Abstract
The objectives were to determine whether single-nucleotide polymorphisms (SNPs) in KCNN3 (encodes the small conductance calcium-activated potassium channel subfamily N, member 3), associate with preterm birth (PTB). In all, 602 preterm families with at least 1 preterm (<37 weeks gestation) infant were studied: DNA from the infant and one or both parents were genotyped for 16 SNPs in KCNN3. A region of interest within KCNN3 was sequenced in 512 Caucasian non-Hispanic mothers (412 with preterm deliveries;100 who delivered at term). Family-based association testing was used for genotyping analysis; Fisher exact test was used for sequencing analysis. Six SNPs (rs1218585, rs4845396, rs12058931, rs1218568, rs6426985, and rs4845394) were associated with PTB (all Ps < .05). These variations were all located within the intronic region between exons 1 and 2. Maternal sequencing revealed an association of 3 SNPs with spontaneous PTB; rs1218585 (P = .007), rs1218584 (P = .05), and a novel SNP at chromosome1:153099353 (P = .02). Polymorphisms in KCNN3 are associated with PTB and investigation into the functional significance of these allelic changes is warranted.
Keywords: KCNN3, SK3, preterm birth, ion channel
Introduction
Preterm birth (PTB) is the leading cause of neonatal morbidity and mortality in the United States and affects over 12% of deliveries annually. The mechanisms underlying prematurity are complex, and these etiologies are often classified into 4 broad categories: (1) uterine distension, (2) decidual hemorrhage, (3) inflammation, and (4) activation of the maternal–fetal hypothalamic pituitary axis.1 The overlap of these categories leads to complexities in investigating the causal pathways of PTB. Genetic factors can affect all of these processes, contributing to the complex gene−environment interactions leading to prematurity. Based on twin studies, up to 40% of PTB is estimated to have a genetic component, with maternal effects thought to be more significant than fetal.2 Additional familial associations of PTB suggest that there is much yet to be learned regarding the genetics of PTB.
Alterations in ion channel expression and regulation are critical to maintaining uterine quiescence during early gestation and lead to increases in uterine excitability at the time of labor.3,4 Myometrial K+ channel activity is particularly important to maintaining uterine relaxation until full-term gestation.5–7 Potassium efflux from myometrial cells results in membrane repolarization and this efflux is the primary ionic current responsible for maintaining the resting membrane potential. In myometrial smooth muscle cells, changes in the expression or activity of K+ channels can translate into an inadequate repolarization leading to aberrant uterine activity. Thus, K+ channel alterations may contribute to certain pathophysiological conditions such as preterm labor and postterm labor. Various K+ channel families have been investigated in this context, although recent studies on the small conductance calcium-activated K+ (SK) channel family suggest that further research efforts should be directed toward these channels.8
One member of the SK channel family has been found to have a unique, and particularly relevant, labor phenotype in a mouse model of SK dysfunction. During normal gestation, the SK3 channel (encoded by the KCNN3 gene) is downregulated from mid-to-late gestation.8 Mice overexpressing the SK3 channel were discovered to have weakened uterine contractility and therefore defective or delayed parturition.9 Therefore, the downregulation of the SK3 channel is thought to be essential for normal delivery in the mouse. Although research investigating the regulation of the SK3 channel during human gestation has been limited, the available evidence similarly indicates that SK3 expression is lower in the pregnant, compared to the nonpregnant, human myometrium as well.10
Investigations into genetic aberrations capable of altering myometrial contractility and relaxation are lacking in the field of PTB. Given the recent increase in attention to the SK channel family in relation to parturition, the KCNN3 gene was a logical candidate to investigate for an influence on PTB in humans. The unique labor phenotype in the overexpressing mouse led us to believe that premature downregulation of SK3 activity could lead to an excitable, contractile uterus. In this study, our goal was to determine whether single-nucleotide polymorphisms (SNPs) in the KCNN3 gene are associated with PTB in humans.
Materials and Methods
This study was carried out in 2 phases. The first involved genotyping 602 preterm families with at least 1 preterm infant and with DNA available for 1 or both parents for 16 known SNPs located within the KCNN3 gene. The second involved sequencing both maternal case and control DNA to identify novel SNPs in areas of interest within the KCNN3 gene.
Sample Population
In the genotyping phase of the analysis, a total of 602 preterm families were genotyped where one or both of parents were included. Blood or buccal swabs from infants admitted to the Neonatal Intensive Care Unit at the University of Iowa and their parent/parents were collected and banked for use in genetic studies investigating diseases of the infant (IRB 199911068). Preterm birth was defined as a delivery occurring prior to 37 weeks gestation, based on best obstetrical estimate (last menstrual period or ultrasound). Spontaneous PTBs were defined by a natural (not induced) onset of contractions or premature rupture of membranes.
