Abstract
AICAr is a cell-permeable nucleotide that has been used in vivo and in vitro to activate AMPK. Our previous findings have shown that AICAr as a single agent induces dose- and time-dependent growth inhibition in acute lymphoblastic leukemia (ALL) cell lines. In addition, the combination of AICAr with antifolates (methotrexate (MTX) or pemetrexed) has been shown to further potentiate AMPK activation and to lead to greater cytotoxicity and growth inhibition in leukemia and other malignant cell types. Our data presented herein demonstrate that sustained ER stress is the predominant mechanism behind the synergistic induction of cell death by the combination of AICAr plus the inhibitor of one-carbon metabolism, MTX, in Bp- and T-ALL, as evidenced by induction of several unfolded protein response markers leading to apoptosis. We also show for the first time that AICAr in combination with MTX significantly induces Akt phosphorylation in ALL. Under these conditions, the concomitant inhibition of Akt, a cellular antagonist of AMPK, leads to further up-regulation of AMPK activity and alleviates AICAr plus MTX-induced ER stress and apoptosis. Therefore, we also demonstrate that the concomitant activation of AMPK actually rescues the cells from AICAr plus MTX-induced ER stress and apoptosis. Our data suggest that the effects of AMPK activation on cell death or survival differ contextually depending on its signaling alterations with related oncogenic pathways and provide insight into the reported paradoxical pro-apoptotic vs. pro-survival effects of AMPK activation.
Keywords: Acute Lymphoblastic Leukemia, AMPK, Akt, AICAR, Methotrexate, ER stress, CHOP, UPR, Salubrinal, N-Acetyl-L-cysteine
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common hematological malignancy affecting children and adolescents and is the number one cause of cancer related mortality in this age group (1). Although cure rates for children with ALL have improved significantly, with an average of 80% survival, event-free survival (EFS) for children and adults diagnosed with chemotherapy resistant phenotypes of ALL or after relapse, continues to be dismal (EFS ~10–20%) (2, 3). Current chemotherapy treatment intensification strategies and/or the use of stem cell transplantation have led to marginal improvements with limited impact on cure rates (3). These facts highlight the need to develop novel molecularly targeted therapies based on an understanding of pathways that are important for cell growth, proliferation and survival.
AMP activated protein kinase (AMPK) is a highly conserved heterotrimeric serine/threonine protein kinase that regulates the intracellular ratio of AMP to ATP, and it is activated under conditions that deplete cellular ATP and increase AMP levels, such as glucose deprivation, heat shock, hypoxia, and ischemia (4, 5). It consists of a catalytic (α) and two regulatory (β and γ) subunits, and requires a conformational change induced by allosteric AMP binding to the α and γ subunits, which in turn allows its phosphorylation at Thr-172 of the α subunit by an upstream protein kinase (6). Consequently, compounds that act as AMP analogues can also induce the conformational change required for AMPK activation under conditions that do not involve changes in the ratio of AMP/ATP. One such compound is AICA riboside (AICAr), a cell permeable nucleoside that is metabolically converted by adenosine kinase to AICA ribotide (AICAR, ZMP), a purine precursor that also exerts cellular effects as a 5′-AMP analogue.
AICAr has been used both in vitro (intact cells) and in vivo (whole animals) to induce AMPK activation (reviewed in (7)). However, a major shortcoming of AICAr is that relatively high concentrations (>200 uM) of the drug are necessary to exert cytotoxic effects (8). A potential strategy to overcome this difficulty or to induce synergy is to combine it with drugs that inhibit one-carbon metabolism such as methotrexate (MTX), which blocks the conversion of AICAR to formyl-AICAR and prevents its subsequent incorporation into the de novo purine biosynthesis pathway. MTX leads to an increased endogenous pool of AICAR and subsequently increases AMP accumulation through inhibition of AMP deaminase and adenosine deaminase (9). Thus, MTX is thought to exert a two-pronged approach to activating AMPK by increasing both the endogenous pool of AICAR and the ratio of AMP/ATP. In addition, ATP, a purine-based nucleotide, inhibits AMPK activity through intrasteric interaction with both α and γ subunits and blocks AMPK activation by AMP (10).
As the master cellular energy switch, AMPK activation promotes catabolic processes while inhibiting energy-consuming anabolic metabolism. In this capacity, AMPK is known to phosphorylate/inhibit Acetyl-CoA carboxylase (ACC) at Ser79, which, in turn, relieves the inhibitory effects of ACC on fatty acid oxidation, an important ATP-producing process (4). AMPK is also known to inhibit phosphorylation of mTOR at its activating residue Ser 2448, thereby shutting down protein synthesis, an ATP-consuming process (5). Our laboratory was the first to show that AICAr induces dose- and time-dependent growth inhibition in Bp- and T-ALL cells, which occurs concomitantly with activation of AMPK, phosphorylation of ACC, downregulation of mTOR, and upregulation of p53 and p27 (8). Recent reports have shown that pretreatment of breast cancer, epidermoid carcinoma and prostate cancer cell lines with MTX significantly potentiates AICAr-induced cell growth inhibition and cell death through increased AMPK activation (11). Pemetrexed, a multitargeted antifolate similar to MTX, in combination with AICAr also causes enhanced cell growth inhibition in CCRF-CEM (T-ALL) cells and correlates with AMPK activation and mTOR inhibition (12). In this study we further characterize the mechanisms of cell death induced by the combination of MTX plus AICAr, and the role of AMPK and Akt in determining cell death. We found that the effects of AMPK activation differ contextually depending on signaling alterations in related pathways, and provide insights into the reported paradoxical pro-apoptotic and pro-survival effects of AMPK activation.
MATERIALS AND METHODS
Cell lines and chemicals
CCRF-CEM (T-lineage ALL) and NALM6 (B-lineage precursor Bp-ALL) were obtained in May 2009 from ATCC (Rockville, MD) and DSMZ (Braunschweig, Germany), respectively. Cells lines were authenticated by the manufacturer (ATCC and DSMZ), were frozen upon receipt and resuscitated every 4 months, using the original frozen stock. Cells were maintained in RPMI 1640 medium (Mediatech, Inc., Herndon, VA) supplemented with 10% FBS (Invitrogen, Carlsbad, CA) and antibiotics at 37°C and 5% CO2 atmosphere, and all drug treatments were done in the presence of FBS. AICAr was purchased from Toronto Research Chemicals (Ontario, Canada), MTX from Sigma-Aldrich Corp. (St. Louis, MO), Akt inhibitor X from EMD Chemicals Inc. (Gibbstown, NJ), Salubrinal from Enzo Life Sciences (Plymouth Meeting, PA), and N-Acetyl-L-cysteine (NAC) from Sigma Aldrich (St. Louis, MO). Accumulation of reactive oxygen species (ROS) was measured using STA-342 OxiSelect™ ROS Assay Kit (Cell Biolabs, Inc., Decatur, GA).
