along with molybdenum to correct for a potential polyatomic interference from 98Mo16O+ at m/z 114.
This analytical method has been validated against NIST SRM 2670a Toxic Elements in Urine, as well as secondary reference materials. The laboratory participates successfully in a number of External Quality Assessment Schemes specifically for trace elements in urine, including those operated by (a) L’Institut National de Santé Publique du Québec, Centre de Toxicologie du Québec, Canada, (b) Friedrich-Alexander University, Erlangen, Germany, and (c) the University of Surrey, Guildford, UK Trace Elements scheme. The laboratory organizes the NYS Department of Health’s Proficiency Testing program for trace elements in urine. Quality control during the course of the study included analysis of four concentration levels of urine-based internal quality control (IQC) materials after calibration and every ten specimens and at the end of every analytical run. The coefficient of variation of the mean of the first and last IQC sample per day on 9 days over the 6 month period in which these samples were assayed was 9.1% at 0.08 μg/L, 9.1% at 2.06 μg/L, 6.2% at 6.97 μg/L, and 1.6% at 10.43 μg/L. The method detection limit for cadmium in urine, calculated from data obtained over 20 independent runs, was 0.02 μg/L, while the limit of quantitation was 0.07 μg/L. The possibility of a polyatomic interference from the presence of high levels of molybdenum on low concentrations of cadmium in urine was reported recently.[9] This can result in the formation of molybdenum oxide in the argon plasma leading to a polyatomic overlap at all the major isotopes for cadmium. Therefore, we measured molybdenum concentration in urine to provide the option to use a mathematical correction based on the molybdenum concentration in each urine sample.
Blood lead was measured with an Hitachi 8100 Zeeman background-corrected atomic absorption spectrophotometer [10] (Hitachi Ltd. Instruments, Tokyo, Japan) at the Institute of Industrial Medicine, a certified reference laboratory for lead in South Korea. Tibia lead levels were assessed via a 30-minute measurement of the left mid-tibia diaphysis using 109Cd in a back-scatter geometry to fluoresce the K-shell X-rays of lead. The lead X-rays were recorded with a radiation detector and then quantified and compared to calibration data to estimate the concentration of lead in bone.[11-13]
Serum and urine creatinine were measured via a Dimension® clinical chemistry system using a Flex reagent cartridge in a modified kinetic Jaffe assay (model RxL; Dade Behring, Glasgow, DE, USA). Measured creatinine clearance, in mL/minute, was defined as: ([urinary creatinine in mg/dl × urine volume in ml]/serum creatinine in mg/dl) / collection time in minutes. Glomerular filtration rate, in mL/min/1.73 m2, was estimated using the abbreviated Modification of Diet in Renal Disease (MDRD) formula: 186.3 × (serum creatinine)−1.154 × (age)−0.203 × 0.742 (if the participant was female) [14, 15]. Creatinine clearance was calculated with the Cockcroft-Gault equation ([140 - age] × weight in kg)/(72 × serum creatinine) for males; multiplied by 0.85 for females.[16] NAG activity (expressed in μmol substrate converted per hour) was measured using the P.P.R. NAG test kit (P.P.R. Diagnostics, Ltd.; London, UK).
Statistical analysis
The goals of the analysis were to: 1) evaluate associations between urine cadmium levels and kidney outcomes (serum creatinine, eGFR, measured and calculated creatinine clearances, and NAG) in current and former lead workers, while controlling for covariates; and 2) to evaluate effect modifiers of those associations, also controlling for covariates. Statistical analysis was completed using SAS/STAT and SAS/GRAPH software, Version 9.1 of the SAS System for Windows, Copyright © 2002-2003 SAS Institute Inc (Cary, NC, USA).
Initially, variable distributions were examined. Cadmium adjusted for urine creatinine (μg/g creatinine) was right skewed and thus was ln-transformed to minimize influential outliers. The NAG distribution also showed departures from normality and levels were thus ln-transformed; the adequacy of this transformation was subsequently confirmed by examination of the residuals from the final regression models. In linear regression models, associations of urine cadmium with outcomes were evaluated in two ways: the traditional approach in which cadmium concentration is adjusted for urine dilution by dividing by urine creatinine; and a more recent approach in which urine cadmium and creatinine are both included as separate covariates in the model.[17] The latter approach has been recommended for study populations that include groups likely to differ by muscle mass and both men and women with a wide age range are represented in our study population. Analyses using urine cadmium corrected for molybdenum concentration were similar to those with uncorrected data, therefore results from uncorrected data are presented.