For the sequencing phase of the analysis, a total of 512 Caucasian non-Hispanic mothers were included. This included 412 cases (preterm delivery prior to 37 weeks, 0 days gestation) and 100 controls (delivery on or after 37 weeks, 0 days gestation). In addition to maternal samples from Iowa (405 mothers), maternal samples from collaborating sites (Wake Forest University, Winston-Salem, North Carolina [47 mothers]; University of Rochester, Rochester, New York [22 mothers]; University of Pittsburgh and Magee Womens Hospital, Pittsburgh, Pennsylvania [38 mothers]) were sequenced in accordance with the multicenter project protocol (IRB 200506792).
DNA Processing, Genotyping, and Sequencing
Infant DNA was extracted from umbilical cord blood; parental DNA was obtained from either venous blood or buccal swabs. Allelic variation was determined using the TaqMan genotyping system (Applied Biosystems, Foster City, California). Genotyping reactions were completed in 384-well plates containing dried sample DNA. Polymerase chain reactions (PCRs) were performed on an ABI GeneAmp9700 thermocycler using conditions set by the manufacturer. Allelic determination was carried out on an ABI 7900HT using the Sequence Detection Systems software (version 2.2, Applied Biosystems). Genotype data were uploaded into a Progeny database (Progeny Software, LLC, South Bend, Indiana) containing demographic and clinical information for subsequent statistical analysis. Commercial sequencing was completed (Functional Biosciences, Inc, Madison, Wisconsin) for specified regions of the KCNN3 gene. Primer sequences are available upon request.
Single-Nucleotide Polymorphism Selection
Single-nucleotide polymorphisms were chosen to maximize the coverage of the KCNN3 gene, based on linkage disequilibrium data available from the International HapMap Project (www.hapmap.org). Selection was biased toward either the functionality or the conservation of the particular SNP. A minor allele frequency of 0.10 was chosen as a lower cutoff for a SNP to ensure than an adequate number of individuals within the population would be carriers of the minor allele. A complete list of the selected SNPs, along with their dbSNP identification (“rs”) numbers, chromosome 1 base pair location, and minor allele frequency can be found in Table 1 .
Table 1.
Single-Nucleotide Polymorphisms (SNPs) Within KCNN3 That Were Selected for Genotyping
| SNP | Chr 1 Location | MAF |
|---|---|---|
| rs10128027 | 152958069 | 0.34 |
| rs11264254 | 152981386 | 0.38 |
| rs1051614 | 153011431 | 0.42 |
| rs883319 | 153024796 | 0.21 |
| rs11584635 | 153027589 | 0.26 |
| rs1218602 | 153057696 | 0.26 |
| rs1218553 | 153065251 | 0.32 |
| rs4845394 | 153074661 | 0.43 |
| rs6426985 | 153080243 | 0.40 |
| rs1218568 | 153083632 | 0.45 |
| rs12058931 | 153091717 | 0.25 |
| rs4845396 | 153094783 | 0.49 |
| rs1218585 | 153099285 | 0.20 |
| rs6699080 | 153101285 | 0.40 |
| rs6683557 | 153118931 | 0.47 |
| rs4845680 | 153129819 | 0.46 |
Abbreviations: MAF, minor allele frequency.
Statistical Analyses
The genotyping data for all SNPs were assessed using the PedCheck program to check for any departures from Mendelian inheritance patterns. Alleles at each marker were tested for an association with PTB with the infant as the risk case, using the Family-Based Association Test (FBAT).11–13 Preterm deliveries at various gestational ages (early [22-27 weeks; n = 130 families], middle [28-33 weeks; n = 302 families], and late [34-36 weeks; n = 217 families]) were analyzed in subgroups to determine whether the associations uncovered were dependent on gestational age. Preterm delivery was also subdivided into spontaneous and nonspontaneous labor to determine whether associations were impacted by the type of labor. Additionally, when appropriate, haplotype FBAT (HBAT) analyses were performed for sliding windows of 2, 3, and 4 SNPs across the gene to evaluate for regional associations to prematurity. Haplotypes with a minor allele frequency of <0.05 were not considered. Case−control analysis using Fisher exact test was used for maternal sequencing results, with the mother as the risk case. Preterm deliveries at various gestational ages (early [22-27 weeks; n = 84 infants], middle [28-33 weeks; n = 171 infants], and late [34-36 weeks; n = 155 infants]) were also evaluated. In addition, preterm delivery was subdivided into spontaneous (n = 320 infants) and nonspontaneous (92 infants). Simple statistical significance was used at an α of .05; however, none of the SNPs passed multiple testing corrections. Hardy-Weinberg Equilibrium (HWE) was evaluated for each marker using Fisher exact tests. Hardy-Weinberg Equilibrium using Fisher exact tests was determined in cases and controls for each SNP. Haplotype analysis using sliding windows of 2, 3, and 4 SNPs across the region was used to evaluate associations with prematurity. For sequencing analyses, haplotypes were considered regardless of minor allele frequency. Case−control analyses were performed in PLINK (http://pngu.mgh.harvard.edu/purcell/plink/).14
Results
Initial FBAT analysis of the genotyping data revealed that 6 SNPs of the KCNN3 gene appeared to be associated with PTB (Table 2 ). Two types of analyses were completed: 1 for all preterm deliveries and another subdivided by type of preterm labor either spontaneous or nonspontaneous. In all, 3 SNPs (rs1218568, rs12058931, and rs1218585) were positively associated with preterm delivery and 4 SNPs (rs4845394, rs6426985, rs12058931, and rs4845396) were positively associated with spontaneous preterm delivery, while none associated with nonspontaneous delivery (Table 2).