Cell proliferation assays
Nalm6 and CCRF-CEM cell lines were grown to mid log phase cultures, diluted to 0.5 × 106 cells/ml, and treated for 24, 48 and 72 hours with each drug alone or in combination. Apoptosis was evaluated using the Annexin V-FITC/PI Apoptosis Detection Kit I following the manufacturer’s recommendations (BD Biosciences, San Jose, CA) and analyzed by flow cytometry. For temporary inhibition of Akt with Akt inhibitor X (AIX), cells were treated for 24 hrs, washed with PBS 1X, and resuspended in drug-free media. We used this experimental design in this set of studies in order to assess if commitment to cell death occurred early after drug treatment, and apoptosis was measured following 24 hrs of drug exposure, and following an additional 24 hrs of incubation in drug-free media. For these experiments AICAr was used at 200 μM; Akt inhibitor X (AIX) at 12 μM; MTX at EC50 concentrations, i.e. 100 nM and 50 nM for CCRF-CEM and Nalm6 cells, respectively. Salubrinal at 10 μM was used to inhibit ER stress, and NAC at 5 mM for Nalm6 and 2 mM for CCRF-CEM in rescue experiments following induction of ROS by AICAr and MTX for a total of 72 hrs.
Immunoblots
AICAr, MTX or AICAr plus MTX treated cells were harvested at the specified time points, washed with PBS 1X, and sonicated in 50 mM Tris-HCl (pH 7.4) containing protease cocktail inhibitors (Thermo). Proteins (50 μg/lane) were resolved by SDS-PAGE electrophoresis and transferred onto PVDF membranes as previously described following manufacturer’s recommendation (8). Proteins were detected using the enhanced chemiluminescence (ECL) detection kit (Amersham Biosciences).
Lentiviral Transduction
Lentiviral particles carrying Scramble shRNA (sc-108080) and AMPKα1 shRNA (sc-29673-V) were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Aliquots of 2 × 105 cells, grown in RPMI 1640 media to log phase cultures and resuspended in 1 ml media, were placed in 2 ml Falcon tubes and spun in the presence of 4 μg/ml polybrene and lentiviral particles (MOI=5) for 20 min at 20°C and 2,000 RPM in desktop Beckman centrifuge GS-6R. The cells were resuspended in the polybrene+lentivirus media, incubated for 24 hrs, then washed and resuspended in polybrene-free RPM1 media and incubated for additional 48 hrs at 37°C. Puromycin was added 72 hrs after transduction at the initial concentration of 2 μg/ml, which was gradually increased to 6 μg/ml to select for stable transductants. Western Blot was used to confirm total AMPK knockdown in ALL transductants (~75–85%). ALL cell lines were grown to log phase cultures and were then diluted to 0.5 × 106 cells/ml and treated for 24 hours with each drug alone or in combination. After 24 hrs of incubation, both drugs were washed, and cells were resuspended in drug-free media. Cell death was assessed by trypan blue exclusion using the ViCell XR cell viability analyzer (Beckman Coulter). This shorter incubation design (24 hrs) was used for these experiments too, to evaluate if commitment to cell death occurred early during treatment.
Statistical Analysis
Multiple comparisons of cell proliferation and cell viability/death were assessed by one-way ANOVA followed by the Newman-Keuls multiple comparison test. Pair comparisons were achieved using a two-tailed, paired t test of correlated samples using the Graph Pad PRISM software version 2 (GraphPad Software, Inc., San Diego, CA). The data are expressed as mean ± SE.
RESULTS
AICAr potentiates MTX-induced cytotoxicity in ALL cells lines
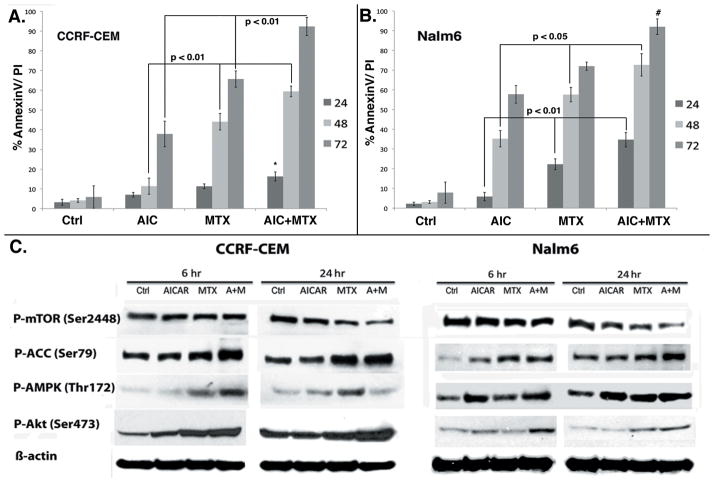
Our laboratory had previously shown that AICAr inhibited cell proliferation and induced cell cycle arrest and apoptosis in several ALL cell models (8). Others have shown that MTX can enhance the cytotoxic effects of AICAr in breast cancer, epidermoid carcinoma and prostate cancer cell lines (11). Herein, we tested whether AICAr in combination with MTX exerted increased cytotoxicity in representative Bp- and T-ALL cell line models. AICAr was used at a concentration of 200 μM for both cell lines, while MTX was used at EC50 concentrations for each cell line, i.e. 100 nM for CCRF-CEM (T-ALL) and 50 nM for Nalm6 (Bp-ALL) cells. Due to differences in intracellular metabolism of antifolates between Bp- and T-ALL, the former are known to be more sensitive to MTX (13). Our data show that MTX in combination with AICAr inhibited cell proliferation (data not shown) and induced significantly higher apoptotic cell death in both CCRF-CEM (Fig. 1A; p <0.01) and Nalm6 (Fig. 1B; p <0.01 and <0.02 for 24 and 48 hrs, respectively) cell line models, compared to each drug alone. Although the combination MTX plus AICAr induced similar degree of apoptosis in both cell lines, this effect was apparent at 24 hrs in Nalm6 cells whereas only at 48 hrs in CCRF-CEM cells (likely due to lower FPGS expression and lengthier exposure required for sufficient accumulation of the active polyglutamated metabolites of MTX (MTX-PG) in CCRF-CEM cells) (14). In addition, our data show that AICAr was also able to overcome the known relative resistance of CCRF-CEM cells to single agent MTX, which is demonstrated by the ability of this combination to induce the same degree of cell death in both CCRF-CEM (MTX resistant) and Nalm6 (MTX sensitive) cells at 72 hrs with the combination.