Covariate selection utilized a priori variables (age, gender, and BMI [weight in kilograms divided by the square of height in meters]) in modeling that initially included urine cadmium with other biologically relevant variables in separate models. Variables were retained in the final model if they substantially changed either the urine cadmium regression coefficient or the explanatory value (r2) of the model for any of the kidney outcomes; were statistically significant, or were relevant based on a priori knowledge or hypotheses inherent to this study (e.g., blood and tibia lead and enrollee status [phase I vs. II study entry]). Additional covariates that were assessed for inclusion using this approach were diabetes and hypertension (both based on participant report of physician diagnosis or medication use); regular analgesic use (based on questionnaire data on medication usage); self-reported work status (current vs. former lead worker); enrollee status (phase I vs. II study entry), systolic and diastolic blood pressure (average of three measurements); tobacco use (smoking status: never, former, current; smoking dose [cigarettes per day × years of smoking) in quartiles for current smokers and dichotomized for former smokers; alcohol consumption (never, former, current); education (< middle school graduate, < high school graduate, high school graduate, > high school) and annual income (≤ 10, 10-20, 20-30, 30-40, and > 40 million won). Blood and tibia lead were added to final models after all other covariates were selected. Associations between urine cadmium and kidney outcomes were also examined in three groups stratified by tertile of eGFR in order to determine whether associations were potentially consistent with reverse causality i.e., present only in participants with reduced renal function. Finally, in order to examine the impact of lead-related hyperfiltration, models were examined in former workers. In a sensitivity analysis, models were assessed in 684 lead workers with the 28 primary lead smelter workers removed since a wider range of metal exposures are encountered in primary smelters. Results were consistent with the larger population (data not shown). As in previous analyses,[7] models were evaluated for linear regression assumptions and the presence of outlying points using added variable plots[18]. When applicable, models were repeated without outliers. Models were also assessed for collinearity through examination of variance inflation factors and conditional indices.
RESULTS
Selected Demographics, Exposure, and Health Outcome Measures
Information on demographics, cadmium and lead biomarkers, kidney outcomes, and selected covariates from the fourth evaluation is presented in Table 1 for all 712 lead workers and separately for the three age and current/former lead worker status groups. Women and former lead workers comprised 149 (20.9%) and 234 (32.9%), respectively, of the population. Mean (SD) urine cadmium and blood and tibia lead levels, in all lead workers, were 1.15 (0.66) μg/g creatinine, 23.1 (14.1) μg/dL, and 26.6 (28.9) μg Pb/g bone mineral, respectively. Mean values for the glomerular filtration measures were in the normal range.
Table 1.
Selected demographic, exposure, and health outcome measures from the fourth evaluation in current and former lead workers (n = 712)
| Characteristic | N (%) |
|---|---|
| Female | 149 (20.9) |
| Diabetes | 27 (3.8) |
| Hypertension | 86 (12.1) |
| Regular analgesic use | 17 (2.4) |
| Smoking | |
| Never | 242 (34.0) |
| Current | 310 (43.5) |
| Former | 160 (22.5) |
| Alcohol use | |
| Never | 109 (15.3) |
| Current | 571 (80.2) |
| Former | 32 (4.5) |
| Education | |
| < Middle school graduate | 180 (25.3) |
| < High school graduate | 182 (25.6) |
| High school graduate | 294 (41.3) |
| Median | Mean (SD) | Range | |
|---|---|---|---|
| Age, years | 46.7 | 47.6 (7.9) | 24.1-71.3 |
| BMI, kg/m2 | 24.2 | 24.2 (2.8) | 15.6-33.3 |
| Systolic blood pressure, mm Hg | 121.5 | 123.7 (15.5) | 90.5-213.3 |
| Diastolic blood pressure, mm Hg | 74.3 | 75.2 (12.0) | 46.0-147.0 |
| Urine cadmium, μg/g creatinine | 0.98 | 1.15 (0.66) | 0.25-4.92 |
| Blood lead, μg/dl | 21.4 | 23.1 (14.1) | 1.9-74.4 |
| Tibia lead, μg Pb/g bone mineral* | 19 | 26.6 (28.9) | −12 -231 |
| Lead job duration, years | 13.2 | 13.1 (7.3) | 0.23-37.4 |
| Serum creatinine, mg/dl | 0.87 | 0.87 (0.15) | 0.42-1.53 |
| Estimated GFR, ml/min/1.73 m2 | 95.5 | 97.4 (19.2) | 23.6-189.7 |
| Measured creat. clearance, ml/min | 110.8 | 111.1(30.7) | 9.9-222.9 |
| Calculated creat. clearance, ml/min | 93.6 | 95.2 (21.9) | 30.5-209.9 |
| NAG, μmol/h/g creatinine | 317.0 | 383.5 (278.7) | 46.1-3778.5 |
Similar information was also compared by phase I and II study enrollment status (Table 1 online supplement). As expected, the proportion of former lead workers was higher among returning phase I study enrollees. The two groups also differed by current smoking, alcohol ingestion, education, and proportion of women. Urine cadmium levels were similar, however, phase II enrollees had lower lead dose levels.