Table 2.
Genotyping Family-Based Association Test (FBAT) Analysis for Association of SNPs in the KCNN3 Gene With Preterm (All), Spontaneous Preterm (Spontaneous), and Nonspontaneous (Preterm) Births
| SNP | # Informative Families (All) | P Value (All) | P Value (Spontaneous) | P Value (Non spontaneous) |
|---|---|---|---|---|
| rs10128027 | 174 | .307 | .753 | .261 |
| rs11264254 | 191 | .685 | .411 | .644 |
| rs1051614 | 203 | .292 | 1.000 | .107 |
| rs883319 | 210 | .084 | .230 | .233 |
| rs11584635 | 145 | .452 | .644 | .377 |
| rs1218602 | 101 | .508 | .680 | .414 |
| rs1218553 | 112 | .596 | .443 | .714 |
| rs4845394 | 227 | .293 | .039 | .267 |
| rs6426985 | 178 | .072 | .028 | .918 |
| rs1218568 | 158 | .049 | .065 | .433 |
| rs12058931 | 142 | .020 | .033 | .286 |
| rs4845396 | 212 | .205 | .027 | .609 |
| rs1218585 | 141 | .026 | .134 | .072 |
| rs6699080 | 238 | .685 | .174 | .219 |
| rs6683557 | 186 | .813 | .756 | .458 |
| rs4845680 | 195 | .685 | .651 | .927 |
Abbreviations: FBAT, family-based association test; SNP, single nucleotide polymorphism. Shaded Areas Correspond to P Values ≤ .05.
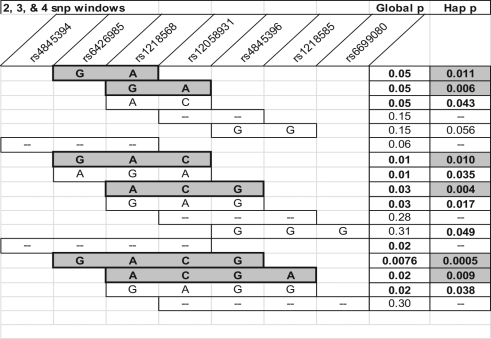
Haplotype analysis of the genotyped SNPs was then performed to examine whether combinations of alleles might be associated with PTB. In this analysis, the presence of G, A, C, and G alleles at rs6426985, rs1218568, rs12058931, and rs4845396, respectively, was associated with PTB (P = .0005; Figure 1 ). Several other SNP windows in this region are associated with PTB, both based on the global P value for the haplotype window and the haplotype P value, which reflects specific allelic combinations in the region. Additional haplotype associations were associated with both nonspontaneous and spontaneous PTB, but they did not reach the level of significance of that shown in Figure 1 and were not pursued further in this study. Data for haplotype analysis of infants affected by spontaneous PTB were similar to those shown in Figure 1.
Figure 1.
Haplotype family-based association test (HBAT) analysis for association of SNPs in the KCNN3 gene with preterm birth. Global P values refer to an overall association of the single-nucleotide polymorphisms (SNPs) in the window with PTB. Specific allele combinations yielding a haplotype P value of .01 or less are shaded gray, and P values are indicated by “Hap p.” Global p = Overall P value for association between PTB and the indicated haplotype window. Hap p (assoc) = P value for association between PTB and a specific haplotye composed of the alleles indicated in the bars. SNPs indicate single nucleotide polymorphisms; PTB, preterm birth.