Figure 1. MTX potentiates AICAR-induced cytotoxicity, AMPK and Akt activation, and mTOR downregulation in ALL cell lines.
Cells were treated for 24, 48 and 72 hrs with AICAr, MTX and AICAr plus MTX, and apoptosis was assayed at each time point by AnnexinV/PI staining using flow cytometry. AICAr plus MTX lead to significant enhancement of apoptosis in CCRF-CEM (p < 0.01 at 48 and 72 hrs; A) and Nalm6 (p< 0.01 and 0.02 at 24 and 48 hrs, respectively; B) cells compared to each drug alone. Cells treated as described under Materials and Methods for 6 and 24 hrs were analyzed by Western blotting at each time point. MTX potentiates AICAr-induced AMPK activation, mTOR inhibition and Akt activation in ALL cell lines (C). Graphs represent mean and SE derived from 5 independent experiments. p values were calculated by one-way ANOVA analysis of each drug and the combination at 48 and 72 hrs time points.
MTX potentiates AICAr-induced AMPK activation, downstream mTOR downregulation and Akt activation
Our previous findings indicated that AMPK activation plays a key role in AICAr-induced ALL cytotoxicity. Therefore, we determined whether the increased induction of apoptosis and cell growth inhibition exhibited by cells treated with the combination of AICAr plus MTX resulted from enhanced AMPK activation. The effects of AICAr, MTX and the combination AICAr plus MTX on the phosphorylation of AMPK, ACC and mTOR, were examined by Western blot analysis in CCRF-CEM and Nalm6 cells following 6 hrs and 24 hrs drug exposure. These early time points were chosen to determine if signaling alterations and ensuing commitment to cell death occurred early during drug treatment. We found that in both ALL cell lines, AMPK phosphorylation/activation at Thr172 was greater after treatment with the combination of AICAr plus MTX compared to single drug treatment, and led to a more robust inhibition of p-mTOR (decreased p-mTOR Ser2448) (Fig. 1C). Expression of p-ACC (Ser79), a surrogate measure of AMPK activity, was also significantly increased following combination treatment. We repeatedly observed that AICAr plus MTX-induced AMPK activation appeared most pronounced in CCRF-CEM cells at the earlier time point (6 hrs) and returned to baseline at 24 hrs for the combination compared to NALM6 cells. The biological relevance of this finding is not clear as the downstream effects of AMPK activation on p-ACC and p-mTOR persisted in both cell lines (Fig. 1C). Nevertheless, this signaling pattern would be consistent with the constitutive activation of Akt signaling, a known AMPK cellular antagonist, in the PTEN mutant CCRF-CEM cells (15).
Our published data shows that AICAr induces phosphorylation of Akt at Ser473 in a dose-dependent manner, and this represents a compensatory survival mechanism in cells exposed to AICAr (8). Therefore, we investigated the effects of the combination of AICAr plus MTX on the expression of p-Akt. Western blot analysis of lysates from cells treated with AICAr, MTX and the combination demonstrated that treatment with AICAr plus MTX significantly potentiates AICAr-induced Akt phosphorylation in both ALL cell lines (Fig. 1C). As expected, baseline levels of p-Akt were higher in the PTEN mutant CCRF-CEM cells when compared to the WT PTEN Nalm6 cells (Fig. 1C). We concluded that the increased expression of p-Akt resulted in part from increased level of AMPK activity on IGF1R/IRS1 signaling (16), the AMPK-induced mTOR inhibition through TSC2 (17), and the relief from the known mTOR-mediated feedback-loop inhibition mechanism (5, 8).
AICAr potentiates MTX-induced Endoplasmic Reticulum stress in ALL cell lines
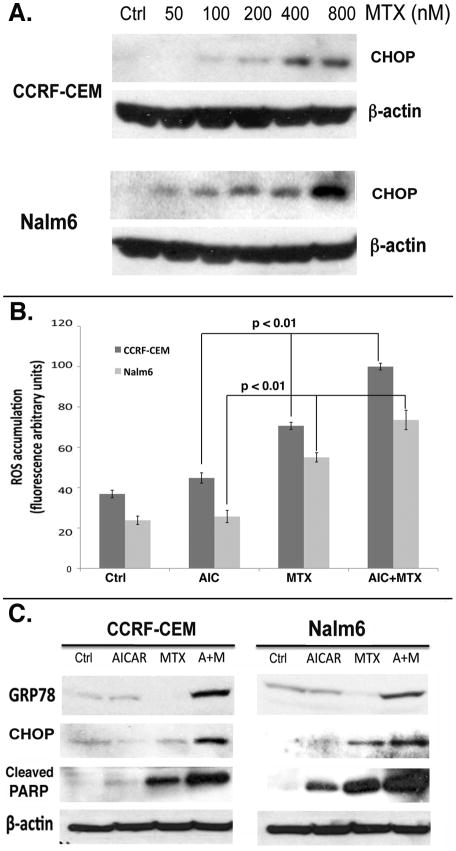
One of the mechanisms by which MTX has been shown to induce apoptosis is through production of reactive oxygen species (ROS) leading to oxidative stress (18). Generation of ROS accumulation in the cytoplasm has been shown to trigger endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) (19), leading to C/EBP homologous protein (CHOP) (20) and GRP78 (21) expression, cleavage of poly(ADP-ribose) polymerase (PARP) and apoptosis (22). To investigate the molecular mechanisms that lead to apoptotic death in ALL cells treated with AICAr plus MTX, and the roles of AMPK and Akt signaling in these processes, we first determined whether MTX as a single agent led to ER stress/UPR in CCRF-CEM and Nalm6 cells. For this, CCRF-CEM and Nalm6 cells were treated with increasing concentrations of MTX (0–800 nM) for 24 hrs, and analyzed by Western blot for expression of CHOP. Our data show a dose-dependent induction of CHOP expression in both ALL cell lines (Fig. 2A). The induction of CHOP at lower doses in Nalm6 cells correlated with their higher sensitivity to MTX compared to CCRF-CEM cells. Next, we analyzed CCRF-CEM and Nalm6 cells treated with AICAr, MTX and AICAr plus MTX, for accumulation of ROS. Consistent with our data and published results, treatment with MTX for 24 hrs induced increased ROS accumulation in both ALL cell lines (Fig. 2B) (18). Of note, exposure of ALL cells to AICAr alone at 200 μM for 24 hrs was insufficient to induce any appreciable increase of ROS (Fig. 2B) or CHOP (Fig. 2C), but in contrast, when these same cells were exposed to AICAr at higher concentrations and for longer time (48 hrs), a substantial increase in ROS accumulation was also observed (data not shown). As shown in Figure 2B, treatment with MTX plus AICAr at 200 μM significantly increased ROS generation compared to treatment with MTX alone (ANOVA, p <0.01 for both cell lines), and resulted in significant potentiation of CHOP and GRP78 expression, leading to the concomitant cleavage of PARP in both CCRF-CEM and Nalm6 cells (Fig. 2C). Therefore, the statistically significant increase in apoptosis induced by the combination of AICAr plus MTX in ALL cells (Fig. 1A and B) appears mediated via ROS-induced ER stress and UPR-dependent induction of apoptotic cell death markers.