In all 712 lead workers, urine cadmium was positively correlated with tibia lead and age but negatively correlated with blood lead (rs = 0.08, 0.46, and −0.11 respectively, p < 0.05 for each). However, when examined by worker status, urine cadmium remained associated with age in all three groups but was only correlated with the lead biomarkers in younger, current workers; both associations were positive (rs = 0.19; p = 0.003 and r = 0.12; p = 0.06 for blood and tibia lead, respectively; Table 2).
Table 2.
Spearman correlation coefficients for age and cadmium and lead biomarkers in 712 current and former lead workers
|
Current workers Age ≤ 44.8 years mineral |
Urine cadmium μg/g |
Blood lead μg/dL |
Tibia lead μg/ g bone |
|---|---|---|---|
| Age | 0.34# | 0.04 | 0.13* |
| Urine cadmium | 0.19** | 0.12 | |
| Blood lead | 0.45# |
|
Current workers Age > 44.8 years mineral |
Urine cadmium μg/g |
Blood lead μg/dL |
Tibia lead μg/ g bone |
|---|---|---|---|
| Age | 0.17** | 0.17** | 0.19** |
| Urine cadmium | −0.02 | −0.07 | |
| Blood lead | 0.66# |
|
Former workers mineral |
Urine cadmium μg/g | Blood lead μg/dL | Tibia lead μg/ g bone |
|---|---|---|---|
| Age | 0.33# | 0.21** | 0.40# |
| Urine cadmium | −0.001 | 0.07 | |
| Blood lead | 0.61# |
p-value < 0.05
p-value < 0.01
p-value < 0.001
Associations of Urine Cadmium with Kidney Outcomes
In all 712 lead workers, after adjustment, ln urine cadmium was significantly (p < 0.05) associated with three of the four glomerular filtration measures in a paradoxical pattern; higher urine cadmium was associated with lower serum creatinine, higher calculated creatinine clearance and higher eGFR (Table 3).
Table 3.
Associations between urine cadmium, modeled with and without urine creatinine adjustment, and kidney outcomes in 712 lead workersa
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Kidney Outcome | β coeff (95 % CI) | Model r2 | β coeff (95 % CI) | Model r2 | β coeff (95 % CI) | Model r2 |
| Serum Creatinine, mg/dl | ||||||
| Ln-Urine Cadmium μg/g creat. | −0.066 (−0.090, −0.042)# | 0.34 | ||||
| Ln-Urine Cadmium μg/L | −0.066 (−0.091, −0.041)# | 0.34 | −0.018 (−0.032, −0.004)* | 0.31 | ||
| Ln-Urine Creatinine, mg/dl | 0.065 (0.038, 0.093)# | NA | ||||
| Calculated Creatinine Clearance, ml/min | ||||||
| Ln-Urine Cadmium μg/g creat. | 6.3 (3.3, 9.2)# | 0.50 | ||||
| Ln-Urine Cadmium μg/L | 6.6 (3.6, 9.6)# | 0.50 | 2.8 (1.1, 4.5)** | 0.50 | ||
| Ln-Urine Creatinine, mg/dl | −5.1 (−8.4, −1.8)** | NA | ||||
| Est. Glomerular Filtration Rate, ml/min/1.73 m2 | ||||||
| Ln-Urine Cadmium μg/g creat. | 8.7 (5.4, 12.0)# | 0.17 | ||||
| Ln-Urine Cadmium μg/L | 8.7 (5.4, 12.1)# | 0.17 | 2.3 (0.42, 4.2)* | 0.15 | ||
| Ln-Urine Creatinine, mg/dl | −8.6 (−12.3, −4.9)# | NA | ||||
| Measured Creatinine Clearance, ml/min | ||||||
| Ln-Urine Cadmium μg/g creat. | −3.4 (−8.5, 1.7) | 0.24 | ||||
| Ln-Urine Cadmium μg/L | −1.4 (−6.4, 3.6) | 0.29 | 7.1 (4.3, 10.0)# | 0.27 | ||
| Ln-Urine Creatinine, mg/dl | 11.5 (5.9, 17.1)# | NA | ||||
| Ln-NAG, μmol/h/g creatinine | ||||||
| Ln-Urine Cadmium μg/g creat. | 0.27 (0.17, 0.37)# | 0.20 | ||||
| Ln-Urine Cadmium μg/L | 0.26 (0.16, 0.36)# | 0.20 | 0.03 (−0.03, 0.09) | 0.16 | ||
| Ln-Urine Creatinine, mg/dl | −0.31 (−0.42, −0.20)# | NA | ||||
Models also adjusted for age, gender, BMI, work status (current vs. former lead worker), smoking dose [cigarettes per day × years of smoking) in quartiles for current smokers and ex-smoker status, diastolic blood pressure; alcohol consumption (never, former, current); education (< middle school graduate, < high school graduate, high school graduate, > high school), annual income (≤ 10, 10-20, 20-30, 30-40, and > 40 million won), enrollee status (phase I vs. II study entry), and blood and tibia lead.