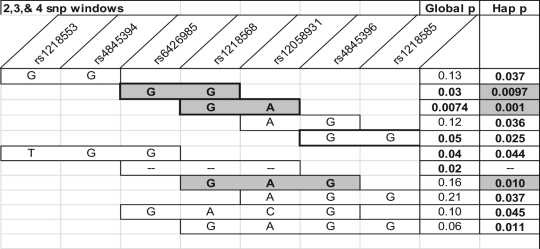
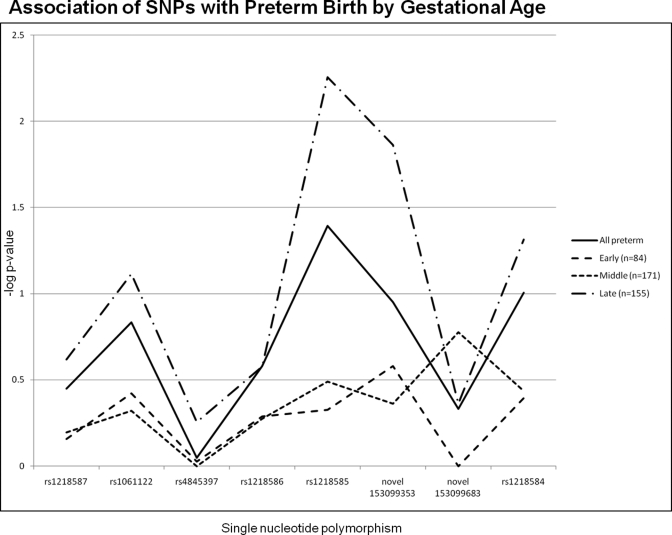
Because etiologies of PTB vary based on gestational age at delivery, we analyzed the initial FBAT results in 3 gestational age groupings, early (less than 28 weeks), middle (28-33 weeks), and late (34-36 weeks). Two SNPs of interest in the overall FBAT analysis, rs12058931 and rs1218585, seem to be more closely associated with the middle and early gestational age groupings, respectively (Figure 2 ). In the early preterm group, rs883319 had an association with PTB as well. Haplotype analysis of the genotyped SNPs was also performed across the gestational groupings. Haplotype analysis determines whether there is an association of adjacent SNPs, and therefore a region of the gene, with the trait of interest (PTB). The largest region of highest association with preterm delivery in the middle gestation grouping was the same region of SNPs that is shown in Figure 2, with several global and haplotype P values of less than .05 across 2, 3, and 4 SNP windows (Figure 3 ). A smaller region of association was identified in the late gestational age group, however this did not reach the significance levels seen with the middle gestational age group. There were no associations in the early gestational age group.
Figure 2.
Graphic representation of results of the family-based association test (FBAT) for association of single-nucleotide polymorphisms (SNPs) with preterm birth, according to gestational age. Early = 22 to 27 weeks gestation, middle = 28 to 33 weeks gestation, late = 34 to 36 weeks gestation.
Figure 3.
Haplotype family-based association test (HBAT) analysis for association of single-nucleotide polymorphisms (SNPs) in the KCNN3 gene with preterm birth in the middle gestational age range (between 28 and 33 weeks gestation). Global P values refer to an overall association of the SNPs in the window with preterm birth. Specific allele combinations yielding a haplotype P value (Hap-p) of .01 or less are shaded gray. Global p = Overall P value for association between PTB and the indicated haplotype window. Hap p (assoc) = P value for association between PTB and a specific haplotye composed of the alleles indicated in the bars.
In the second phase of the study, genomic sequencing was performed in the region of rs1218585 on Caucasian maternal DNA samples from the 412 cases and 100 controls. Sequencing results for this region are reported for 6 known SNPs and 2 novel SNPs (at base pair locations of 153099353 and 153099683 on chromosome 1). The A allele at novel site 153099353 was associated with both PTB (P = .03) and spontaneous PTB (P = .02; Table 3 ). Genotypic association at rs1218585 was identified with PTB (P = .04) and spontaneous PTB (P = .007). Genotypic association at rs1218584 was also identified with spontaneous PTB (P = .05). None of these sequenced SNPs were associated with nonspontaneous PTB.
Table 3.
Genotype Sequencing Case−Control Analysis for Association of SNPs in the KCNN3 Gene With Preterm Birth (All), Spontaneous Preterm Birth (Spontaneous), and Nonspontaneous Birtha
| SNP | All preterm | Spontaneous | Nonspontaneous |
|---|---|---|---|
| rs1218587 | 0.35 | 0.18 | 0.37 |
| rs1061122 | 0.15 | 0.04 | 0.31 |
| rs4845397 | 0.90 | 0.57 | 0.31 |
| rs1218586b | 0.26 | 0.47 | 0.23 |
| rs1218585b | 0.04 | 0.007 | 0.62 |
| novel 153099353 | 0.11 (A 0.03) | 0.07 (A 0.02) | 0.67 |
| novel 153099683 | 0.47 | 0.49 | 0.45 |
| rs1218584 | 0.10 | 0.05 | 0.14 |
Abbreviations: HWE, Hardy-Weinberg equilibrium; SNP, single nucleotide polymorphism.
a Values are listed as global associations of the SNP with preterm birth. Specific allelic associations are listed in parentheses when they are statistically significant.
b rs1218586 deviates from HWE in controls (P = .002) and rs1218585 deviates from HWE in cases (P = .04). Shaded Areas Correspond to P Values ≤ .05.