Figure 2. AICAr potentiates MTX-induced ROS accumulation and induction of ER stress in ALL cell lines.
Cells were treated with increasing concentrations of MTX for 24 hrs, lysed, immunoblotted and probed with antibody against CHOP (A). Cells were then treated with AICAr, MTX and AICAr plus MTX combination for 24 hrs and assayed for ROS accumulation (B), and the ER stress-specific CHOP, GRP78 and cleaved PARP expression (C), as described in the Materials and Methods. Data in the graphs represent mean and SE from 3 independent experiments. The p values shown on the graph were calculated by one-way ANOVA analysis of each drug and the combination at 24 hr time points. Immunoblots shown are representative of these 3 independent experiments.
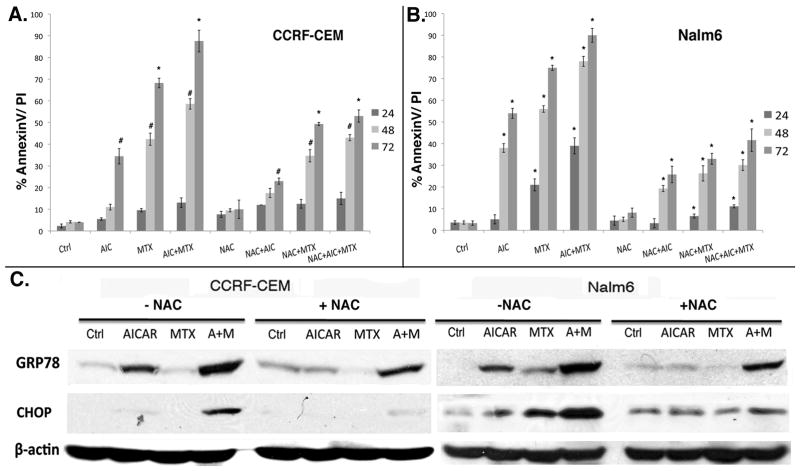
N-Acetyl-L-cysteine alleviates AICAr plus MTX-induced ER stress and apoptosis in ALL
To test whether ROS accumulation was indeed involved in AICAr and MTX-induced ER stress and apoptosis in ALL cells, we employed N-Acetyl-L-cysteine (NAC), a powerful antioxidant known to scavenge and neutralize ROS (23). For this, CCRF-CEM and Nalm6 cells were treated with AICAr, MTX, or AICAr plus MTX (as described under Materials and Methods) in the presence or absence of NAC (2 mM for CCRF-CEM and 5 mM for Nalm6). Cells were harvested after 24 hrs of drug exposure and levels of GRP78 and CHOP expression were determined by Western blotting to assess if commitment to cell death occurred early after drug treatment. Apoptosis was determined by AnnexinV/PI staining after 24, 48 and 72 hrs of continuous exposure. Our data shows that NAC significantly reduced AICAr and MTX-induced apoptosis in both CCRF-CEM and Nalm6 cells as compared to controls (Fig. 3A, B; ANOVA, p <0.01). In addition, NAC reduced AICAr and MTX-induced ER stress/UPR, as evidenced by decreased CHOP and GRP78 expression in cells co-treated with the antioxidant. The effect of NAC was most pronounced in Nalm6 cells and evidenced as early as 24 hrs in comparison to cells treated with MTX or AICAr+MTX in the presence of absence of NAC (Fig. 3B, ANOVA, p <0.01). As it was the case for the cytotoxicity experiments (Fig. 1), CCRF-CEM cells only showed significant reduction in apoptosis after 48 and 72 hrs co-treatment with NAC (Fig. 3A, ANOVA, p< 0.05 and p<0.01, respectively).
Figure 3. N-Acetyl-L-cysteine alleviates AICAr plus MTX-induced ER stress and apoptosis in ALL.
Cells were treated with AICAr, MTX, or AICAr plus MTX (as described under Materials and Methods) in the presence or absence of 2 mM or 5 mM NAC for CCRF-CEM (A) and Nalm6 (B), respectively. Cells were harvested after 24 hrs of drug exposure and levels of GRP78 and CHOP were determined by Western blotting (C). Apoptosis was determined by AnnexinV staining after 24, 48 and 72 hrs of continuous exposure. Data in the graphs represent mean and SE of 3 independent experiments. P values: * corresponds to p<0.01 and # to p<0.05. p values were calculated by one-way ANOVA analysis of each drug/combination in the presence vs. absence of NAC at each time point.