p-value < 0.05
p-value < 0.01
p-value < 0.001
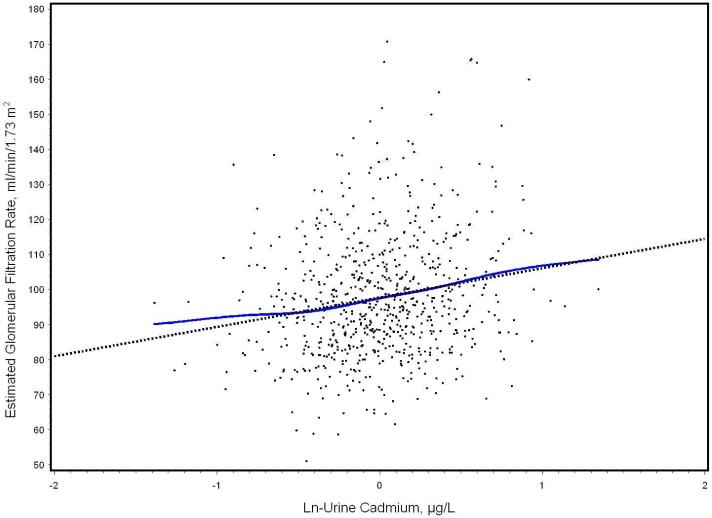
The added variable plot for the fully adjusted association between ln-urine cadmium and eGFR illustrates the direction of this association (Figure 1). In contrast, urine cadmium was not associated with measured creatinine clearance. However, urine cadmium was significantly and positively associated with NAG, the sole kidney outcome in this analysis that does not reflect glomerular filtration but rather is a proximal tubular marker. Associations were consistent in models with cadmium and urine creatinine entered as separate covariates without ln-transformation (data not shown). Associations between urine cadmium and kidney measures were not substantially altered by adjustment for lead dose. These paradoxical associations were apparent early in model building. Higher ln-urine cadmium (modeled as μg/g creatinine) was associated with lower serum creatinine in unadjusted models; the beta coefficient declined but remained significant in a priori models adjusted for age, sex and BMI (data not shown). Similarly, higher ln-urine cadmium was associated with higher eGFR in unadjusted models although the association was not statistically significant unless adjusted for either sex or age. In contrast, higher ln-urine cadmium was significantly associated with lower calculated creatinine clearance in unadjusted models. Adjustment for age resulted in significant associations in the paradoxical direction observed in the final models
Figure 1.
Added variable plot of the association of ln urine cadmium with eGFR, after full adjustment in 712 Korean lead workers (model results in Table 3). The adjusted regression line (dotted) and the smoothed line (solid) determined by a scatter plot smoothing method (cubic spline).[40], which was estimated using the scatterplot smoothing method (SAS/GRAPH software), are shown with the residuals. Both lines have been adjusted for covariates. For ease of interpretation, the Y axis has been scaled, so that the plotted residuals are centered around the eGFR mean, rather than zero.
Ln urine creatinine was associated with all kidney outcomes analyzed but in the opposite direction compared to associations between ln urine cadmium and each outcome (Table 3). In models without adjustment for urine dilution, ln urine cadmium was associated with all kidney outcomes except NAG (Table 3). However, associations were three to four-fold higher with adjustment for ln urine creatinine for all of the filtration measures except measured creatinine clearance. In order to evaluate whether selection bias could have contributed to the observed associations, we adjusted for enrollee status in regression models. Although phase II enrollees had significantly lower glomerular filtration measures, associations between cadmium and those measures were unchanged.
Urine Cadmium Associations in eGFR Subgroups
Models in the population stratified by tertile of eGFR were examined in order to determine whether associations were present only in participants with reduced kidney function and thus potentially consistent with reverse causality (lower levels in urine due to lack of cadmium excretion). After adjustment, paradoxical associations between urine cadmium and eGFR were observed in participants in the highest (≥ 103.0 ml/min/1.73 m2) as well as the lowest eGFR tertile (< 88.9 ml/min/1.73 m2); however, no significant association was observed in the middle tertile (Table 4). In contrast, associations with NAG were consistently positive in the expected direction in all three eGFR groups.
Table 4.