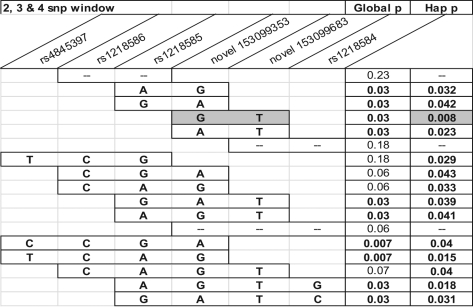
Haplotype analysis was performed on the sequencing data results, to determine whether neighboring allelic combinations were associated with prematurity. Several adjacent SNPs in this region have an association with spontaneous PTB (Figure 4 ). Specifically, the inheritance of a G and T allele at novel SNP site 153099353 and novel SNP site 153099683, respectively, appears to be associated with spontaneous PTB (P = .008; Figure 4). The results were similar when the total preterm delivery group was analyzed. No haplotype associations were found in the nonspontaneous group.
Figure 4.
Haplotype case−control analysis (Fisher exact test) for association of single-nucleotide polymorphisms (SNPs) in the KCNN3 gene with spontaneous preterm birth. Global P values refer to an overall association of the SNPs in the window with spontaneous preterm birth. Specific allele combinations yielding a haplotype P value (Hap-p) of .01 or less are shaded gray. Global p = Overall P value for association between PTB and the indicated haplotype window. Hap p (assoc) = P value for association between PTB and a specific haplotye composed of the alleles indicated in the bars.
Sequencing results were also analyzed based on gestational age, using the early, middle, and late groupings described above. Figure 5 shows that both rs1218585 and novel SNP at position 153099353 are associated with late PTB. Other sequenced SNPs were associated with late PTB when gestational age was considered (Table 4 ). Further haplotype analysis revealed significant regions in late spontaneous PTB; however the small sample sizes make interpretation difficult.
Figure 5.
Sequencing results for the case−control analysis of the association of single-nucleotide polymorphisms (SNPs) in the KCNN3 gene with preterm birth, according to gestational age. Early = 22 to 27 weeks, middle = 28 to 33 weeks, late = 34 to 36 weeks gestation.
Table 4.
Genotype Sequencing Case−Control Analysis for Association of SNPs in the KCNN3 Gene With Late, Middle, and Early Gestational Age Spontaneous Preterm Birth
| SNP | Early | Middle | Late |
|---|---|---|---|
| rs1218587 | 0.62 | 0.33 | 0.21 |
| rs1061122 | 0.16 | 0.21 | 0.05 |
| rs4845397 | 0.71 | 0.80 | 0.40 |
| rs1218586a | 0.20 | 0.86 | 0.63 |
| rs1218585a | 0.31 | 0.09 | 0.004 |
| novel 153099353 | 0.12 | 0.27 | 0.02 (A 0.008) |
| novel 153099683 | 1.00 | 0.09 | 0.61 |
| rs1218584 | 0.31 | 0.14 | 0.07 |
Abbreviations: HWE, Hardy-Weinberg equilibrium; SNP, single nucleotide polymorphism.
a rs1218586 deviates from HWE in controls (P = .002) and rs1218585 deviates from HWE in cases (P = .04).
Shaded Areas Correspond to P Values ≤ .05.
Discussion
Investigating the mechanisms underlying PTB are challenging for several reasons. Although defining a birth as preterm is relatively straightforward in most clinical cases, the overlap in etiologies makes it virtually impossible to identify a single underlying mechanism. The final common pathway of the etiologies leading to preterm contractions is the override of the mechanisms that maintain uterine quiescence. Potassium channels within the myometrium are known to be the major mediators of uterine relaxation.5 Transgenic mice overexpressing SK3 channels have compromised parturition.15 This is likely a result of the hyperpolarizing potential of the SK3 channel, which maintains the myometrial cells in a hypoexcitable state. Overexpression of the hyperpolarizing SK3 channel in mice results in attenuated myometrial contractions leading to inefficient labor.9,16 Based on this mouse studies, we sought to determine whether the converse is true whereby polymorphisms in this channel would associate with PTB. Downregulation of the SK3 channel occurs with advancing gestation in the mouse model of parturition and also in humans.9,17 Therefore, we chose to investigate the genetics of the SK3 channel (encoded by the KCNN3 gene) in relation to PTB in humans.