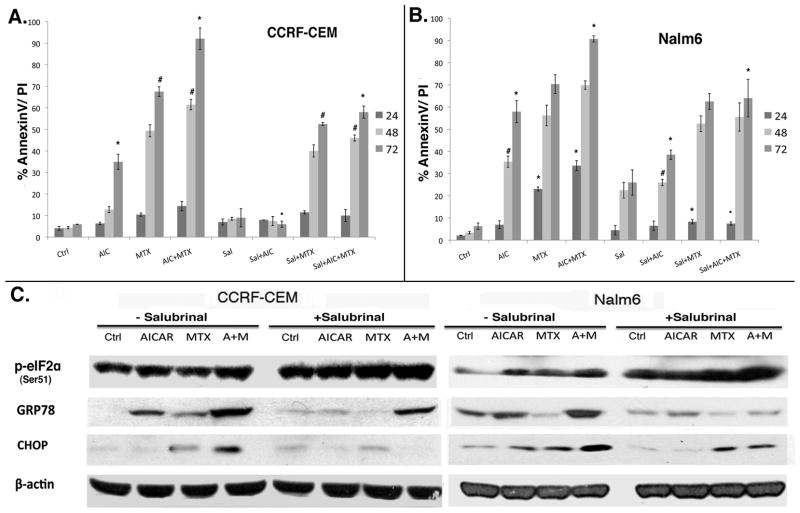
Salubrinal blocks AICAr plus MTX-induced ER stress and alleviates apoptosis in ALL
To assess the role of ER stress in AICAr plus MTX-induced apoptosis in ALL cell models, we used Salubrinal to pharmacologically block ER stress. Salubrinal has been shown to decrease ER stress-induced apoptosis in response to various ER-stress inducers in multiple cell models by inhibiting dephosphorylation of eIF2α and subsequently blocking global protein synthesis to reduce the strain on the ER lumen (24–26). CCRF-CEM and Nalm6 cells were treated with AICAr, MTX, or AICAr plus MTX in the presence or absence of 10 μM Salubrinal. Apoptosis was determined by AnnexinV/PI staining after 24, 48 and 72 hrs of continuous drug exposure. Our data shows that Salubrinal significantly reduced AICAr and MTX-induced ER-stress and apoptosis in both CCRF-CEM and Nalm6 cells (p <0.01 at 72 hrs for both cell lines; Fig. 4). The rescue effect of Salubrinal was evident in CCRF-CEM only after 48 hrs of continuous exposure, whereas in Nalm6 cells the rescue effects were observed as early as 24 hrs, and persisted throughout the 72 hrs exposure (Fig. 4A and B).
Figure 4. ER-stress inhibition alleviates AICAr plus MTX-induced ER stress and apoptosis in ALL.
CCRF-CEM (A) and Nalm6 (B) ALL cells were treated with AICAr, MTX, or AICAr plus MTX (as described under Materials and Methods) in the presence or absence of 10 μM Salubrinal, harvested after 24 hrs of drug exposure, and levels of GRP78, CHOP and eIF2α were determined by Western blotting (C). Apoptosis was determined by AnnexinV staining after 24, 48 and 72 hrs of continuous exposure. Data in the graphs represent mean and SE of 3 independent experiments. P values: * corresponds to p<0.01 and # to p<0.05. p values were calculated by one-way ANOVA analysis of each drug/combination in the presence vs. absence of Salubrinal at a given time point.
To confirm that the rescue effects of Salubrinal were mediated by alleviation of ER stress/UPR, expression of p-eIF2α, GRP78 and CHOP were assayed by Western blotting. Co-treatment with Salubrinal resulted in decreased expression of the ER stress/UPR markers GRP78 and CHOP, and consistent with the known mechanism of action of this agent, expression of p-eIF2α was increased in Salubrinal treated cells (Fig. 4C). These data demonstrated that AICAr and MTX-induced ER stress/UPR is the mechanism responsible for apoptotic cell death in CCRF-CEM and Nalm6 cells treated with this combination.
Inhibition of Akt and the concomitant potentiation of AMPK alleviate AICAr plus MTX-induced ER stress and cytotoxicity in ALL cells
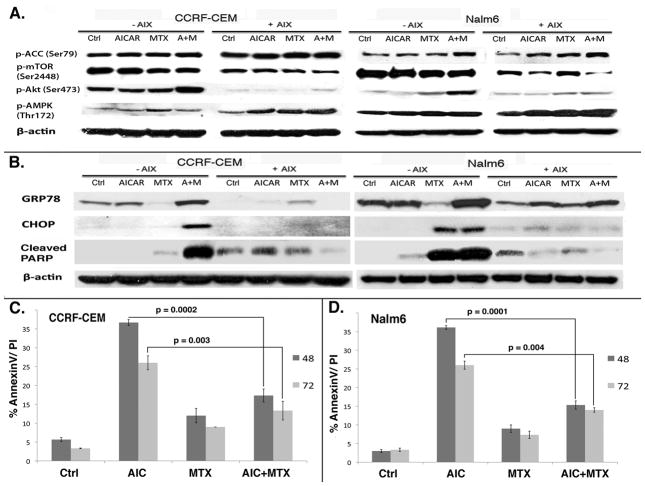
Akt has been shown to be an effective cellular antagonist of AMPK (27–29). We hypothesized that the observed AICAr plus MTX-induced mTOR inhibition, ER stress/UPR signaling pathways, and increased cell death would be potentiated by inhibition of Akt signaling. To test this hypothesis we determined the role of Akt activation in ALL cells treated with AICAr plus MTX. CCRF-CEM and Nalm6 cells were treated with AICAr, MTX, or AICAr plus MTX (as described under Materials and Methods) in the presence or absence of 12μM Akt inhibitor X (AIX) for 24 hrs and expression of p-AMPK, p-ACC, p-Akt, p-mTOR were determined by Western blotting. As shown in Figure 5A, co-treatment with AIX (+AIX blots) led to significant abrogation of activated Akt (p-Akt) in both cells lines under all treatment conditions. Akt inhibition after co-treatment with AIX for 24 hrs resulted in significant increase in p-AMPK/p-ACC in CCRF-CEM and Nalm6 cells treated with AICAR, MTX or the combination, and to a more significant downregulation of p-mTOR compared to single drug treatment (Fig. 5A). These signaling changes are consistent with the known effects of Akt on the AMPK signaling factors examined (27–30).
Figure 5. Inhibition of Akt and the concomitant potentiation of AMPK alleviate AICAr plus MTX-induced ER stress and cytotoxicity in ALL cell lines.