Associations between urine cadmium and selected kidney outcomes in models stratified by eGFR tertile (n = 712)a
|
eGFR < 88.9 ml/min/1.73 m2 (n=228) |
eGFR 88.9-102.9 ml/min/1.73 m2 (n=240) |
eGFR ≥ 103.0 ml/min/1.73 m2 (n=234) |
||||
|---|---|---|---|---|---|---|
| Kidney Function Measure | β coeff (95 % CI) | Model r2 | β coeff (95 % CI) | Model r2 | β coeff (95 % CI) | Model r2 |
| Est. Glomerular Filtration Rate, ml/min/1.73 m2 | ||||||
| Ln-Urine Cadmium μg/L | 3.1 (0.7, 5.4)* | 0.26 | 0.92 (−0.5, 2.3) | 0.10 | 6.5 (1.7, 11.3)** | 0.13 |
| Ln-Urine Creatinine, mg/dl | −3.2 (−5.8, −0.7)* | −0.6 (−2.1, 0.9) | −4.9 (−10.4, 0.7) | |||
| Ln-NAG, μmol/h/g creatinine | ||||||
| Ln-Urine Cadmium μg/L | 0.22 (0.06, 0.39)** | 0.32 | 0.18 (−0.02, 0.38) | 0.23 | 0.34 (0.17, 0.51)# | 0.28 |
| Ln-Urine Creatinine, mg/dl | −0.25 (−0.43, −0.07)** | −0.26 (−0.47, −0.05)* | −0.43 (−0.62, −0.23)# | |||
Models also adjusted for age, gender, BMI, ln-urine creatinine, work status (current vs. former lead worker), smoking dose [cigarettes per day × years of smoking) in quartiles for current smokers and ex-smoker status, diastolic blood pressure; alcohol consumption (never, former, current); education (< middle school graduate, < high school graduate, high school graduate, > high school) and annual income (≤ 10, 10-20, 20-30, 30-40, and > 40 million won), enrollee status (phase I vs. II study entry), and blood and tibia lead.
p-value < 0.05
p-value < 0.01
p-value < 0.001
Associations in Former Lead Workers
In order to determine whether the paradoxical cadmium associations could be due to lead-related hyperfiltration, fully adjusted models were examined in 230 former lead workers whose blood lead levels were negatively associated with eGFR, a pattern consistent with traditional lead-related nephrotoxicity (β coeff [95 % CI] = −0.38 [−0.74, −0.03]. However, urine cadmium remained positively associated with eGFR in these workers as well (β coeff [95 % CI] = 8.7 [3.2, 14.2]).
DISCUSSION
We compared associations of urine cadmium with four measures of glomerular filtration and NAG, a proximal tubular early biologic effect marker, to determine the impact of cadmium co-exposure on kidney function in the presence of occupational lead exposure. In 712 lead workers, higher urine cadmium was associated with higher NAG. Paradoxically, higher urine cadmium was also associated with lower serum creatinine and higher eGFR and calculated creatinine clearance. In subgroup analyses stratified by tertile of eGFR, these associations were not confined to participants with eGFR in the lowest tertile. Urine cadmium was positively associated with eGFR even in former workers in whom lead-related hyperfiltration was not apparent.
Although the study population is occupationally exposed to lead, mean and median urine cadmium levels are consistent with environmental exposure in the Korean general population. Median blood cadmium was 1.55 μg/L in 1902 participants in the Korean National Health and Nutrition Examination Survey conducted in 2005.[19] Our cadmium levels were measured in urine, however, in US NHANES data, median urine cadmium levels in adults, although lower, are similar to median blood cadmium (0.27 μg/g creatinine and 0.4 μg/L, respectively, in NHANES from 2003-4).[20] Questionnaire data in our study supports the non-occupational nature of cadmium exposure. Only twelve participants reported occupational exposure on questionnaire; seven used cadmium as a stabilizer in plastics and two were among the 28 participants employed in a primary lead smelter where occupational cadmium exposure is likely (median urine cadmium in those 28 employees was 1.47 μg/g creatinine).
Cadmium, at higher levels of exposure, is a well established nephrotoxicant associated with decreased glomerular filtration and chronic kidney disease.[1] However, few studies have evaluated associations between low-level cadmium dose and glomerular filtration. Both blood and urine cadmium were associated with lower creatinine clearance and serum cystatin C based eGFR after adjustment for sociodemographic factors, CKD risk factors, and blood lead in 820 Swedish women 53–64 years of age.[2] In a general US population, blood cadmium was associated with lower eGFR after adjustment.[4] In contrast, neither blood nor urine cadmium was associated with measured or calculated creatinine clearance in the baseline Cadmibel study, at least in models that included blood lead.[21] Similarly, blood cadmium was not associated with serum creatinine in a study of 500 adults;[22] neither blood nor urine cadmium was significantly associated with serum cystatin C in 200 adolescents.[23] Urine cadmium was obtained in a subset of lead workers (n=191) in the first evaluation of the current longitudinal study and was significantly associated with NAG but not blood urea nitrogen, serum creatinine, measured or calculated creatinine clearance or retinol-binding protein.[7]
Two studies have reported associations between urine cadmium and glomerular filtration measures in the same paradoxical direction observed herein. After adjustment, higher urine but not blood cadmium was associated with higher measured creatinine clearance in the five year follow-up of the Cadmibel study although the level of statistical significance was not reported. [24] Similarly, in European children, in models that adjusted for sex, lead, mercury, arsenic, and significant metal cross-products, higher urine cadmium was associated with lower serum creatinine but was not significantly associated with serum β2 microglobulin or cystatin C [25]. Blood cadmium was not associated with any of these measures. Higher blood lead was associated with lower levels of all three, consistent with hyperfiltration.