We began our investigation by selecting representative SNPs throughout the KCNN3 gene. Initially, 10 SNPs were genotyped; subsequently 6 additional SNPs were added to fine-map the gene. Of these 16 SNPs, 6 were associated with preterm or spontaneous PTB, at a level of P < .05. All of these SNPs were located in the intronic region between exons 1 and 2. Additional haplotype analysis was used to verify that combinations of neighboring SNPs (and therefore regions of the gene) that were also associated with prematurity. These supporting results led us to investigate the large 47 kb region between exons 1 and 2 more closely.
While none of the SNPs of interest from our genotyping analysis are located in exons, we found that rs1218585 is located between 2 regions of the KCNN3 gene that are highly conserved among mammalian species. One of these regions (but not the same SNP) has recently been identified by genome-wide association studies to associate with lone atrial fibrillation.18 This highly conserved region corresponds to a unique promoter and alternate exon 1c, which encodes an alternative isoform of the channel. A study describes this isoform as a dominant-negative suppressor of the native (1a) form of the SK3 channel.19 Its inhibitory action is believed to occur by sequestering the native SK3 channel intracellularly, thereby decreasing its activity at the cell surface. The discovery of this alternate (inhibitory) isoform of the channel fits with our hypothesis of an association between KCNN3 and prematurity. Given that downregulation of the channel is important for labor to occur,9,17 premature inhibition of the native SK3 channel could lead to preterm uterine excitability and contractions.
We next sequenced the 1400-bp region of the KCNN3 gene, which surrounds exon 1c and SNP rs1218585 in mothers with preterm deliveries and in mothers who delivered at term, and compared the results. We chose to use maternal DNA for sequencing, as we suspected that SK3 downregulation in the mother, rather than the fetus, was physiologically relevant to the timing of birth. Sequencing results were obtained for 6 known SNPs and 2 novel SNPs in this region. Again, we found that rs1218585 is associated with spontaneous PTB, as is rs1218584 and the novel SNP at 153099353. The minor allele frequency of this novel SNP was 0.05. Due to the low prevalence of the heterozygous (AG) and homozygous recessive (AA) genotypes, there was concern that the presence of this mutation in a small number of families was potentially skewing the data. We excluded this possibility by verifying that the individuals of these genotypes were not clustered but rather spread across several unrelated families.
Haplotype analysis was also performed on our sequencing results to establish whether neighboring SNPs are associated with prematurity. Several allelic combinations in the sequenced region were associated with spontaneous PTB, with a P value of less than .05. This observation also supports the individual SNP sequencing data suggesting that sequence alterations in this region of the KCNN3 gene are associated with PTB in humans.
Because the etiology of PTB varies according to gestational age at delivery, we analyzed our results across 3 gestational age groupings: early (less than 28 weeks), middle (28-33 weeks), and late (34-36 weeks). These subdivisions correspond to commonly used gestational age groupings, with our early group often regarded as “extreme or very-early PTB” and our late group considered “late preterm birth.” Our genotyping data suggest that rs1218585 is associated with early PTB, yet our sequencing data suggest that rs1218585 is associated with late PTB. Superficially, these results may appear contradictory. However, the genotyping data were analyzed by FBAT, in which the underlying assumption is that the infant is the “affected” individual. The sequencing data, in contrast, were generated from maternal DNA; this approach assumes that the mother is the “affected” individual contributing to the PTB. The sequencing (maternal) association of rs1218585 with late PTB fits the general assumption that myometrial stretch phenomena typically are a factor related to late PTB. We can only speculate that it is the complexity of the fetal–maternal gene interplay that would suggest a fetal association of rs1218585 with early PTB.
The complex genetics of PTB are another challenge in investigating this phenotype. Heritability studies suggest that both maternal and fetal components are important in establishing gestational age at delivery.2,20 Identifying whether it is the maternal genotype itself or the maternal contribution to the fetal genotype that determines prematurity is inherently difficult. However, unique maternal and fetal contributions to the timing of delivery could also explain the difference in our genotyping and sequencing results with regard to the influence of gestational age. Aberrant myometrial stretch, as seen in multiple gestations, is usually thought of as contributing to late PTB. Although several signal transduction pathways are activated in response to myometrial stretch during pregnancy, the downstream effectors have not been fully elucidated.21–24 Both in vivo and in vitro studies have shown that stretch of myometrial tissue during pregnancy leads to the stimulation of both labor-associated proteins (eg, cyclooxygenase 2 [COX-2]) and receptors for prostaglandin and oxytocin.24–26 Another protein class that can be influenced by stretch is ion channels.27 In theory, premature downregulation of myometrial SK3 activity via encoding a nonfunctional isoform of the channel (as is suspected with this SNP) could be a phenomenon that contributes to PTB at late gestational ages.