CCRF-CEM and Nalm6 cells were treated as described under Materials and Methods in the presence 12 μM Akt inhibitor X (+AIX) and absence (−AIX). Equal aliquots of protein extracts were analyzed by Western blotting for AMPK (Thr172), ACC (Ser79), mTOR (Ser2448), Akt (Ser472), CHOP, GRP78 and cleaved PARP (A, B). CCRF-CEM and Nalm6 cell death was assessed by AnnexinV staining and flow cytometry at 48 and 72 hrs (C, D). The data represent mean and SE from 3 independent experiments. The p values shown on the graphs were calculated with two-tailed paired t-Test analysis of AICAR plus MTX combination in the presence vs. absence of AIX at each time point.
We then determined how Akt inhibition and upregulation of AMPK influenced ER stress and UPR induction in AICAr plus MTX treated cells. For this, we analyzed expression of the ER stress/UPR markers CHOP, GRP78 and the apoptotic marker PARP in ALL cells co-treated with AIX. As shown in Figure 5B, when CCRF-CEM and Nalm6 cells were treated with AICAr, MTX, AICAr/MTX in the presence or absence of AIX, expression of CHOP, GRP78, and cleavage of PARP were significantly decreased suggesting that upregulation of AMPK and downregulation of Akt may be important regulators of ER stress/UPR in ALL cells. This decrease in ER stress/UPR markers correlated with a 50% abrogation of cell death in both cell lines (Fig. 5C and D). Consequently, our data suggest that Akt inhibition concomitant to AICAr plus MTX-induced upregulation of AMPK relieves ER stress/UPR- induced apoptosis in these ALL models, indicating that the contextual cross-talk between AMPK and Akt are important in determining ALL cell fate through ER stress/UPR related mechanisms.
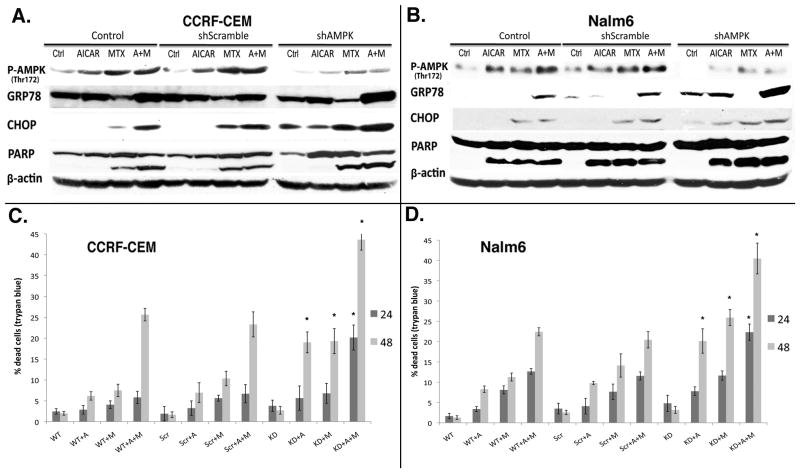
shRNA knockdown of AMPK promotes ER stress and sensitizes ALL cells to AICAr plus MTX-induced cytotoxicity
In order to gain additional insight into the paradoxical pro-apoptotic and pro-survival functions ascribed to AMPK and its role in the relief of ER stress/UPR and cytotoxicity under our experimental conditions, we employed lentivirus encoding shRNA against the α1 and α2 subunits of AMPK to knockdown total AMPK protein levels in both ALL cell lines. Lentiviral-transduced ALL cells were then treated with AICAr, MTX and AICAr plus MTX in combination for 24 hrs (as described under Materials and Methods), lysed, immunoblotted and probed with antibodies against p-AMPK (Thr172), GRP78, CHOP and PARP. We found that lentiviral shRNA-mediated knockdown of AMPK significantly sensitized both ALL cell lines to AICAr plus MTX induced ER stress/UPR, as assessed by increased CHOP and GRP78 expression (Fig. 6A, B). Consequently, AMPK knockdown potentiated the AICAr, MTX and AICAr plus MTX induction of apoptosis, as assessed by increased PARP cleavage (Fig. 6A, B) and increased cell death (Fig. 6C, D). Therefore, in these ALL cell line models AMPK signaling also appears necessary in the regulation of ER stress/UPR. These data and that presented in Figure 5 suggest that under these experimental conditions, concomitant activation AMPK protects ALL cells from ER stress-induced cytotoxicity.
Figure 6. shRNA knockdown of AMPK promotes AICAr plus MTX-induced ER stress and apoptosis in both ALL cell lines.
CCRF-CEM (A) and Nalm6 (B) cells were transduced with lentivirus encoding shScramble (Scr) or shAMPK (Knock-down, KD), and treated as described under Materials and Methods. Mock-transduced cells were used as control. Cells were lysed, immunoblotted and probed with antibodies specific to p-AMPK (Thr172), GRP78, CHOP and PARP (A, B). Cytotoxicity data was obtained by an automated trypan blue exclusion assay as described in the Materials and Methods (C, D). Data in the graphs represent mean and SE of 3 independent experiments. P values were calculated by one-way ANOVA followed by the Newman-Keuls multiple comparison test for each drug or combination in shScramble- vs. shAMPK-expressing cells at a given time point. * corresponds to p<0.01.
DISCUSSION
The anti-proliferative properties of AMPK have become evident with the discovery of the upstream kinase LKB1 and the downstream factor TSC2. Mutations in either LKB1 or TSC2 result in similar clinical syndromes: increased incidence of gastrointestinal polyps, benign tumors, and higher chances of malignant tumor formation, although phenotypes are restricted to different organs (31, 32). Although AMPK modulated activity can be tissue-specific, in general, AMPK activation results in inhibition of lipid, glycogen, and protein synthesis as well as cell growth inhibition and proliferation, while fatty acid oxidation and glucose uptake are concomitantly stimulated (reviewed in (7)). In certain cells and under certain conditions, these AMPK-perturbed processes result in protective and pro-survival effects. For instance, AICAr has been shown to protect cardiac cells from injury and apoptosis caused by myocardial ischemia in humans (33). In most cited cases, AMPK exerts protective effects in cells with a resting phenotype where a decrease in metabolic rate does not alter the balance between survival and death; however, in fast-growing and cancer cells that exhibit elevated anabolic rates, inhibition of ATP-catabolic processes by AMPK is incompatible with survival and often leads to apoptosis (reviewed in (17). Nevertheless, AMPK activation has been shown to promote metabolic changes to maintain cell proliferation and survival in human prostate cancer cells (34), and to protect epithelial cells from TRAIL-induced apoptosis (35). Therefore, paradoxical pro-survival and pro-apoptotic functions have been described for AMPK, which tend to be tissue-specific, and highlight the regulatory complexities of this kinase and related signaling pathways.