NAG is commonly used in research on the nephrotoxicity of low-level cadmium exposure. Associations between urine cadmium and higher NAG, as observed in our data, have been reported in a number of populations [2, 26-29], including in the European children in whom higher urine cadmium was associated with lower serum creatinine [25]; in longitudinal data [24]; and, as noted above, in the first evaluation of this study.[7]
A number of hypotheses for the paradoxical associations (higher urine cadmium with lower serum creatinine and higher glomerular filtration) observed in these data must be considered. Given the similarities between lead and cadmium, it is possible that these associations represent cadmium-induced hyperfiltration. However, we are not aware of longitudinal animal or human data with cadmium exposure showing an initial increase in glomerular filtration followed by subsequent decline similar to the data of Khalil-Manesh, et al. [30, 31] that was critical to our understanding of the lead associations we observed in these workers.
Adjustment of cadmium dose for urine dilution using urine creatinine may also be a factor. Although there is a large literature on optimal approaches to adjustment of urinary biomarkers for urine dilution, [17, 32, 33] the additional complexity of assessing associations between urine markers and kidney outcomes is rarely addressed. In our data, urine cadmium was adjusted with the same urine creatinine value that was used in the measured creatinine clearance calculation. This may be a factor in the inconsistent associations with measured creatinine clearance compared to the three other filtration measures. However, urine creatinine was also significantly associated with serum creatinine, calculated creatinine clearance, eGFR, and NAG in fully adjusted models. The correlation between creatinine levels from the four-hour urine collection and those from the spot urine used to adjust NAG (rs = 0.49; p < 0.0001) is a likely explanatory factor in the association between urine creatinine and NAG. The explanation for associations between urine creatinine and the serum creatinine based kidney outcomes is less clear since these associations were adjusted for age, sex and BMI.
In our data, urine creatinine was positively associated with serum creatinine and calculated and measured creatinine clearances but not eGFR in simple linear regression models (data not shown). After adjustment for age, sex and BMI, urine creatinine remained significantly associated only with measured creatinine clearance; the direction of the association was still positive. However, as shown in Table 3, when urine cadmium was further added to the model, urine creatinine was again significant. These results raise concerns regarding associations between urine biomarkers and kidney function when urine creatinine is used in both and potentially for kidney outcomes using only serum creatinine. Few data are available to address this issue. Akesson et al. reported no major impact on their results when urine cadmium was adjusted with urine creatinine instead of density.[2] Studies in which non-creatinine based measures of glomerular function were assessed reported higher cadmium dose associations with higher cystatin C and lower cystatin C-based eGFR [2] but no significant associations were observed with serum cystatin C in two other studies.[23, 25]
It is also possible that these associations reflect reverse causality, a mechanism usually defined as an increase in blood concentration of a nephrotoxicant as a result of decreased excretion in CKD due to a cause unrelated to the nephrotoxicant. However, in our data, urine cadmium was positively associated with eGFR in the highest as well as the lowest tertile. Thus, if reverse causality is involved, urine cadmium levels reflect filtration over a wider range which would imply that urine cadmium, at these low environmental exposure levels, reflects kidney function as well as or perhaps even more than exposure. This seems unlikely given the widespread use of urine cadmium as an internal dose measure and associations reported in research to date such as the association with lung cancer observed prospectively in the Cadmibel study.[34] The positive association between urine cadmium and NAG in our population also suggests toxicity rather than renal filtration. Urinary NAG is generated by proximal tubular cells, reflecting necrosis in the case of the NAG-B isoenzyme [35] and milder forms of proximal tubular alteration for the NAG-A isoenzyme which contributes more to the total NAG used in this study. [36] Thus, NAG, although a sensitive marker of cadmium nephrotoxicity, is not filtered at the glomerulus and so would not be expected to increase with GFR. However, as noted above, the NAG associations may be related to urine creatinine. Paradoxical cadmium associations may also represent a secondary effect of lead hyperfiltration. However, cadmium was positively associated with glomerular filtration measures in populations in which lead-related hyperfiltration was not observed: former lead workers in this study and in the Cadmibel general population study. Alterations in metal excretion due to protein binding may also be involved in paradoxical metal-kidney associations.[37]
Finally, limited ability to assess kidney function accurately in this population may also be a factor. The MDRD estimating equation underestimates GFR in the normal range which is relevant for most occupational populations including these lead workers. Furthermore, greater bias with this equation in Chinese and Japanese compared to black and white individuals at levels < 60 ml/min per 1.73 m2 has been reported.[38] However, a small study in a Korean population compared several eGFR estimating techniques with measured GFR using 99mTcDTPA renal clearance and found that eGFR estimated with the MDRD equation performed better than measured creatinine clearance or serum creatinine but less well than calculated creatinine clearance [39] Therefore, we have reported results with a range of kidney outcome assessment techniques in this study.