One drawback of this study is that our population was of limited diversity; most of the maternal and fetal DNA samples were obtained at the University of Iowa, where the patient population is predominantly Caucasian and lives in a rural or suburban environment. Our data reflect only Caucasian non-Hispanic women. Replication of these results in at least one unique population would be necessary for confirmation across races. Alternatively, one could compare these results to those from larger, genome-wide association studies that have evaluated the genetic associations of PTB in other populations. We note that we do not expect to find the same associations across all populations, as the etiologies of PTB are not identical across diverse populations.
A notable strength of this study is that the number of individuals represented in the genotyping and sequencing analyses is large. The benefit of a large sample size is in conflict with the large number of analyses and subsequent observations generated. When multiple observations are identified, it becomes difficult to achieve statistically stringent P values. Although this point is relevant to an overall understanding of the statistical analysis of the associations, our main goal in evaluating the KCNN3 gene was to generate hypotheses that could then be evaluated physiologically. In this regard, we clearly achieved our goal.
One of the overwhelming strengths of this study is the use of the abnormal labor phenotype in the mouse model of SK3 overexpressors to guide genetic investigations in the KCNN3 gene and PTB in humans.15 While our belief is that the SK3 channel is modulating contractile function, this channel is widely expressed throughout tissues that are essential for pregnancy and parturition.28 For example, SK3 is expressed in the hypothalamus,28 where it could alter hormone secretion, thereby leading to compromised parturition. Oxytocin, a hormone that stimulates uterine contractions during labor,29 is released from neurons in the supraoptic nucleus, a region that contains both neurons and astrocytes that express SK3 channels.30 Regardless, this study bridges the physiologic mechanisms with an analysis of genetic inheritance, in addition to connecting the human and mouse models of parturition. These collaborative, multidisciplinary efforts are essential to advancing our knowledge of PTB.
A logical step in the investigation of the role of SK3 in the timing of parturition would be to investigate the alterations in physiologic mechanisms that occur by these genetic alterations. This would include seeking to identify potential gene regulatory elements—whether at the level of promoter, splicing, or transcription—that may be located in this region. Ultimately, it will be necessary to undertake functional studies to evaluate whether the alternate SK3-1c isoform is important in regulating uterine excitability.
Acknowledgments
First, we thank the families who generously agreed to participate in this study. Our gratitude is extended to our research nurses: Susan Berends, Gretchen Cress, Karen Johnson, Laura Knosp, Nancy Krutzfield, Ruthann Schrock, and Sara Scott. We appreciate the editorial support of Kristi Borowski, MD, Christine Blaumueller, PhD, and Monali Sawai, PhD. We also extend special thanks to Keegan Kelsey and Allison Momany for technical assistance.
This study was conducted at the University of Iowa.
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: March of Dimes (21-FY08-566) and NIH (HD-037831) to Sarah K England and NIH (HD-052953 and HD-057192), March of Dimes (FY06-575 and FY05-126) to Jeffrey C. Murray, NIH T32 training grant (HL-007638-24) to Kelli K Ryckman and NIH T32 training grant (HL 07638-23) to Lori J. Day.
References
- 1. Simhan HN, Caritis SN. Prevention of preterm delivery. N Engl J Med. 2007;357(5):477–487 [DOI] [PubMed] [Google Scholar]
- 2. Boyd HA, Poulsen G, Wohlfahrt J, Murray JC, Feenstra B, Melbye M. Maternal contributions to preterm delivery. Am J Epidemiol. 2009;170(11):1358–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown A, Cornwell T, Korniyenko I, et al. Myometrial expression of small conductance Ca2+-activated K+ channels depresses phasic uterine contraction. Am J Physiol Cell Physiol. 2007;292(2):C832–C840 [DOI] [PubMed] [Google Scholar]
- 4. Khan RN, Smith SK, Morrison JJ, Ashford ML. Properties of large-conductance K+ channels in human myometrium during pregnancy and labour. Proc Biol Sci. 1993;251(1330):9–15 [DOI] [PubMed] [Google Scholar]
- 5. Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol. 2007;18(3):332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kimura T, Ogita K, Kusui C, Ohashi K, Azuma C, Murata Y. What knockout mice can tell us about parturition. Rev Reprod. 1999;4(2):73–80 [DOI] [PubMed] [Google Scholar]