Our laboratory has previously described the cytotoxic effects of AICAr on Bp- and T-ALL cell lines, and shown that AICAr inhibited cell proliferation, induced cell cycle arrest and apoptosis in multiple ALL cell lines. Using the adenosine inhibitor iodotubericidin, which blocks conversion of exogenous AICAr to AICAR (ZMP), we confirmed AICAr’s conversion to the AMP-mimetic ZMP is essential for AICAr-induced cytotoxicity in ALL (8). Beckers et al. have shown that MTX markedly sensitizes AMPK for activation by AICAr, and enhances AICAr-induced inhibition of tumor-associated anabolism and colony formation in breast cancer, epidermoid carcinoma and prostate cancer cell lines (11). Herein we demonstrated that similarly, MTX treatment resulted in enhanced AICAr-induced AMPK activation in T- and Bp-ALL cell lines, and that these effects translated to downstream targets such as activation of ACC (p-ACC) and inhibition of mTOR (Fig. 1C). For each of these signaling factors, the combination of AICAr plus MTX resulted in increased perturbations of their phosphorylation status and correlated with a greater induction of cell death compared to each drug alone. Nevertheless, while Bp-ALL cells exhibited increased apoptosis after combination therapy as early as 24 hrs (Fig. 1B), T-ALL cells required at least 48 hrs for increased apoptosis (Fig. 1A). These differences may result from differences in signaling within AMPK and related PI3K/Akt pathways in the PTEN mutant CCRF-CEM cells and/or could be secondary to known differences in the metabolism of drugs such as MTX in Bp-and T-ALL.
Our data also highlights the importance of the cross-talk between AMPK and oncogenic networks such as PI3K/Akt and mTOR. We previously observed that cells treated with increasing concentrations of AICAr exhibited higher levels of Akt phosphorylation as compared to untreated/control cells, and this effect was dose-dependent (8). In this report we uncovered for the first time, that AICAr in combination with MTX significantly induces Akt phosphorylation at residue Ser473 in both CCRF-CEM and Nalm6 cells (Fig. 1C), and phosphorylation at Thr308 in CCRF-CEM cell line only (data not shown). We postulate that the differences in p-Akt expression in these cell lines reflects differences in PTEN expression and resulting downstream Akt activations, since CCRF-CEM cells express an inactivating mutation in PTEN leading to higher constitutive activation of Akt signaling, whereas Nalm6 cells express wild-type PTEN. Furthermore, we have shown that inhibition of Akt with the pharmacological inhibitor of Akt (AIX), leads to a significant increase in AMPK activation in both ALL cell lines (Fig. 5A). We recently reported that the cross-talk between AMPK and Akt is bidirectional, and that AMPK-induced activation of Akt by AICAr is mediated by IGF-1R dependent and independent mechanisms in acute lymphoblastic leukemia (36).
In this report, we demonstrated that MTX induces a dose-dependent induction of CHOP expression in ALL cells, an ER-stress/UPR marker indicative of commitment to apoptosis (Fig. 2A). These data is consistent with the recently reported induction of UPR in the promyelocytic leukemia cell line HL60 following NFkB targeting with MTX (37). More important, employing the antioxidant N-Acetyl-L-cysteine and ER-stress inhibitor Salubrinal, we demonstrated for the first time that the significant increase in induction of apoptotic death attributed to the combination of AICAr plus MTX in ALL cells results from enhanced accumulation of ROS/oxidative stress leading to induction of UPR/ER stress (Figs. 2, 3 and 4).
Altogether our findings describe the ability of AICAr and MTX to perturb three major cellular pathways: AMPK, Akt and UPR/ER stress pathways. MTX potentiates the ability of the AMP-analogue AICAR to activate AMPK by increasing the total levels of AMP and by decreasing the total levels of ATP (10, 11, 38). At the same time, AICAr potentiates MTX-induced ER stress via upregulation of ROS accumulation. Consequently, we postulate that AICAr plus MTX-induced ER stress in conjunction with the upregulation of Akt, which negatively regulates AMPK, leads to prolonged ER stress and subsequent cell death. In this context, activated Akt exerts a pro-apoptotic effect in our experimental model, i.e. when Akt is inhibited and AMPK activity is increased, AICAr plus MTX-induced ER stress is alleviated decreasing cell death (Fig. 5). Therefore, we suggest that the described paradoxical pro-survival and pro-apoptotic responses following AMPK and Akt activation result from the intracellular signaling context within these pathways in cells at the time of drug-induced injury. In conclusion, our data underscore the complexity of the interactions within these pathways, and suggests that the contextual relationship between these signaling proteins and cross-talk with related pathways are critical determinants of cellular responses and fate (survival vs. death) in ALL cells. Consequently, our findings provide a strong rationale for co-targeting strategies that achieve maximal induction of apoptotic death, as an important strategy for clinical translation in future ALL trials.
Acknowledgments
The authors would like to thank Dr. Bart Kamen for insightful discussions, Joanna DeSalvo and Dr. Jianfeng Du for helpful input.
This investigation was supported by the National Cancer Institute (NCI R01-CA098152) and the Leukemia & Lymphoma Society Translational Research Program Grant (6168-09).