In conclusion, after adjustment, higher ln-urine cadmium was associated with higher ln-NAG but, paradoxically, also with lower serum creatinine and higher eGFR and calculated creatinine clearance. These unexpected associations were present in participants in the highest as well as the lowest eGFR tertiles and remained even in former workers in whom lead-related hyperfiltration was not apparent. Potential explanations for these results include a normal physiologic response in which renal filtration affects urine cadmium levels, the impact of adjustment for urine dilution with creatinine in kidney outcome models, and cadmium-related hyperfiltration. Additional research is required to determine which of these hypotheses is involved; analyses using specific gravity to adjust for urine dilution, cystatin C outcomes, and other metals to assess the potential impact of metal-protein binding would be very helpful as would analysis of prospective data. These associations have important implications for cadmium risk assessment related to kidney outcomes but may also have relevance for any toxicant research involving associations between urine biomarkers and kidney outcomes.
What this paper adds.
We performed this analysis because few studies have examined the impact of low-level cadmium exposure in workers who are occupationally exposed to other nephrotoxicants such as lead.
We report our analysis of associations between urine cadmium levels and kidney function in lead workers.
Higher urine cadmium levels were associated with higher levels of glomerular filtration measures.
We considered a range of hypotheses for this finding which may have relevance for the use of urinary biomarkers other than cadmium in kidney research.
Supplementary Material
Acknowledgments
This research was supported by NIEHS grant 2 ES007198 (Dr. Weaver) and KRF-2000-00545 (Dr. Lee) from the Korea Research Foundation.
Abbreviations
- BMI
body mass index
Footnotes
Competing Interest: None declared.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in OEM and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://oem.bmj.com/ifora/licence.pdf).
REFERENCES
- 1.Kido T, Nordberg GF, Roels HA. Cadmium-induced renal effects. In: De Broe ME, Porter GA, Bennett WM, Verpooten GA, editors. Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals. 2nd ed. Kluwer Academic Publishers; Dordrecht: 2003. pp. 507–30. [Google Scholar]
- 2.Akesson A, Lundh T, Vahter M, et al. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect. 2005;113:1627–31. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellstrom L, Elinder CG, Dahlberg B, et al. Cadmium exposure and end-stage renal disease. Am J Kidney Dis. 2001;38:1001–8. doi: 10.1053/ajkd.2001.28589. [DOI] [PubMed] [Google Scholar]
- 4.Navas-Acien A, Tellez-Plaza M, Guallar E, et al. Blood Cadmium and Lead and Chronic Kidney Disease in US Adults: A Joint Analysis. Am J Epidemiol. 2009;170:1156–64. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int. 2006;70:2074–84. doi: 10.1038/sj.ki.5001809. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz BS, Lee BK, Lee GS, et al. Associations of blood lead, dimercaptosuccinic acid-chelatable lead, and tibia lead with neurobehavioral test scores in South Korean lead workers. Am J Epidemiol. 2001;153:453–64. doi: 10.1093/aje/153.5.453. [DOI] [PubMed] [Google Scholar]
- 7.Weaver VM, Lee BK, Ahn KD, et al. Associations of lead biomarkers with renal function in Korean lead workers. Occup Environ Med. 2003;60:551–62. doi: 10.1136/oem.60.8.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minnich MG, Miller DC, Parsons PJ. Determination of As, Cd, Pb, and Hg in urine using inductively coupled plasma mass spectrometry with the direct injection high efficiency nebulizer. Spectrochimica Acta Part B: Atomic Spectroscopy. 2008;63:389–95. [Google Scholar]
- 9.Jarrett JM, Xiao G, Caldwell KL, et al. Eliminating molybdenum oxide interference in urine cadmium biomonitoring using ICP-DRC-MS. Journal of Analytical Atomic Spectrometry. 2008;23:962–67. [Google Scholar]
- 10.Fernandez FJ. Micromethod for lead determination in whole blood by atomic absorption, with use of the graphite furnace. Clin Chem. 1975;21:558–61. [PubMed] [Google Scholar]
- 11.Todd AC. Contamination of in vivo bone-lead measurements. Phys Med Biol. 2000;45:229–40. doi: 10.1088/0031-9155/45/1/316. [DOI] [PubMed] [Google Scholar]
- 12.Todd AC, Chettle DR. Calculating the uncertainty in lead concentration for in vivo bone lead x-ray fluorescence. Phys Med Biol. 2003;48:2033–9. doi: 10.1088/0031-9155/48/13/314. [DOI] [PubMed] [Google Scholar]
- 13.Todd AC, Parsons PJ, Carroll S, et al. Measurements of lead in human tibiae. A comparison between K-shell x-ray fluorescence and electrothermal atomic absorption spectrometry. Phys Med Biol. 2002;47:673–87. doi: 10.1088/0031-9155/47/4/309. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Greene T, Kusek JW, et al. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. [Abstract] 2000;11:A0828. [Google Scholar]