- 7. Lundgren DW, Moore JJ, Chang SM, Collins PL, Chang AS. Gestational changes in the uterine expression of an inwardly rectifying K+ channel, ROMK. Proc Soc Exp Biol Med. 1997;216(1):57–64 [DOI] [PubMed] [Google Scholar]
- 8. Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition?. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R525–R545 [DOI] [PubMed] [Google Scholar]
- 9. Pierce SL, Kresowik JD, Lamping KG, England SK. Overexpression of SK3 channels dampens uterine contractility to prevent preterm labor in mice. Biol Reprod. 2008;78(6):1058–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazzone JN, Kaiser RA, Buxton IL. Calcium-activated potassium channel expression in human myometrium: effect of pregnancy. Proc West Pharmacol Soc. 2002;45:184–186 [PubMed] [Google Scholar]
- 11. Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype—phenotype associations. Eur J Hum Genet. 2001;9(4):301–306 [DOI] [PubMed] [Google Scholar]
- 12. Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidemiol. 2000;19(suppl 1):S36–S42 [DOI] [PubMed] [Google Scholar]
- 13. Rabinowitz D, Laird N. A unified approach to adjusting association tests for population admixture with arbitrary pedigree structure and arbitrary missing marker information. Hum Hered. 2000;50(4):211–223 [DOI] [PubMed] [Google Scholar]
- 14. Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bond CT, Sprengel R, Bissonnette JM, et al. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science. 2000;289(5486):1942–1946 [DOI] [PubMed] [Google Scholar]
- 16. Pierce SL, Kutschke W, Cabeza R, England SK. In vivo measurement of intrauterine pressure by telemetry: a new approach for studying parturition in mouse models. Physiol Genomics. 2010; 42(2):310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pierce SL, England SK. SK3 channel expression during pregnancy is regulated through estrogen and Sp factor-mediated transcriptional control of the KCNN3 gene. Am J Physiol Endocrinol Metab. 2010;299(4):E640–E646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ellinor PT, Lunetta KL, Glazer NL, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42(3):240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolski-Andreaco A, Tomita H, Shakkottai VG, et al. SK3-1C, a dominant-negative suppressor of SKCa and IKCa channels. J Biol Chem. 2004;279(8):6893–6904 [DOI] [PubMed] [Google Scholar]
- 20. Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol. 2007;165(7):734–741 [DOI] [PubMed] [Google Scholar]
- 21. Oldenhof AD, Shynlova OP, Liu M, Langille BL, Lye SJ. Mitogen-activated protein kinases mediate stretch-induced c-fos mRNA expression in myometrial smooth muscle cells. Am J Physiol Cell Physiol. 2002;283(5):C1530–C1539 [DOI] [PubMed] [Google Scholar]
- 22. Shynlova OP, Oldenhof AD, Liu M, Langille L, Lye SJ. Regulation of c-fos expression by static stretch in rat myometrial smooth muscle cells. Am J Obstet Gynecol. 2002;186(6):1358–1365 [DOI] [PubMed] [Google Scholar]
- 23. Sooranna SR, Engineer N, Loudon JA, Terzidou V, Bennett PR, Johnson MR. The mitogen-activated protein kinase dependent expression of prostaglandin H synthase-2 and interleukin-8 messenger ribonucleic acid by myometrial cells: the differential effect of stretch and interleukin-1{beta}. J Clin Endocrinol Metab. 2005;90(6):3517–3527 [DOI] [PubMed] [Google Scholar]
- 24. Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod. 2004;10(2):109–113 [DOI] [PubMed] [Google Scholar]
- 25. Loudon JA, Sooranna SR, Bennett PR, Johnson MR. Mechanical stretch of human uterine smooth muscle cells increases IL-8 mRNA expression and peptide synthesis. Mol Hum Reprod. 2004;10(12):895–899 [DOI] [PubMed] [Google Scholar]
- 26. Terzidou V, Sooranna SR, Kim LU, Thornton S, Bennett PR, Johnson MR. Mechanical stretch up-regulates the human oxytocin receptor in primary human uterine myocytes. J Clin Endocrinol Metab. 2005;90(1):237–246 [DOI] [PubMed] [Google Scholar]
- 27. Dalrymple A, Mahn K, Poston L, Songu-Mize E, Tribe RM. Mechanical stretch regulates TRPC expression and calcium entry in human myometrial smooth muscle cells. Mol Hum Reprod. 2007;13(3):171–179 [DOI] [PubMed] [Google Scholar]
- 28. Chen MX, Gorman SA, Benson B, et al. Small and intermediate conductance Ca(2+)-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(6):602–615 [DOI] [PubMed] [Google Scholar]
- 29. Blanks AM, Thornton S. The role of oxytocin in parturition. BJOG. 2003;110(suppl 20):46–51 [DOI] [PubMed] [Google Scholar]
- 30. Armstrong WE, Rubrum A, Teruyama R, Bond CT, Adelman JP. Immunocytochemical localization of small-conductance, calcium-dependent potassium channels in astrocytes of the rat supraoptic nucleus. J Comp Neurol. 2005;491(3):175–185 [DOI] [PubMed] [Google Scholar]