Abbreviation list
- ALL
Acute Lymphoblastic Leukemia
- AMPK
AMP activated protein kinase
- ACC
Acetyl-CoA Carboxylase
- CHOP
C/EBP homologous protein
- ER stress
endoplasmic reticulum stress
- MTX
Methotrexate
- AICAr
5-Aminoimidazole-4-carboxamide ribonucleoside
- AICAR (ZMP)
5-Aminoimidazole-4-carboxamide ribonucleotide
- NAC
N-Acetyl-L-cysteine
- UPR
unfolded protein response
References
- 1.Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339:605–15. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 2.Greaves M. Childhood leukaemia. Bmj. 2002;324:283–7. doi: 10.1136/bmj.324.7332.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 4.Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–73. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 5.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: The AMP-activated protein kinase. Trends Biochem Sci. 1999;24:22–5. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 6.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guigas B, Bertrand L, Taleux N, et al. 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes. 2006;55:865–74. doi: 10.2337/diabetes.55.04.06.db05-1178. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta TK, Leclerc GM, Hsieh Kinser TT, Leclerc GJ, Singh I, Barredo JC. Cytotoxic effect of 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) on childhood acute lymphoblastic leukemia (ALL) cells: Implication for targeted therapy. Mol Cancer. 2007;6:46. doi: 10.1186/1476-4598-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–82. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams J, Chen ZP, Van Denderen BJ, et al. Intrasteric control of AMPK via the γ1 subunit AMP allosteric regulatory site. Protein Sci. 2004;13:155–65. doi: 10.1110/ps.03340004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckers A, Organe S, Timmermans L, et al. Methotrexate enhances the antianabolic and antiproliferative effects of 5-aminoimidazole-4-carboxamide riboside. Mol Cancer Ther. 2006;5:2211–7. doi: 10.1158/1535-7163.MCT-06-0001. [DOI] [PubMed] [Google Scholar]
- 12.Racanelli AC, Rothbart SB, Heyer CL, Moran RG. Therapeutics by cytotoxic metabolite accumulation: Pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res. 2009;69:5467–74. doi: 10.1158/0008-5472.CAN-08-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barredo JC, Synold TW, Laver J, et al. Differences in constitutive and post-methotrexate folylpolyglutamate synthetase activity in B-lineage and T-lineage leukemia. Blood. 1994;84:564–9. [PubMed] [Google Scholar]
- 14.Galpin AJ, Schuetz JD, Masson E, et al. Differences in folylpolyglutamate synthetase and dihydrofolate reductase expression in human B-lineage versus T-lineage leukemic lymphoblasts: Mechanisms for lineage differences in methotrexate polyglutamylation and cytotoxicity. Mol Pharmacol. 1997;52:155–63. doi: 10.1124/mol.52.1.155. [DOI] [PubMed] [Google Scholar]
- 15.Palomero Teresa, Luisa Sulis M, Cortina Maria, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276:46912–6. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- 17.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman S, Zurgil N, Deutsch M. Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res. 2005;54:273–80. doi: 10.1007/s00011-005-1355-8. [DOI] [PubMed] [Google Scholar]
- 19.Guan L, Han B, Li Z, et al. Sodium selenite induces apoptosis by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction in human acute promyelocytic leukemia NB4 cells. Apoptosis. 2009;14:218–25. doi: 10.1007/s10495-008-0295-5. [DOI] [PubMed] [Google Scholar]
- 20.Terai K, Hiramoto Y, Masaki M, et al. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–75. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai MT, Huang KL, Chang WM, Lai YK. Geldanamycin induction of grp78 requires activation of reactive oxygen species via ER stress responsive elements in 9L rat brain tumour cells. Cell Signal. 2003;15:585–95. doi: 10.1016/s0898-6568(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 22.Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–9. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- 23.Spagnuolo G, D’Antò V, Cosentino C, Schmalz G, Schweikl H, Rengo S. Effect of N-Acetyl-l-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials. 2006;27:1803–9. doi: 10.1016/j.biomaterials.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Eom KS, Kim HJ, So HS, Park R, Kim TY. Berberine-induced apoptosis in human glioblastoma T98G cells is mediated by endoplasmic reticulum stress accompanying reactive oxygen species and mitochondrial dysfunction. Biol Pharm Bull. 2010;33:1644–9. doi: 10.1248/bpb.33.1644. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Jiang H, Fan Y, et al. Phosphorylation of the α-subunit of the eukaryotic initiation factor-2 (eIF2α) alleviates benzo[a]pyrene-7,8-diol-9,10-epoxide induced cell cycle arrest and apoptosis in human cells. Environ Toxicol Pharmacol. doi: 10.1016/j.etap.2010.08.005. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 26.Zbidi H, Redondo PC, López JJ, Bartegi A, Salido GM, Rosado JA. Homocysteine induces caspase activation by endoplasmic reticulum stress in platelets from type 2 diabetics and healthy donors. Thromb Haemost. 2010;103:1022–32. doi: 10.1160/TH09-08-0552. [DOI] [PubMed] [Google Scholar]
- 27.Horman S, Vertommen D, Heath R, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006 Mar 3;281(9):5335–40. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 28.Berggreen C, Gormand A, Omar B, Degerman E, Goransson O. Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes. Am J Physiol Endocrinol Metab. 2009;296:E635–46. doi: 10.1152/ajpendo.90596.2008. [DOI] [PubMed] [Google Scholar]
- 29.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–9. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 31.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in peutz-jeghers syndrome. Nature. 1998;391:184–7. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi C, Yamaguchi T, Iijima T, et al. Germline mutation of the LKB1/STK11 gene with loss of the normal allele in an aggressive breast cancer of peutz-jeghers syndrome. Oncology. 2004;67:476–9. doi: 10.1159/000082933. [DOI] [PubMed] [Google Scholar]
- 33.Mullane K. Acadesine: The prototype adenosine regulating agent for reducing myocardial ischaemic injury. Cardiovasc Res. 1993;27:43–7. doi: 10.1093/cvr/27.1.43. [DOI] [PubMed] [Google Scholar]
- 34.Park HU, Suy S, Danner M, et al. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol Cancer Ther. 2009;8:733–41. doi: 10.1158/1535-7163.MCT-08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrero-Martin Griselda, Hoyer-Hansen Maria, Garcia-Garcia Celina, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leclerc GM, Leclerc GJ, Fu G, Barredo JC. AMPK-induced activation of Akt by AICAR is mediated by IGF-1R dependent and independent mechanisms in acute lymphoblastic leukemia. J Mol Signal. 2010;5:15. doi: 10.1186/1750-2187-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal NK, Mueller GA, Mueller C, Streich JH, Asif AR, Dihazi H. Expression proteomics of acute promyelocytic leukaemia cells treated with methotrexate. Biochim Biophys Acta. 2010;1804:918–28. doi: 10.1016/j.bbapap.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Swinnen JV, Beckers A, Brusselmans K, et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–8. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]