- 16.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 17.Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisberg S. Applied linear regression. John Wiley & Sons; New York: 1985. [Google Scholar]
- 19.Eum KD, Lee MS, Paek D. Cadmium in blood and hypertension. Sci Total Environ. 2008;407:147–53. doi: 10.1016/j.scitotenv.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Fourth National Report on Human Exposure to Environmental Chemicals. 2009 December; Available from: http://www.cdc.gov/exposurereport/
- 21.Staessen JA, Lauwerys RR, Buchet JP, et al. Impairment of renal function with increasing blood lead concentrations in the general population. The Cadmibel Study Group. N Engl J Med. 1992;327:151–6. doi: 10.1056/NEJM199207163270303. [DOI] [PubMed] [Google Scholar]
- 22.de Burbure C, Buchet JP, Bernard A, et al. Biomarkers of renal effects in children and adults with low environmental exposure to heavy metals. J Toxicol Environ Health A. 2003;66:783–98. doi: 10.1080/15287390306384. [DOI] [PubMed] [Google Scholar]
- 23.Staessen JA, Nawrot T, Hond ED, et al. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: a feasibility study of biomarkers. Lancet. 2001;357:1660–9. doi: 10.1016/s0140-6736(00)04822-4. [DOI] [PubMed] [Google Scholar]
- 24.Hotz P, Buchet JP, Bernard A, et al. Renal effects of low-level environmental cadmium exposure: 5-year follow-up of a subcohort from the Cadmibel study. Lancet. 1999;354:1508–13. doi: 10.1016/s0140-6736(99)91145-5. [DOI] [PubMed] [Google Scholar]
- 25.de Burbure C, Buchet JP, Leroyer A, et al. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect. 2006;114:584–90. doi: 10.1289/ehp.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchet JP, Lauwerys R, Roels H, et al. Renal effects of cadmium body burden of the general population. Lancet. 1990;336:699–702. doi: 10.1016/0140-6736(90)92201-r. [DOI] [PubMed] [Google Scholar]
- 27.Noonan CW, Sarasua SM, Campagna D, et al. Effects of exposure to low levels of environmental cadmium on renal biomarkers. Environ Health Perspect. 2002;110:151–5. doi: 10.1289/ehp.02110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas LD, Hodgson S, Nieuwenhuijsen M, et al. Early kidney damage in a population exposed to cadmium and other heavy metals. Environ Health Perspect. 2009;117:181–4. doi: 10.1289/ehp.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamagami T, Ezaki T, Moriguchi J, et al. Low-level cadmium exposure in Toyama City and its surroundings in Toyama prefecture, Japan, with references to possible contribution of shellfish intake to increase urinary cadmium levels. Sci Total Environ. 2006;362:56–67. doi: 10.1016/j.scitotenv.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 30.Khalil-Manesh F, Gonick HC, Cohen AH. Experimental model of lead nephropathy. III. Continuous low-level lead administration. Arch Environ Health. 1993;48:271–8. doi: 10.1080/00039896.1993.9940372. [DOI] [PubMed] [Google Scholar]
- 31.Khalil-Manesh F, Gonick HC, Cohen AH, et al. Experimental model of lead nephropathy. I. Continuous high-dose lead administration. Kidney Int. 1992;41:1192–203. doi: 10.1038/ki.1992.181. [DOI] [PubMed] [Google Scholar]
- 32.Suwazono Y, Akesson A, Alfven T, et al. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005;10:117–26. doi: 10.1080/13547500500159001. [DOI] [PubMed] [Google Scholar]
- 33.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am Ind Hyg Assoc J. 1993;54:615–27. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 34.Nawrot T, Plusquin M, Hogervorst J, et al. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol. 2006;7:119–26. doi: 10.1016/S1470-2045(06)70545-9. [DOI] [PubMed] [Google Scholar]
- 35.Bernard A, Thielemans N, Roels H, et al. Association between NAG-B and cadmium in urine with no evidence of a threshold. Occup Environ Med. 1995;52:177–80. doi: 10.1136/oem.52.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Amico G, Bazzi C. Urinary protein and enzyme excretion as markers of tubular damage. Curr Opin Nephrol Hypertens. 2003;12:639–43. doi: 10.1097/01.mnh.0000098771.18213.a6. [DOI] [PubMed] [Google Scholar]
- 37.Bernard A. Biomarkers of metal toxicity in population studies: research potential and interpretation issues. J Toxicol Environ Health A. 2008;71:1259–65. doi: 10.1080/15287390802211885. [DOI] [PubMed] [Google Scholar]
- 38.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–57. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 39.Kang YS, Han KH, Han SY, et al. Characteristics of population with normal serum creatinine impaired renal function and: the validation of a MDRD formula in a healthy general population. Clin Nephrol. 2005;63:258–66. doi: 10.5414/cnp63258. [DOI] [PubMed] [Google Scholar]
- 40.Reinsch CH. Smoothing by Spline Functions. Numerische Mathematik. 1967;10:177–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.